Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Amani Baidwan, Kendyl Egizi and Alysha Payne
Darrell Jackson, 81 year old male, came to the Emergency Department at Los Robles Hospital by ambulance after he collapsed in a coffee shop. Upon arrival he presented with left sided weakness, facial drooping, and aphasia. He was diagnosed with an ischemic stroke, right humerus head fracture, and right wrist fracture. The priority of care upon initial presentation to the Emergency Department included a CT scan, frequent monitoring of vital signs, starting a peripheral IV, drawing labs, assessing blood glucose, and an EKG. The nurse in the Emergency Department continuously monitored Mr. Jackson’s neurological status, changes in level of consciousness and signs and symptoms of complications.
After much discussion with the family, consent was given for tissue plasminogen activator (tPA). After tPA was given, Mr. Jackson converted to a hemorrhagic stroke, which is one of many risks associated with administration of tPA. He was His computed tomographic scans (CT) revealed intraparenchymal hematoma in both cerebral hemispheres and a large hemorrhage in the left parietal lobe. In the Intensive care Unit, Mr. Jackson was on a ventilator, had a RASS score of -5 and was only responsive to noxious stimuli. Priority in plan of care included airway management and a CPAP trial to begin weaning protocols. The CPAP trial failed, and a tracheostomy was placed. Mr.Jackson was then transferred to the Progressive Care Unit to continue treatment where the NG tube was removed and a PEG tube was inserted. Mr. Jackson has no known allergies and has a history of hypertension, dementia, Parkinson’s disease, stroke, diabetes, GERD, BPH, hypophosphatemia and anemia.
Collaborative interventions are necessary from all healthcare providers, such as physicians, nurses, physical therapy, occupational therapy, speech therapy, case management and social work, to adequate;y care for Mr. Jackson. Case management has been working closely with the family to provide necessary resources to continue care for Mr. Jackson after discharge from the hospital. Mr. Jackson was discharged home with home health after 3 weeks in the hospital. His condition prior to discharge was as follows: A/O x 3 with mild cognitive deficits, speech impairment and left sided weakness.
Discussion Questions
- What is the difference between an ischemic stroke and hemorrhagic stroke?
- What are some of the risk associated with tissue plasminogen activator (tPA) that the nurse would need to assess for and educate the patient about?
- What are some of the psychological needs the nurse should anticipate for a patient who has experienced a stroke?
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.

Share This Book
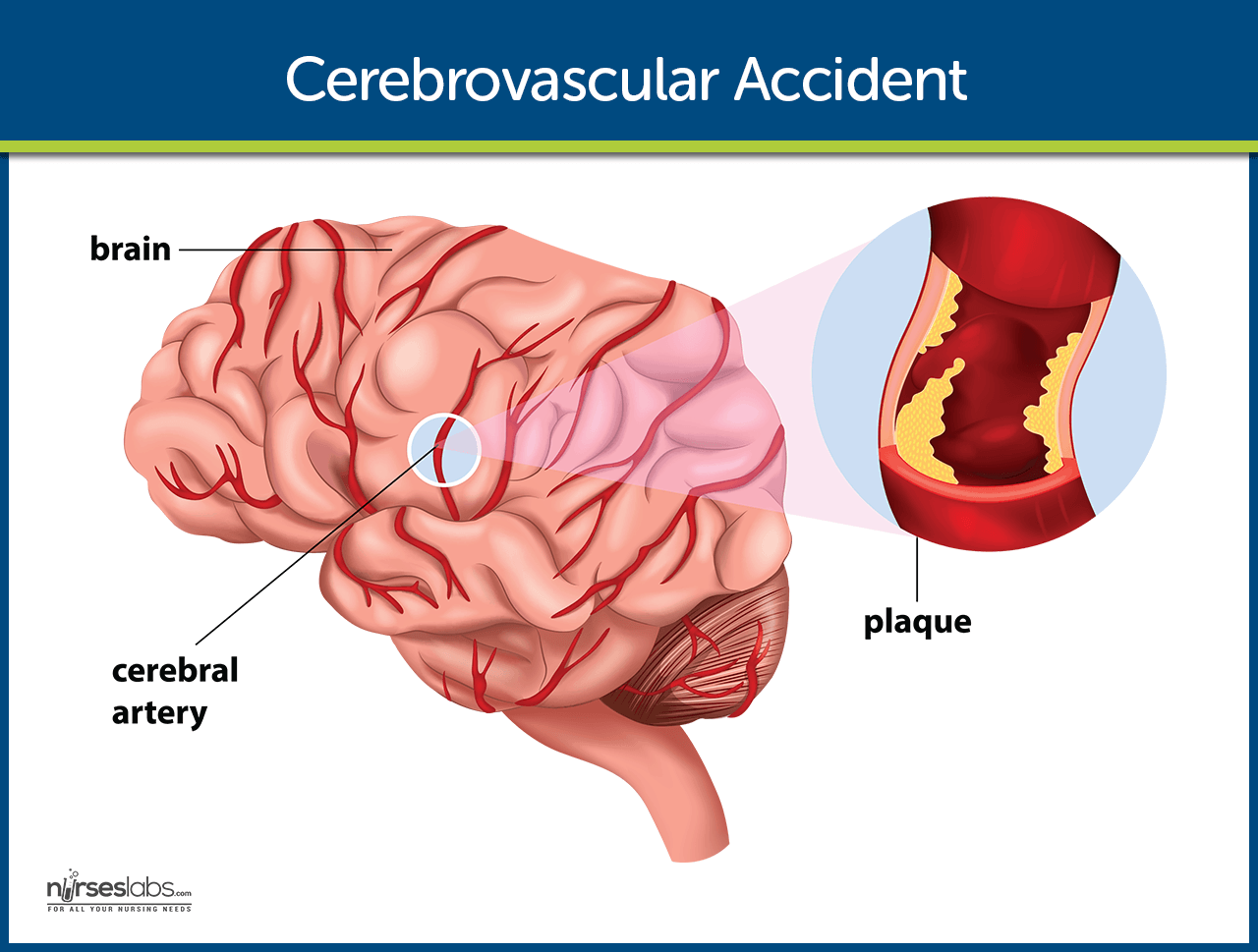
Cerebrovascular Accident (Stroke)

Learn about the nursing care management of patients with cerebrovascular accident in this nursing study guide .
Table of Contents
- What is Cerebrovascular Accident?
Classification
Risk factors, pathophysiology, statistics and epidemiology, clinical manifestations, complications, assessment and diagnostic findings, medical management, surgical management, nursing assessment, nursing diagnosis, nursing care planning & goals, nursing interventions, discharge and home care guidelines, documentation guidelines, what is cerebrovascular accident.
A cerebrovascular accident (CVA), an ischemic stroke or “ brain attack,” is a sudden loss of brain function resulting from a disruption of the blood supply to a part of the brain.
- Cerebrovascular accident or stroke is the primary cerebrovascular disorder in the United States.
- A cerebrovascular accident is a sudden loss of brain functioning resulting from a disruption of the blood supply to a part of the brain.
- It is a functional abnormality of the central nervous system .
- Cryptogenic strokes have no known cause, and other strokes result from causes such as illicit drug use, coagulopathies, migraine, and spontaneous dissection of the carotid or vertebral arteries.
- The result is an interruption in the blood supply to the brain, causing temporary or permanent loss of movement, thought, memory , speech, or sensation.
Strokes can be divided into two classifications.
- Ischemic stroke. This is the loss of function in the brain as a result of a disrupted blood supply.
- Hemorrhagic stroke. Hemorrhagic strokes are caused by bleeding into the brain tissue, the ventricles, or the subarachnoid space.
The following are the nonmodifiable and modifiable risk factors of Cerebrovascular accident:
Nonmodifiable
- Advanced age (older than 55 years)
- Gender (Male)
- Race (African American)
- Hypertension
- Atrial fibrillation
- Hyperlipidemia
- Asymptomatic carotid stenosis and valvular heart disease (eg, endocarditis, prosthetic heart valves)
- Periodontal disease
The disruption in the blood flow initiates a complex series of cellular metabolic events.
- Decreased cerebral blood flow. The ischemic cascade begins when cerebral blood flow decreases to less than 25 mL per 100g of blood per minute.
- Aerobic respiration. At this point, neurons are unable to maintain aerobic respiration.
- Anaerobic respiration. The mitochondria would need to switch to anaerobic respiration, which generates large amounts of lactic acid , causing a change in pH and rendering the neurons incapable of producing sufficient quantities of ATP.
- Loss of function. The membrane pumps that maintain electrolyte balances fail and the cells cease to function.
Stroke is a worldwide phenomenon suffered through all walks of life.
- Morbidity: In 2005, prevalence of stroke was estimated at 2.3 million males and 3.4 million females; many of the approximately 5.7 million U.S. stroke survivors have permanent stroke-related disabilities.
- Mortality: In 2004, stroke ranked fifth as the cause of death for those aged 45 to 64 years and third for those aged 65 years or older (National Heart, Lung and Blood Institute [NHLBI], 2007), with 150,000 deaths (American Heart Association and American Stroke Association, 2008); hemorrhagic strokes are more severe, and mortality rates are higher than ischemic strokes, with a 30-day mortality rate of 40% to 80%.
- Cost: Estimated direct and indirect cost for 2008 was $65.5 billion (American Heart Association and American Stroke Association, 2008).
- Stroke is the third leading cause of death after heart disease and cancer .
- Approximately 780, 000 people experience a stroke each year in the United States.
- Approximately 600, 000 of these are new strokes, and 180, 000 are recurrent strokes.
- About 5.6 million noninstitutionalized stroke survivors are alive today.
- Stroke is the leading cause of serious, long-term disability in the United States.
- Direct and indirect costs for stroke cost $65.5 billion in 2008.
- Strokes are usually hemorrhagic (15%) or ischemic/nonhemorrhagic (85%).
- Ischemic strokes are categorized according to their cause: large artery thrombotic strokes (20%), small penetrating artery thrombotic strokes (25%), cardiogenic embolic strokes (20%), cryptogenic strokes (30%), and other (5%).
Strokes are caused by the following:
- Large artery thrombosis . Large artery thromboses are caused by atherosclerotic plaques in the large blood vessels of the brain.
- Small penetrating artery thrombosis. Small penetrating artery thrombosis affects one or more vessels and is the most common type of ischemic stroke.
- Cardiogenic emboli. Cardiogenic emboli are associated with cardiac dysrhythmias, usually atrial fibrillation .
Stroke can cause a wide variety of neurologic deficits, depending on the location of the lesion, the size of the area of inadequate perfusion, and the amount of the collateral blood flow. General signs and symptoms include numbness or weakness of face, arm, or leg (especially on one side of the body); confusion or change in mental status; trouble speaking or understanding speech; visual disturbances; loss of balance, dizziness, difficulty walking ; or sudden severe headache.
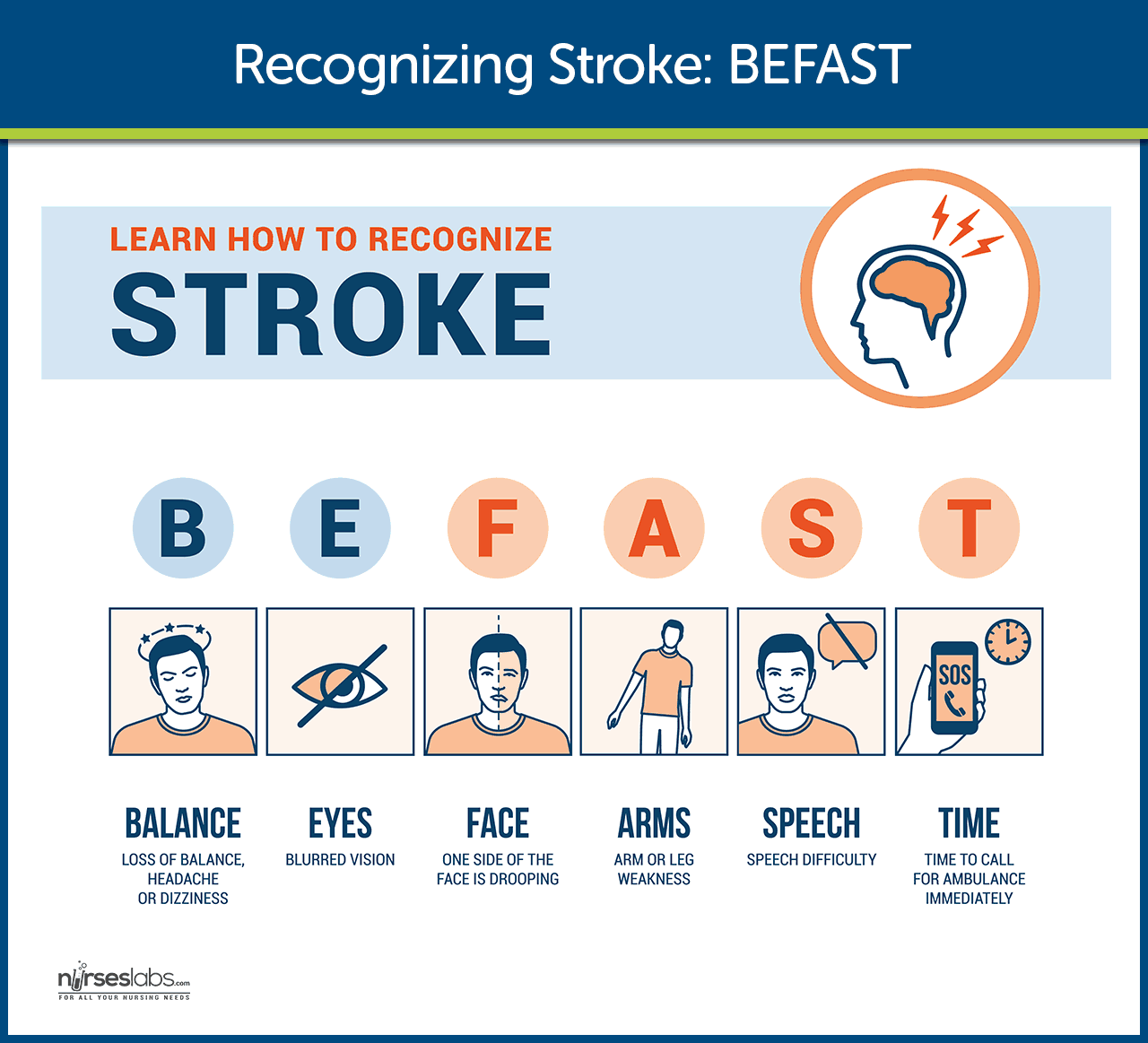
General signs and symptoms include numbness or weakness of face, arm, or leg (especially on one side of the body); confusion or change in mental status; trouble speaking or understanding speech; visual disturbances; loss of balance, dizziness, difficulty walking ; or sudden severe headache.
- Numbness or weakness of the face. Without adequate perfusion, oxygen is also low, and facial tissues could not function properly without them.
- Change in mental status. Due to decreased oxygen, the patient experiences confusion .
- Trouble speaking or understanding speech. Cells cease to function as a result of inadequate perfusion.
- Visual disturbances. The eyes also need enough oxygen for optimal functioning.
- Homonymous hemianopsia. There is loss of half of the visual field.
- Loss of peripheral vision . The patient experiences difficulty seeing at night and is unaware of objects or the borders of objects.
- Hemiparesis. There is a weakness of the face, arm, and leg on the same side due to a lesion in the opposite hemisphere.
- Hemiplegia. Paralysis of the face, arm, and leg on the same side due to a lesion in the opposite hemisphere.
- Ataxia. Staggering, unsteady gait and inability to keep feet together.
- Dysarthria. This is the difficulty in forming words.
- Dysphagia . There is difficulty in swallowing.
- Paresthesia. There is numbness and tingling of extremities and difficulty with proprioception.
- Expressive aphasia . The patient is unable to form words that is understandable yet can speak in single-word responses.
- Receptive aphasia . The patient is unable to comprehend the spoken word and can speak but may not make any sense.
- Global aphasia. This is a combination of both expressive and receptive aphasia.
- Hemiplegia, hemiparesis
- Flaccid paralysis and loss of or decrease in the deep tendon reflexes (initial clinical feature) followed by (after 48 hours) reappearance of deep reflexes and abnormally increased muscle tone (spasticity)
Communication Loss
- Dysarthria (difficulty speaking)
- Dysphasia (impaired speech) or aphasia (loss of speech)
- Apraxia (inability to perform a previously learned action)
Perceptual Disturbances and Sensory Loss
- Visual-perceptual dysfunctions (homonymous hemianopia [loss of half of the visual field])
- Disturbances in visual-spatial relations (perceiving the relation of two or more objects in spatial areas), frequently seen in patients with right hemispheric damage
- Sensory losses: slight impairment of touch or more severe with loss of proprioception; difficulty in interrupting visual, tactile, and auditory stimuli
Impaired Cognitive and Psychological Effects
- Frontal lobe damage: Learning capacity, memory, or other higher cortical intellectual functions may be impaired. Such dysfunction may be reflected in a limited attention span, difficulties in comprehension, forgetfulness, and lack of motivation.
- Depression , other psychological problems: emotional lability, hostility, frustration, resentment, and lack of cooperation.
Primary prevention of stroke remains the best approach.
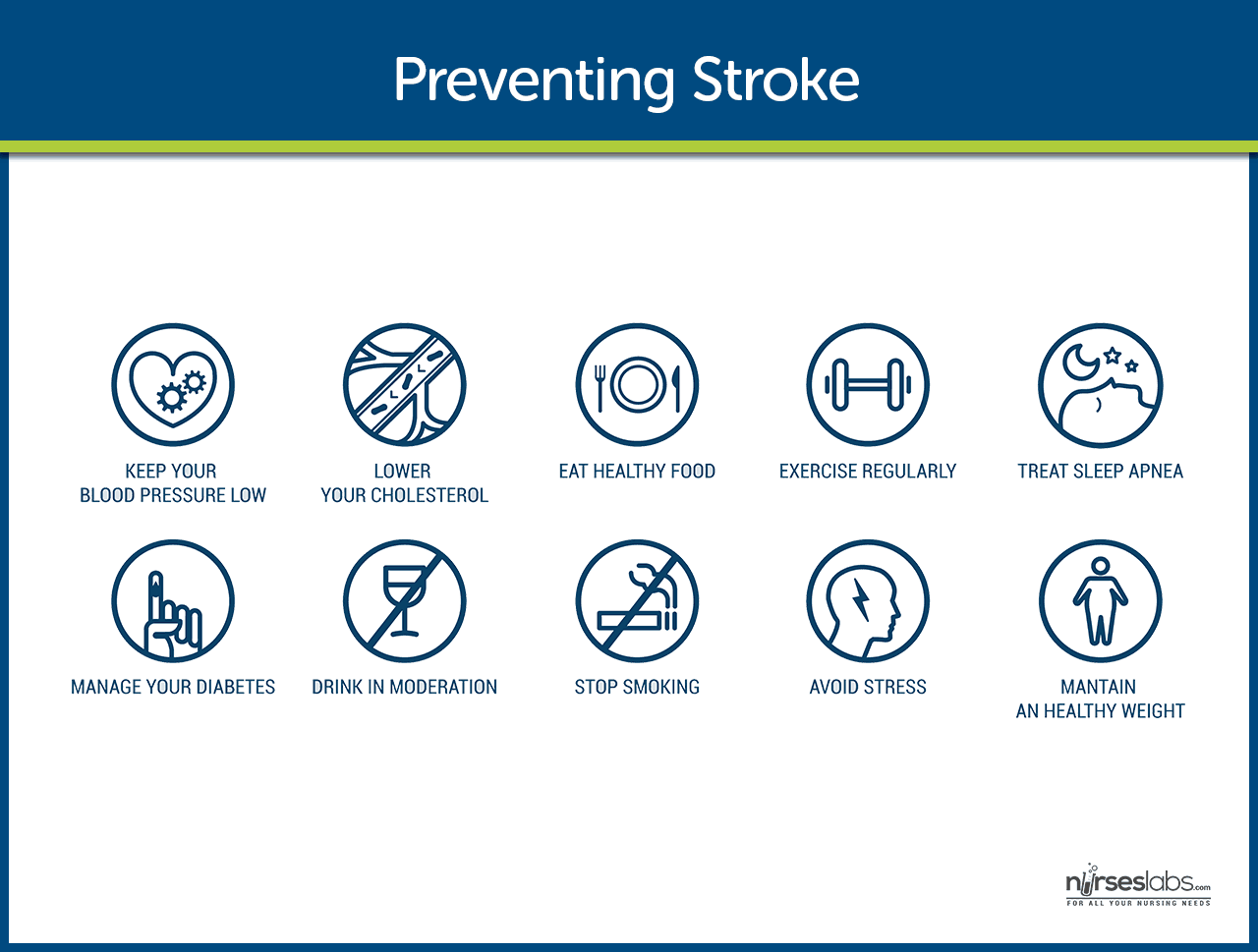
- Healthy lifestyle. Leading a healthy lifestyle which includes not smoking, maintaining a healthy weight, following a healthy diet, and daily exercise can reduce the risk of having a stroke by about one half.
- DASH diet. The DASH ( Dietary Approaches to Stop Hypertension ) diet is high in fruits and vegetables, moderate in low-fat dairy products, and low in animal protein and can lower the risk of stroke.
- Stroke risk screenings. Stroke risk screenings are an ideal opportunity to lower stroke risk by identifying people or groups of people who are at high risk for stroke.
- Education. Patients and the community must be educated about recognition and prevention of stroke.
- Low-dose aspirin . Research findings suggest that low-dose aspirin may lower the risk of stroke in women who are at risk.
If cerebral oxygenation is still inadequate; complications may occur.
- Tissue ischemia . If cerebral blood flow is inadequate, the amount of oxygen supplied to the brain is decreased, and tissue ischemia will result.
- Cardiac dysrhythmias. The heart compensates for the decreased cerebral blood flow, and with too much pumping, dysrhythmias may occur.
Any patient with neurologic deficits needs a careful history and complete physical and neurologic examination.
- CT scan . Demonstrates structural abnormalities, edema , hematomas, ischemia , and infarctions. Demonstrates structural abnormalities, edema , hematomas, ischemia, and infarctions. Note: May not immediately reveal all changes, e.g., ischemic infarcts are not evident on CT for 8–12 hr; however, intracerebral hemorrhage is immediately apparent; therefore, emergency CT is always done before administering tissue plasminogen activator (t-PA). In addition, patients with TIA commonly have a normal CT scan
- PET scan. Provides data on cerebral metabolism and blood flow changes.
- MRI. Shows areas of infarction, hemorrhage , AV malformations, and areas of ischemia.
- Cerebral angiography. Helps determine specific cause of stroke, e.g., hemorrhage or obstructed artery, pinpoints site of occlusion or rupture. Digital subtraction angiography evaluates patency of cerebral vessels, identifies their position in head and neck, and detects/evaluates lesions and vascular abnormalities.
- Lumbar puncture . Pressure is usually normal and CSF is clear in cerebral thrombosis, embolism, and TIA. Pressure elevation and grossly bloody fluid suggest subarachnoid and intracerebral hemorrhage. CSF total protein level may be elevated in cases of thrombosis because of inflammatory process. LP should be performed if septic embolism from bacterial endocarditis is suspected.
- Transcranial Doppler ultrasonography. Evaluates the velocity of blood flow through major intracranial vessels; identifies AV disease, e.g., problems with carotid system (blood flow/presence of atherosclerotic plaques).
- EEG. Identifies problems based on reduced electrical activity in specific areas of infarction; and can differentiate seizure activity from CVA damage.
- Skull x-ray. May show a shift of pineal gland to the opposite side from an expanding mass; calcifications of the internal carotid may be visible in cerebral thrombosis; partial calcification of walls of an aneurysm may be noted in subarachnoid hemorrhage.
- ECG and echocardiography . To rule out cardiac origin as source of embolus (20% of strokes are the result of blood or vegetative emboli associated with valvular disease, dysrhythmias, or endocarditis).
- Laboratory studies to rule out systemic causes: CBC, platelet and clotting studies, VDRL/RPR, erythrocyte sedimentation rate (ESR), chemistries ( glucose , sodium ).
Patients who have experienced TIA or stroke should have medical management for secondary prevention.
- Recombinant tissue plasminogen activator would be prescribed unless contraindicated, and there should be monitoring for bleeding .
- Increased ICP. Management of increased ICP includes osmotic diuretics , maintenance of PaCO2 at 30-35 mmHg, and positioning to avoid hypoxia through elevation of the head of the bed.
- Endotracheal Tube. There is a possibility of intubation to establish patent airway if necessary.
- Hemodynamic monitoring. Continuous hemodynamic monitoring should be implemented to avoid an increase in blood pressure .
- Neurologic assessment to determine if the stroke is evolving and if other acute complications are developing
Surgical management may include prevention and relief from increased ICP.
- Carotid endarterectomy. This is the removal of atherosclerotic plaque or thrombus from the carotid artery to prevent stroke in patients with occlusive disease of the extracranial cerebral arteries.
- Hemicraniectomy. Hemicraniectomy may be performed for increased ICP from brain edema in severe cases of stroke.
Nursing Management
After the stroke is complete, management focuses on the prompt initiation of rehabilitation for any deficits.
During the acute phase , a neurologic flow sheet is maintained to provide data about the following important measures of the patient’s clinical status:
- Change in level of consciousness or responsiveness.
- Presence or absence of voluntary or involuntary movements of extremities.
- Stiffness or flaccidity of the neck.
- Eye opening, comparative size of pupils, and pupillary reaction to light.
- Color of the face and extremities; temperature and moisture of the skin.
- Ability to speak.
- Presence of bleeding.
- Maintenance of blood pressure .
During the postacute phase , assess the following functions:
- Mental status (memory, attention span, perception, orientation, affect, speech/language).
- Sensation and perception (usually the patient has decreased awareness of pain and temperature).
- Motor control (upper and lower extremity movement); swallowing ability, nutritional and hydration status, skin integrity, activity tolerance , and bowel and bladder function.
- Continue focusing nursing assessment on impairment of function in patient’s daily activities.
Based on the assessment data, the major nursing diagnoses for a patient with stroke may include the following:
- Impaired physical mobility related to hemiparesis, loss of balance and coordination , spasticity, and brain injury .
- Acute pain related to hemiplegia and disuse.
- Deficient self-care related to stroke sequelae.
- Disturbed sensory perception related to altered sensory reception, transmission, and/or integration.
- Impaired urinary elimination related to flaccid bladder , detrusor instability, confusion , or difficulty in communicating.
- Disturbed thought processes related to brain damage.
- Impaired verbal communication related to brain damage.
- Risk for impaired skin integrity related to hemiparesis or hemiplegia and decreased mobility .
- Interrupted family processes related to catastrophic illness and caregiving burdens.
- Sexual dysfunction related to neurologic deficits or fear of failure.
Main article: Cerebrovascular Accident (Stroke) Nursing Care Plans
The major nursing care planning goals for the patient and family may include:
- Improve mobility.
- Avoidance of shoulder pain .
- Achievement of self-care .
- Relief of sensory and perceptual deprivation.
- Prevention of aspiration .
- Continence of bowel and bladder.
- Improved thought processes.
- Achieving a form of communication .
- Maintaining skin integrity .
- Restore family functioning.
- Improve sexual function.
- Absence of complications.
Nursing care has a significant impact on the patient’s recovery. In summary, here are some nursing interventions for patients with stroke:
- Positioning. Position to prevent contractures, relieve pressure, attain good body alignment, and prevent compressive neuropathies.
- Prevent flexion . Apply splint at night to prevent flexion of the affected extremity.
- Prevent adduction. Prevent adduction of the affected shoulder with a pillow placed in the axilla.
- Prevent edema. Elevate affected arm to prevent edema and fibrosis.
- Full range of motion. Provide full range of motion four or five times a day to maintain joint mobility.
- Prevent venous stasis. Exercise is helpful in preventing venous stasis, which may predispose the patient to thrombosis and pulmonary embolus .
- Regain balance. Teach patient to maintain balance in a sitting position, then to balance while standing and begin walking as soon as standing balance is achieved.
- Personal hygiene . Encourage personal hygiene activities as soon as the patient can sit up.
- Manage sensory difficulties. Approach patient with a decreased field of vision on the side where visual perception is intact.
- Visit a speech therapist. Consult with a speech therapist to evaluate gag reflexes and assist in teaching alternate swallowing techniques.
- Voiding pattern. Analyze voiding pattern and offer urinal or bedpan on patient’s voiding schedule.
- Be consistent in patient’s activities. Be consistent in the schedule, routines, and repetitions; a written schedule, checklists, and audiotapes may help with memory and concentration, and a communication board may be used.
- Assess skin. Frequently assess skin for signs of breakdown, with emphasis on bony areas and dependent body parts.
Improving Mobility and Preventing Deformities
- Position to prevent contractures; use measures to relieve pressure, assist in maintaining good body alignment, and prevent compressive neuropathies.
- Apply a splint at night to prevent flexion of affected extremity.
- Prevent adduction of the affected shoulder with a pillow placed in the axilla.
- Elevate affected arm to prevent edema and fibrosis.
- Position fingers so that they are barely flexed; place hand in slight supination. If upper extremity spasticity is noted, do not use a hand roll; dorsal wrist splint may be used.
- Change position every 2 hours; place patient in a prone position for 15 to 30 minutes several times a day.
Establishing an Exercise Program
- Provide full range of motion four or five times a day to maintain joint mobility, regain motor control, prevent contractures in the paralyzed extremity, prevent further deterioration of the neuromuscular system, and enhance circulation. If tightness occurs in any area, perform a range of motion exercises more frequently.
- Exercise is helpful in preventing venous stasis, which may predispose the patient to thrombosis and pulmonary embolus.
- Observe for signs of pulmonary embolus or excessive cardiac workload during exercise period (e.g., shortness of breath, chest pain , cyanosis , and increasing pulse rate ).
- Supervise and support the patient during exercises; plan frequent short periods of exercise, not longer periods; encourage the patient to exercise unaffected side at intervals throughout the day.
Preparing for Ambulation
- Start an active rehabilitation program when consciousness returns (and all evidence of bleeding is gone, when indicated).
- Teach patient to maintain balance in a sitting position, then to balance while standing (use a tilt table if needed).
- Begin walking as soon as standing balance is achieved (use parallel bars and have a wheelchair available in anticipation of possible dizziness).
- Keep training periods for ambulation short and frequent.
Preventing Shoulder Pain
- Never lift patient by the flaccid shoulder or pull on the affected arm or shoulder.
- Use proper patient movement and positioning (e.g., flaccid arm on a table or pillows when patient is seated, use of sling when ambulating).
- Range of motion exercises are beneficial, but avoid over strenuous arm movements.
- Elevate arm and hand to prevent dependent edema of the hand; administer analgesic agents as indicated.
Enhancing Self Care
- Encourage personal hygiene activities as soon as the patient can sit up; select suitable self-care activities that can be carried out with one hand.
- Help patient to set realistic goals; add a new task daily.
- As a first step, encourage patient to carry out all self-care activities on the unaffected side.
- Make sure patient does not neglect affected side; provide assistive devices as indicated.
- Improve morale by making sure patient is fully dressed during ambulatory activities.
- Assist with dressing activities (e.g., clothing with Velcro closures; put garment on the affected side first); keep environment uncluttered and organized.
- Provide emotional support and encouragement to prevent fatigue and discouragement.
Managing Sensory-Perceptual Difficulties
- Approach patient with a decreased field of vision on the side where visual perception is intact; place all visual stimuli on this side.
- Teach patient to turn and look in the direction of the defective visual field to compensate for the loss; make eye contact with patient, and draw attention to affected side.
- Increase natural or artificial lighting in the room; provide eyeglasses to improve vision.
- Remind patient with hemianopsia of the other side of the body; place extremities so that patient can see them.
Assisting with Nutrition
- Observe patient for paroxysms of coughing , food dribbling out or pooling in one side of the mouth , food retained for long periods in the mouth, or nasal regurgitation when swallowing liquids.
- Consult with speech therapist to evaluate gag reflexes; assist in teaching alternate swallowing techniques, advise patient to take smaller boluses of food, and inform patient of foods that are easier to swallow; provide thicker liquids or pureed diet as indicated.
- Have patient sit upright, preferably on chair, when eating and drinking; advance diet as tolerated.
- Prepare for GI feedings through a tube if indicated; elevate the head of bed during feedings, check tube position before feeding , administer feeding slowly, and ensure that cuff of tracheostomy tube is inflated (if applicable); monitor and report excessive retained or residual feeding .
Attaining Bowel and Bladder Control
- Perform intermittent sterile catheterization during the period of loss of sphincter control.
- Analyze voiding pattern and offer urinal or bedpan on patient’s voiding schedule.
- Assist the male patient to an upright posture for voiding.
- Provide highfiber diet and adequate fluid intake (2 to 3 L/day), unless contraindicated.
- Establish a regular time (after breakfast) for toileting.
Improving Thought Processes
- Reinforce structured training program using cognitive, perceptual retraining, visual imagery, reality orientation, and cueing procedures to compensate for losses.
- Support patient: Observe performance and progress, give positive feedback, convey an attitude of confidence and hopefulness; provide other interventions as used for improving cognitive function after a head injury.
Improving Communication
- Reinforce the individually tailored program.
- Jointly establish goals, with the patient taking an active part.
- Make the atmosphere conducive to communication , remaining sensitive to patient’s reactions and needs and responding to them in an appropriate manner; treat the patient as an adult.
- Provide strong emotional support and understanding to allay anxiety ; avoid completing patient’s sentences.
- Be consistent in schedule, routines, and repetitions. A written schedule, checklists, and audiotapes may help with memory and concentration; a communication board may be used.
- Maintain patient’s attention when talking with the patient, speak slowly, and give one instruction at a time; allow the patient time to process.
- Talk to aphasic patients when providing care activities to provide social contact.
Maintaining Skin Integrity
- Frequently assess skin for signs of breakdown, with emphasis on bony areas and dependent body parts.
- Employ pressure relieving devices; continue regular turning and positioning (every 2 hours minimally); minimize shear and friction when positioning.
- Keep skin clean and dry, gently massage the healthy dry skin and maintain adequate nutrition .
Improving Family Coping
- Provide counseling and support to the family.
- Involve others in patient’s care; teach stress management techniques and maintenance of personal health for family coping.
- Give family information about the expected outcome of the stroke, and counsel them to avoid doing things for the patient that he or she can do.
- Develop attainable goals for the patient at home by involving the total health care team, patient, and family.
- Encourage everyone to approach the patient with a supportive and optimistic attitude, focusing on abilities that remain; explain to the family that emotional lability usually improves with time.
Helping the Patient Cope with Sexual Dysfunction
- Perform indepth assessment to determine sexual history before and after the stroke.
- Interventions for patient and partner focus on providing relevant information, education, reassurance, adjustment
- of medications, counseling regarding coping skills, suggestions for alternative sexual positions, and a means of sexual expression and satisfaction.
Teaching points
- Teach patient to resume as much self care as possible; provide assistive devices as indicated.
- Have occupational therapist make a home assessment and recommendations to help the patient become more independent.
- Coordinate care provided by numerous health care professionals; help family plan aspects of care.
- Advise family that patient may tire easily, become irritable and upset by small events, and show less interest in daily events.
- Make a referral for home speech therapy. Encourage family involvement. Provide family with practical instructions to help patient between speech therapy sessions.
- Discuss patient’s depression with the physician for possible antidepressant therapy.
- Encourage patient to attend community-based stroke clubs to give a feeling of belonging and fellowship to others.
- Encourage patient to continue with hobbies, recreational and leisure interests, and contact with friends to prevent social isolation .
- Encourage family to support patient and give positive reinforcement.
- Remind spouse and family to attend to personal health and wellbeing.
Expected patient outcomes may include the following:
- Improved mobility.
- Absence of shoulder pain .
- Self-care achieved.
- Achieved a form of communication.
- Maintained skin integrity .
- Restored family functioning.
- Improved sexual function.
Patient and family education is a fundamental component of rehabilitation.
- Consult an occupational therapist. An occupational therapist may be helpful in assessing the home environment and recommending modifications to help the patient become more independent.
- Physical therapy. A program of physical therapy may be beneficial, whether it takes place in the home or in an outpatient program.
- Antidepressant therapy. Depression is a common and serious problem in the patient who has had a stroke.
- Support groups . Community-based stroke support groups may allow the patient and the family to learn from others with si milar problems and to share their experiences.
- Assess caregivers . Nurses should assess caregivers for signs of depression, as depression is also common among caregivers of stroke survivors.
The focus of documentation should involve:
- Individual findings including level of function and ability to participate in specific or desired activities.
- Needed resources and adaptive devices.
- Results of laboratory tests, diagnostic studies, and mental status or cognitive evaluation .
- SO/family support and participation.
- Plan of care and those involved in planning .
- Teaching plan.
- Response to interventions, teaching, and actions performed.
- Attainment or progress toward desired outcomes .
- Modifications to plan of care.
Posts related to Cerebrovascular Accident (Stroke):
- 8+ Cerebrovascular Accident (Stroke) Nursing Care Plans
- Drugs Affecting Coagulation
12 thoughts on “Cerebrovascular Accident (Stroke)”
I’m impressed, I have been challenged to read more.
The article was helpful
Am so impressed with the write up am student will wish to develop a research topic in CVA
As a nursing student, I want to thank this article for the valuable information on cerebrovascular accident nursing management. Understanding the importance of proper care and management for stroke patients is a crucial aspect of my education and future practice as a nurse. This article has provided me with a deeper insight into the role of the nurse in promoting positive outcomes for stroke patients, and I am grateful for the opportunity to learn more about this important topic. Thank you!
very presented alihamudulillah i got something
Hi Mugoya, Wonderful to hear you gained something valuable from the study guide! If you’re curious about more or have any questions, feel free to reach out. Always here to help!
well explained great article for students ……… Kindly increase the number of mcqs
Hi Abdur, Thanks for the positive feedback on the article! I’m glad to hear it’s helpful for students. All of our practice questions are available at our Nursing Test Bank page . If there are specific topics you’d like to see more questions on, just drop a suggestion. Your input helps us create better resources!
So interesting, very very good notes.
So interesting topic to learn
Excelente y completo artículo .muchas felicidades y gracias por la información
Very insightful. ⭐⭐⭐⭐
Can you add reference to the work.
Leave a Comment Cancel reply
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals
You are here
- Volume 13, Issue 8
- Clinical course of a 66-year-old man with an acute ischaemic stroke in the setting of a COVID-19 infection
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-7441-6952 Saajan Basi 1 , 2 ,
- Mohammad Hamdan 1 and
- Shuja Punekar 1
- 1 Department of Stroke and Acute Medicine , King's Mill Hospital , Sutton-in-Ashfield , UK
- 2 Department of Acute Medicine , University Hospitals of Derby and Burton , Derby , UK
- Correspondence to Dr Saajan Basi; saajan.basi{at}nhs.net
A 66-year-old man was admitted to hospital with a right frontal cerebral infarct producing left-sided weakness and a deterioration in his speech pattern. The cerebral infarct was confirmed with CT imaging. The only evidence of respiratory symptoms on admission was a 2 L oxygen requirement, maintaining oxygen saturations between 88% and 92%. In a matter of hours this patient developed a greater oxygen requirement, alongside reduced levels of consciousness. A positive COVID-19 throat swab, in addition to bilateral pneumonia on chest X-ray and lymphopaenia in his blood tests, confirmed a diagnosis of COVID-19 pneumonia. A proactive decision was made involving the patients’ family, ward and intensive care healthcare staff, to not escalate care above a ward-based ceiling of care. The patient died 5 days following admission under the palliative care provided by the medical team.
- respiratory medicine
- infectious diseases
- global health
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bcr-2020-235920
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is a new strain of coronavirus that is thought to have originated in December 2019 in Wuhan, China. In a matter of months, it has erupted from non-existence to perhaps the greatest challenge to healthcare in modern times, grinding most societies globally to a sudden halt. Consequently, the study and research into SARS-CoV-2 is invaluable. Although coronaviruses are common, SARS-CoV-2 appears to be considerably more contagious. The WHO figures into the 2003 SARS-CoV-1 outbreak, from November 2002 to July 2003, indicate a total of 8439 confirmed cases globally. 1 In comparison, during a period of 4 months from December 2019 to July 2020, the number of global cases of COVID-19 reached 10 357 662, increasing exponentially, illustrating how much more contagious SARS-CoV-2 has been. 2
Previous literature has indicated infections, and influenza-like illness have been associated with an overall increase in the odds of stroke development. 3 There appears to be a growing correlation between COVID-19 positive patients presenting to hospital with ischaemic stroke; however, studies investigating this are in progress, with new data emerging daily. This patient report comments on and further characterises the link between COVID-19 pneumonia and the development of ischaemic stroke. At the time of this patients’ admission, there were 95 positive cases from 604 COVID-19 tests conducted in the local community, with a predicted population of 108 000. 4 Only 4 days later, when this patient died, the figure increased to 172 positive cases (81% increase), illustrating the rapid escalation towards the peak of the pandemic, and widespread transmission within the local community ( figure 1 ). As more cases of ischaemic stroke in COVID-19 pneumonia patients arise, the recognition and understanding of its presentation and aetiology can be deciphered. Considering the virulence of SARS-CoV-2 it is crucial as a global healthcare community, we develop this understanding, in order to intervene and reduce significant morbidity and mortality in stroke patients.
- Download figure
- Open in new tab
- Download powerpoint
A graph showing the number of patients with COVID-19 in the hospital and in the community over time.
Case presentation
A 66-year-old man presented to the hospital with signs of left-sided weakness. The patient had a background of chronic obstructive pulmonary disease (COPD), atrial fibrillation and had one previous ischaemic stroke, producing left-sided haemiparesis, which had completely resolved. He was a non-smoker and lived in a house. The patient was found slumped over on the sofa at home on 1 April 2020, by a relative at approximately 01:00, having been seen to have no acute medical illness at 22:00. The patients’ relative initially described disorientation and agitation with weakness noted in the left upper limb and dysarthria. At the time of presentation, neither the patient nor his relative identified any history of fever, cough, shortness of breath, loss of taste, smell or any other symptoms; however, the patient did have a prior admission 9 days earlier with shortness of breath.
The vague nature of symptoms, entwined with considerable concern over approaching the hospital, due to the risk of contracting COVID-19, created a delay in the patients’ attendance to the accident and emergency department. His primary survey conducted at 09:20 on 1 April 2020 demonstrated a patent airway, with spontaneous breathing and good perfusion. His Glasgow Coma Scale (GCS) score was 15 (a score of 15 is the highest level of consciousness), his blood glucose was 7.2, and he did not exhibit any signs of trauma. His abbreviated mental test score was 7 out of 10, indicating a degree of altered cognition. An ECG demonstrated atrial fibrillation with a normal heart rate. His admission weight measured 107 kg. At 09:57 the patient required 2 L of nasal cannula oxygen to maintain his oxygen saturations between 88% and 92%. He started to develop agitation associated with an increased respiratory rate at 36 breaths per minute. On auscultation of his chest, he demonstrated widespread coarse crepitation and bilateral wheeze. Throughout he was haemodynamically stable, with a systolic blood pressure between 143 mm Hg and 144 mm Hg and heart rate between 86 beats/min and 95 beats/min. From a neurological standpoint, he had a mild left facial droop, 2/5 power in both lower limbs, 2/5 power in his left upper limb and 5/5 power in his right upper limb. Tone in his left upper limb had increased. This patient was suspected of having COVID-19 pneumonia alongside an ischaemic stroke.
Investigations
A CT of his brain conducted at 11:38 on 1 April 2020 ( figure 2 ) illustrated an ill-defined hypodensity in the right frontal lobe medially, with sulcal effacement and loss of grey-white matter. This was highly likely to represent acute anterior cerebral artery territory infarction. Furthermore an oval low-density area in the right cerebellar hemisphere, that was also suspicious of an acute infarction. These vascular territories did not entirely correlate with his clinical picture, as limb weakness is not as prominent in anterior cerebral artery territory ischaemia. Therefore this left-sided weakness may have been an amalgamation of residual weakness from his previous stroke, in addition to his acute cerebral infarction. An erect AP chest X-ray with portable equipment ( figure 3 ) conducted on the same day demonstrated patchy peripheral consolidation bilaterally, with no evidence of significant pleural effusion. The pattern of lung involvement raised suspicion of COVID-19 infection, which at this stage was thought to have provoked the acute cerebral infarct. Clinically significant blood results from 1 April 2020 demonstrated a raised C-reactive protein (CRP) at 215 mg/L (normal 0–5 mg/L) and lymphopaenia at 0.5×10 9 (normal 1×10 9 to 3×10 9 ). Other routine blood results are provided in table 1 .
CT imaging of this patients’ brain demonstrating a wedge-shaped infarction of the anterior cerebral artery territory.
Chest X-ray demonstrating the bilateral COVID-19 pneumonia of this patient on admission.
- View inline
Clinical biochemistry and haematology blood results of the patient
Interestingly the patient, in this case, was clinically assessed in the accident and emergency department on 23 March 2020, 9 days prior to admission, with symptoms of shortness of breath. His blood results from this day showed a CRP of 22 mg/L and a greater lymphopaenia at 0.3×10 9 . He had a chest X-ray ( figure 4 ), which indicated mild radiopacification in the left mid zone. He was initially treated with intravenous co-amoxiclav and ciprofloxacin. The following day he had minimal symptoms (CURB 65 score 1 for being over 65 years). Given improving blood results (declining CRP), he was discharged home with a course of oral amoxicillin and clarithromycin. As national governmental restrictions due to COVID-19 had not been formally announced until 23 March 2020, and inconsistencies regarding personal protective equipment training and usage existed during the earlier stages of this rapidly evolving pandemic, it is possible that this patient contracted COVID-19 within the local community, or during his prior hospital admission. It could be argued that the patient had early COVID-19 signs and symptoms, having presented with shortness of breath, lymphopaenia, and having had subtle infective chest X-ray changes. The patient explained he developed a stagnant productive cough, which began 5 days prior to his attendance to hospital on 23 March 2020. He responded to antibiotics, making a full recovery following 7 days of treatment. This information does not assimilate with the typical features of a COVID-19 infection. A diagnosis of community-acquired pneumonia or infective exacerbation of COPD seem more likely. However, given the high incidence of COVID-19 infections during this patients’ illness, an exposure and early COVID-19 illness, prior to the 23 March 2020, cannot be completely ruled out.
Chest X-ray conducted on prior admission illustrating mild radiopacification in the left mid zone.
On the current admission, this patient was managed with nasal cannula oxygen at 2 L. By the end of the day, this had progressed to a venturi mask, requiring 8 L of oxygen to maintain oxygen saturation. He had also become increasingly drowsy and confused, his GCS declined from 15 to 12. However, the patient was still haemodynamically stable, as he had been in the morning. An arterial blood gas demonstrated a respiratory alkalosis (pH 7.55, pCO 2 3.1, pO 2 6.7 and HCO 3 24.9, lactate 1.8, base excess 0.5). He was commenced on intravenous co-amoxiclav and ciprofloxacin, to treat a potential exacerbation of COPD. This patient had a COVID-19 throat swab on 1 April 2020. Before the result of this swab, an early discussion was held with the intensive care unit staff, who decided at 17:00 on 1 April 2020 that given the patients presentation, rapid deterioration, comorbidities and likely COVID-19 diagnosis he would not be for escalation to the intensive care unit, and if he were to deteriorate further the end of life pathway would be most appropriate. The discussion was reiterated to the patients’ family, who were in agreement with this. Although he had evidence of an ischaemic stroke on CT of his brain, it was agreed by all clinicians that intervention for this was not as much of a priority as providing optimal palliative care, therefore, a minimally invasive method of treatment was advocated by the stroke team. The patient was given 300 mg of aspirin and was not a candidate for fibrinolysis.
Outcome and follow-up
The following day, before the throat swab result, had appeared the patient deteriorated further, requiring 15 L of oxygen through a non-rebreather face mask at 60% FiO 2 to maintain his oxygen saturation, at a maximum of 88% overnight. At this point, he was unresponsive to voice, with a GCS of 5. Although, he was still haemodynamically stable, with a blood pressure of 126/74 mm Hg and a heart rate of 98 beats/min. His respiratory rate was 30 breaths/min. His worsening respiratory condition, combined with his declining level of consciousness made it impossible to clinically assess progression of the neurological deficit generated by his cerebral infarction. Moreover, the patient was declining sharply while receiving the maximal ward-based treatment available. The senior respiratory physician overseeing the patients’ care decided that a palliative approach was in this his best interest, which was agreed on by all parties. The respiratory team completed the ‘recognising dying’ documentation, which signified that priorities of care had shifted from curative treatment to palliative care. Although the palliative team was not formally involved in the care of the patient, the patient received comfort measures without further attempts at supporting oxygenation, or conduction of regular clinical observations. The COVID-19 throat swab confirmed a positive result on 2 April 2020. The patient was treated by the medical team under jurisdiction of the hospital palliative care team. This included the prescribing of anticipatory medications and a syringe driver, which was established on 3 April 2020. His antibiotic treatment, non-essential medication and intravenous fluid treatment were discontinued. His comatose condition persisted throughout the admission. Once the patients’ GCS was 5, it did not improve. The patient was pronounced dead by doctors at 08:40 on 5 April 2020.
SARS-CoV-2 is a type of coronavirus that was first reported to have caused pneumonia-like infection in humans on 3 December 2019. 5 As a group, coronaviruses are a common cause of upper and lower respiratory tract infections (especially in children) and have been researched extensively since they were first characterised in the 1960s. 6 To date, there are seven coronaviruses that are known to cause infection in humans, including SARS-CoV-1, the first known zoonotic coronavirus outbreak in November 2002. 7 Coronavirus infections pass through communities during the winter months, causing small outbreaks in local communities, that do not cause significant mortality or morbidity.
SARS-CoV-2 strain of coronavirus is classed as a zoonotic coronavirus, meaning the virus pathogen is transmitted from non-humans to cause disease in humans. However the rapid spread of SARS-CoV-2 indicates human to human transmission is present. From previous research on the transmission of coronaviruses and that of SARS-CoV-2 it can be inferred that SARS-CoV-2 spreads via respiratory droplets, either from direct inhalation, or indirectly touching surfaces with the virus and exposing the eyes, nose or mouth. 8 Common signs and symptoms of the COVID-19 infection identified in patients include high fevers, severe fatigue, dry cough, acute breathing difficulties, bilateral pneumonia on radiological imaging and lymphopaenia. 9 Most of these features were identified in this case study. The significance of COVID-19 is illustrated by the speed of its global spread and the potential to cause severe clinical presentations, which as of April 2020 can only be treated symptomatically. In Italy, as of mid-March 2020, it was reported that 12% of the entire COVID-19 positive population and 16% of all hospitalised patients had an admission to the intensive care unit. 10
The patient, in this case, illustrates the clinical relevance of understanding COVID-19, as he presented with an ischaemic stroke underlined by minimal respiratory symptoms, which progressed expeditiously, resulting in acute respiratory distress syndrome and subsequent death.
Our case is an example of a new and ever-evolving clinical correlation, between patients who present with a radiological confirmed ischaemic stroke and severe COVID-19 pneumonia. As of April 2020, no comprehensive data of the relationship between ischaemic stroke and COVID-19 has been published, however early retrospective case series from three hospitals in Wuhan, China have indicated that up to 36% of COVID-19 patients had neurological manifestations, including stroke. 11 These studies have not yet undergone peer review, but they tell us a great deal about the relationship between COVID-19 and ischaemic stroke, and have been used to influence the American Heart Associations ‘Temporary Emergency Guidance to US Stroke Centres During the COVID-19 Pandemic’. 12
The relationship between similar coronaviruses and other viruses, such as influenza in the development of ischaemic stroke has previously been researched and provide a basis for further investigation, into the prominence of COVID-19 and its relation to ischaemic stroke. 3 Studies of SARS-CoV-2 indicate its receptor-binding region for entry into the host cell is the same as ACE2, which is present on endothelial cells throughout the body. It may be the case that SARS-CoV-2 alters the conventional ability of ACE2 to protect endothelial function in blood vessels, promoting atherosclerotic plaque displacement by producing an inflammatory response, thus increasing the risk of ischaemic stroke development. 13
Other hypothesised reasons for stroke development in COVID-19 patients are the development of hypercoagulability, as a result of critical illness or new onset of arrhythmias, caused by severe infection. Some case studies in Wuhan described immense inflammatory responses to COVID-19, including elevated acute phase reactants, such as CRP and D-dimer. Raised D-dimers are a non-specific marker of a prothrombotic state and have been associated with greater morbidity and mortality relating to stroke and other neurological features. 14
Arrhythmias such as atrial fibrillation had been identified in 17% of 138 COVID-19 patients, in a study conducted in Wuhan, China. 15 In this report, the patient was known to have atrial fibrillation and was treated with rivaroxaban. The acute inflammatory state COVID-19 is known to produce had the potential to create a prothrombotic environment, culminating in an ischaemic stroke.
Some early case studies produced in Wuhan describe patients in the sixth decade of life that had not been previously noted to have antiphospholipid antibodies, contain the antibodies in blood results. They are antibodies signify antiphospholipid syndrome; a prothrombotic condition. 16 This raises the hypothesis concerning the ability of COVID-19 to evoke the creation of these antibodies and potentiate thrombotic events, such as ischaemic stroke.
No peer-reviewed studies on the effects of COVID-19 and mechanism of stroke are published as of April 2020; therefore, it is difficult to evidence a specific reason as to why COVID-19 patients are developing neurological signs. It is suspected that a mixture of the factors mentioned above influence the development of ischaemic stroke.
If we delve further into this patients’ comorbid state exclusive to COVID-19 infection, it can be argued that this patient was already at a relatively higher risk of stroke development compared with the general population. The fact this patient had previously had an ischaemic stroke illustrates a prior susceptibility. This patient had a known background of hypertension and atrial fibrillation, which as mentioned previously, can influence blood clot or plaque propagation in the development of an acute ischaemic event. 15 Although the patient was prescribed rivaroxaban as an anticoagulant, true consistent compliance to rivaroxaban or other medications such as amlodipine, clopidogrel, candesartan and atorvastatin cannot be confirmed; all of which can contribute to the reduction of influential factors in the development of ischaemic stroke. Furthermore, the fear of contracting COVID-19, in addition to his vague symptoms, unlike his prior ischaemic stroke, which demonstrated dense left-sided haemiparesis, led to a delay in presentation to hospital. This made treatment options like fibrinolysis unachievable, although it can be argued that if he was already infected with COVID-19, he would have still developed life-threatening COVID-19 pneumonia, regardless of whether he underwent fibrinolysis. It is therefore important to consider that if this patient did not contract COVID-19 pneumonia, he still had many risk factors that made him prone to ischaemic stroke formation. Thus, we must consider whether similar patients would suffer from ischaemic stroke, regardless of COVID-19 infection and whether COVID-19 impacts on the severity of the stroke as an entity.
Having said this, the management of these patients is dependent on the likelihood of a positive outcome from the COVID-19 infection. Establishing the ceiling of care is crucial, as it prevents incredibly unwell or unfit patients’ from going through futile treatments, ensuring respect and dignity in death, if this is the likely outcome. It also allows for the provision of limited or intensive resources, such as intensive care beds or endotracheal intubation during the COVID-19 pandemic, to those who are assessed by the multidisciplinary team to benefit the most from their use. The way to establish this ceiling of care is through an early multidisciplinary discussion. In this case, the patient did not convey his wishes regarding his care to the medical team or his family; therefore it was decided among intensive care specialists, respiratory physicians, stroke physicians and the patients’ relatives. The patient was discussed with the intensive care team, who decided that as the patient sustained two acute life-threatening illnesses simultaneously and had rapidly deteriorated, ward-based care with a view to palliate if the further deterioration was in the patients’ best interests. These decisions were not easy to make, especially as it was on the first day of presentation. This decision was made in the context of the patients’ comorbidities, including COPD, the patients’ age, and the availability of intensive care beds during the steep rise in intensive care admissions, in the midst of the COVID-19 pandemic ( figure 1 ). Furthermore, the patients’ rapid and permanent decline in GCS, entwined with the severe stroke on CT imaging of the brain made it more unlikely that significant and permanent recovery could be achieved from mechanical intubation, especially as the damage caused by the stroke could not be significantly reversed. As hospitals manage patients with COVID-19 in many parts of the world, there may be tension between the need to provide higher levels of care for an individual patient and the need to preserve finite resources to maximise the benefits for most patients. This patient presented during a steep rise in intensive care admissions, which may have influenced the early decision not to treat the patient in an intensive care setting. Retrospective studies from Wuhan investigating mortality in patients with multiple organ failure, in the setting of COVID-19, requiring intubation have demonstrated mortality can be up to 61.5%. 17 The mortality risk is even higher in those over 65 years of age with respiratory comorbidities, indicating why this patient was unlikely to survive an admission to the intensive care unit. 18
Regularly updating the patients’ family ensured cooperation, empathy and sympathy. The patients’ stroke was not seen as a priority given the severity of his COVID-19 pneumonia, therefore the least invasive, but most appropriate treatment was provided for his stroke. The British Association of Stroke Physicians advocate this approach and also request the notification to their organisation of COVID-19-related stroke cases, in the UK. 19
Learning points
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is one of seven known coronaviruses that commonly cause upper and lower respiratory tract infections. It is the cause of the 2019–2020 global coronavirus pandemic.
The significance of COVID-19 is illustrated by the rapid speed of its spread globally and the potential to cause severe clinical presentations, such as ischaemic stroke.
Early retrospective data has indicated that up to 36% of COVID-19 patients had neurological manifestations, including stroke.
Potential mechanisms behind stroke in COVID-19 patients include a plethora of hypercoagulability secondary to critical illness and systemic inflammation, the development of arrhythmia, alteration to the vascular endothelium resulting in atherosclerotic plaque displacement and dehydration.
It is vital that effective, open communication between the multidisciplinary team, patient and patients relatives is conducted early in order to firmly establish the most appropriate ceiling of care for the patient.
- Cannine M , et al
- Wunderink RG
- van Doremalen N ,
- Bushmaker T ,
- Morris DH , et al
- Wang X-G , et al
- Grasselli G ,
- Pesenti A ,
- Wang M , et al
- American Stroke Assocation, 2020
- Zhang Y-H ,
- Zhang Y-huan ,
- Dong X-F , et al
- Li X , et al
- Hu C , et al
- Zhang S , et al
- Jiang B , et al
- Xu J , et al
- British Association of Stroke Physicians
Contributors SB was involved in the collecting of information for the case, the initial written draft of the case and researching existing data on acute stroke and COVID-19. He also edited drafts of the report. MH was involved in reviewing and editing drafts of the report and contributing new data. SP oversaw the conduction of the project and contributed addition research papers.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Next of kin consent obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 7: 10 Real Cases on Transient Ischemic Attack and Stroke: Diagnosis, Management, and Follow-Up
Jeirym Miranda; Fareeha S. Alavi; Muhammad Saad
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Case review, case discussion, clinical symptoms.
- Radiologic Findings
- Full Chapter
- Supplementary Content
Case 1: Management of Acute Thrombotic Cerebrovascular Accident Post Recombinant Tissue Plasminogen Activator Therapy
A 59-year-old Hispanic man presented with right upper and lower extremity weakness, associated with facial drop and slurred speech starting 2 hours before the presentation. He denied visual disturbance, headache, chest pain, palpitations, dyspnea, dysphagia, fever, dizziness, loss of consciousness, bowel or urinary incontinence, or trauma. His medical history was significant for uncontrolled type 2 diabetes mellitus, hypertension, hyperlipidemia, and benign prostatic hypertrophy. Social history included cigarette smoking (1 pack per day for 20 years) and alcohol intake of 3 to 4 beers daily. Family history was not significant, and he did not remember his medications. In the emergency department, his vital signs were stable. His physical examination was remarkable for right-sided facial droop, dysarthria, and right-sided hemiplegia. The rest of the examination findings were insignificant. His National Institutes of Health Stroke Scale (NIHSS) score was calculated as 7. Initial CT angiogram of head and neck reported no acute intracranial findings. The neurology team was consulted, and intravenous recombinant tissue plasminogen activator (t-PA) was administered along with high-intensity statin therapy. The patient was admitted to the intensive care unit where his hemodynamics were monitored for 24 hours and later transferred to the telemetry unit. MRI of the head revealed an acute 1.7-cm infarct of the left periventricular white matter and posterior left basal ganglia. How would you manage this case?
This case scenario presents a patient with acute ischemic cerebrovascular accident (CVA) requiring intravenous t-PA. Diagnosis was based on clinical neurologic symptoms and an NIHSS score of 7 and was later confirmed by neuroimaging. He had multiple comorbidities, including hypertension, diabetes, dyslipidemia, and smoking history, which put him at a higher risk for developing cardiovascular disease. Because his symptoms started within 4.5 hours of presentation, he was deemed to be a candidate for thrombolytics. The eligibility time line is estimated either by self-report or last witness of baseline status.
Ischemic strokes are caused by an obstruction of a blood vessel, which irrigates the brain mainly secondary to the development of atherosclerotic changes, leading to cerebral thrombosis and embolism. Diagnosis is made based on presenting symptoms and CT/MRI of the head, and the treatment is focused on cerebral reperfusion based on eligibility criteria and timing of presentation.
Symptoms include alteration of sensorium, numbness, decreased motor strength, facial drop, dysarthria, ataxia, visual disturbance, dizziness, and headache.
Get Free Access Through Your Institution
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Explore prestigious scientific journals and award and funding opportunities to advance your research.
Access practice-improvement tools, including guidelines, measures, and practice management resources.
Learn to effectively advocate on behalf of neurologists and their patients, and access AAN position and policy statements.
Loading... please wait
We’re experiencing unusually high levels of traffic. Thank you for your patience.
Inpatient Stroke Case Studies
Inpatient e/m case studies.
Case study of a stroke patient at varying visit levels to better understand code selection for inpatient encounters under the revised guidelines for 2024.
67-year-old Female with Stroke
Total time* for Inpatient E/M in 2023
Refer to the following tables for correct code selection when billing based on time for inpatient E/M Services:
| CPT Code | Time (Minutes) | |
| 99221 99222 99223 | 40 55 75 | |
| 99231 99232 99233 | 25 35 50 | |
| 99291 99292 | First 30-74 Each additional 30 |
*Total time includes non face-to-face time on the date of service
Day 1: Critical Care (99291)
A 67-year-old woman with hypertension and diabetes presents to the emergency department with abrupt onset of left hemiparesis 45 minutes ago.
Pre-evaluation : Discussed presentation and vital signs with ED provider (3 mins).
Face-to-face evaluation : Performed medically appropriate history and exam. She has a dense left hemiparesis and an NIH Stroke Scale score of 8. Thrombolysis safety criteria reviewed (7 mins).
Post-evaluation : Non-contrast head CT, CTA of head and neck, and lab results reviewed in the ED. Case discussed with ED provider and thrombolysis recommended. Consultation documented in the ED (25 mins).
Total time : 35 minutes.
| Problems Addressed | Data Reviewed | Patient Management Risk of Complications |
| Acute impairment of a vital organ system with high risk of deterioration | Independent hisotry, review of vitals and lab data, review of head CT and other imaging | Recommendation for thrombolysis |
| High/Critical | High/Critical | High/Critical |
Critical Care Coding
According to the 2024 CPT code set, a provider may bill for critical care when the following requirements are met:
- A critical condition: one that acutely impairs a vital organ system with a high probability of imminent or life-threatening deterioration. This includes, for example, central nervous system failure.
- Direct delivery of critical care: high complexity decision-making to assess, manipulate, and support vital systems to treat organ system failure or prevent further life-threatening deterioration.
- At least 30 minutes of time spent solely in the care of the patient. It does not need to be continuous, and it includes both time at the bedside and time spent on the same floor or unit engaged in work directly related to the patient’s care (e.g., documenting critical care, reviewing test results, discussing care with other providers, obtaining history, or discussing treatments or treatment limitations with surrogates when the patient lacks the capacity to do so).
Specific critical care credentials are not required to bill critical care. Critical care is usually provided in a critical care area such as an intensive care unit or emergency department, but this is not always the case (for example, critical care provided to a deteriorating patient in a non-critical care unit).
Other examples of critical care might include:
- Evaluating a patient with status epilepticus and prescribing anti-epileptic drugs or sedative infusions,
- Evaluating a patient with acute respiratory failure from neuromuscular disease and prescribing plasmapheresis,
- Evaluating a patient with coma after cardiac arrest and discussing prognosis, treatment, and goals of care with surrogates (documenting the patient’s lack of capacity to participate)
| Condition | Treatment | Time |
| Acute ischemic stroke | Thrombolysis | 35 minutes |
Critical care, 30-74 minutes CPT 99291 is justified based on the above documentation, although E&M codes (e.g., 99223) associated with fewer wRVUs and lower reimbursement could be used as well.
Day 2: Subsequent Hospital Inpatient Care
Pre-rounds : Reviewed vitals, labs, and studies (LDL, Hemoglobin A1c, EKG, TTE). Review and document independent interpretation of MRI (8 mins).
On Rounds : Performed medically appropriate history and exam. The patient’s symptoms and findings improved somewhat overnight. Patient counseled about stroke evaluation and secondary prevention (10 mins).
Post-rounds : Order atorvastatin, order diabetes consult for management of diabetes. Document discussion with case management possible need for acute inpatient rehabilitation. Documentation completed (10 mins).
Total time : 28 minutes
| Problems Addressed | Data Reviewed | Patient Management Risk of Complications |
| One acute illness that poses a threat to life or bodily function | Review of test results Independent interpretation of tests Discussion of management | Prescription drug management |
| High | High | Moderate |
| Time | MDM |
| Total time of visit (includes all time on pre-rounds, on round, and post-rounds): Subsequent encounter, level 1: ≥ 25 minutes
| Problems addressed: Data reviewed: Risk of complications: Level 3:
|
In this situation, billing according to MDM would be associated with higher reimbursement.
Day 3: Discharge Day Management (By Primary Service)
Pre-rounds : Reviewed vitals, daily CBC and BMP, nursing notes and PT/OT notes (5 mins).
On Rounds : Performed medically appropriate history and exam. The patient reports continued slight improvement in symptoms and requests counseling on how complementary and alternative medicine might help manage her chronic conditions (15 mins).
Post-rounds : Prescribe antiplatelet agent, antidiabetic medications, and antihypertensives. Prepare discharge paperwork and document discharge summary (15 mins).
Total time : 35 minutes
| Problems Addressed | Data Reviewed | Patient Management Risk of Complications |
| One acute illness that poses a threat to life or bodily function | Limited | Prescription drug management |
| High | Low | Moderate |
Discharge Day Management Coding (Inpatient or Observation)
Discharge CPTs are selected based on total (face-to-face and non-face-to-face) time, not MDM:
- 99238: 30 minutes or less
- 99239: 31 minutes or more
Discharge CPTs would be used by the primary attending service (e.g., a Neurohospitalist service). Consulting services would continue to choose Subsequent Day codes based on time or MDM.
Discharge Day Management, 31 minutes or more CPT 99239
Disclaimer: The billing and coding information provided by the American Academy of Neurology and its affiliates (collectively, “Academy”) are assessments of clinical information provided as an educational service. The information (1) is not clinical advice; (2) does not account for how private payers cover and reimburse procedures or services*; (3) is not continually updated and may not reflect the most current clinical information (new clinical information may emerge between the time information is developed and when it is published or read); and (4) is not a substitute for the independent professional judgment of the treating provider, who is responsible for correctly coding procedures and services.
Using this information is voluntary. The Academy is providing the information on an “as is” basis and makes no warranty, expressed or implied, regarding the information. The Academy specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. The Academy assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information or for any errors or omissions.
*The Academy recommends always checking private payer policies before rendering procedures or services
This presents an analysis of a case of Ischemic stroke in terms of possible etiology, pathophysiology, drug analysis and nursing care
New? Questions? Start Here!
- Information Hub
- Important Notices
- Need Access to Evidence
- Communities & Collections
- Publication Date
- Posting Date
- Subject(CINAHL)
- Item Format
- Level of Evidence
- Research Approach
- Sigma Chapters
- Author Affiliations
- Review Type
- Create an Account
Ischemic stroke: A case study
View file(s).
Author Information
- Martinez, Rudolf Cymorr Kirby P. ;
- Sigma Affiliation
Item Information
Item link - use this link for citations and online mentions..
Clinical Focus: Adult Medical/Surgical
Repository Posting Date
Type information.
| Type | | |
Category Information
| Evidence Level | ; ; |
Original Publication Info
| 2017-12-07 |
Conference Information
| Name |
Rights Holder
All rights reserved by the author(s) and/or publisher(s) listed in this item record unless relinquished in whole or part by a rights notation or a Creative Commons License present in this item record.
All permission requests should be directed accordingly and not to the Sigma Repository.
All submitting authors or publishers have affirmed that when using material in their work where they do not own copyright, they have obtained permission of the copyright holder prior to submission and the rights holder has been acknowledged as necessary.

Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Stroke case study
- Sanaya Batcho
- Kara Kitzmiller
- Emma Overman
Our reason for choosing this disorder
Stroke is the leading cause of disability in the United States. As advanced practice nurses, we anticipate caring for those impacted by strokes in many healthcare settings including emergency rooms, acute care, rehab settings, extended care facilities, and in primary care. Early diagnosis and treatment are imperative in the treatment of a stroke in order to minimize permanent deficits so it is important for advanced practice nurses to be proficient in recognizing clinical manifestations of a stroke. There are also many modifiable risk factors for strokes so advanced practice nurses need to be able to educate patients and families on potential lifestyle changes that can decrease stroke risk.

Case Presentation
Statement of ethics, conflict of interest statement, funding sources, author contributions, ischemic stroke in a 29-year-old patient with covid-19: a case report.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Christian Avvantaggiato , Loredana Amoruso , Maria Pia Lo Muzio , Maria Assunta Mimmo , Michelina Delli Bergoli , Nicoletta Cinone , Luigi Santoro , Lucia Stuppiello , Antonio Turitto , Chiara Ciritella , Pietro Fiore , Andrea Santamato; Ischemic Stroke in a 29-Year-Old Patient with COVID-19: A Case Report. Case Rep Neurol 2 September 2021; 13 (2): 334–340. https://doi.org/10.1159/000515457
Download citation file:
- Ris (Zotero)
- Reference Manager
Increasing evidence reports a greater incidence of stroke among patients with Coronavirus disease 2019 (COVID-19) than the non-COVID-19 population and suggests that SARS-CoV-2 infection represents a risk factor for thromboembolic and acute ischemic stroke. Elderly people have higher risk factors associated with acute ischemic stroke or embolization vascular events, and advanced age is strongly associated with severe COVID-19 and death. We reported, instead, a case of an ischemic stroke in a young woman during her hospitalization for COVID-19-related pneumonia. A 29-year-old woman presented to the emergency department of our institution with progressive respiratory distress associated with a 2-day history of fever, nausea, and vomiting. The patient was transferred to the intensive care unit (ICU) where she underwent a tracheostomy for mechanical ventilation due to her severe clinical condition and her very low arterial partial pressure of oxygen. The nasopharyngeal swab test confirmed SARS-CoV-2 infection. Laboratory tests showed neutrophilic leucocytosis, a prolonged prothrombin time, and elevated D-dimer and fibrinogen levels. After 18 days, during her stay in the ICU after suspension of the medications used for sedation, left hemiplegia was reported. Central facial palsy on the left side, dysarthria, and facial drop were present, with complete paralysis of the ipsilateral upper and lower limbs. Computed tomography (CT) of the head and magnetic resonance imaging of the brain confirmed the presence of lesions in the right hemisphere affecting the territories of the anterior and middle cerebral arteries, consistent with ischemic stroke. Pulmonary and splenic infarcts were also found after CT of the chest. The age of the patient and the absence of serious concomitant cardiovascular diseases place the emphasis on the capacity of SARS-CoV-2 infection to be an independent cerebrovascular risk factor. Increased levels of D-dimer and positivity to β2-glycoprotein antibodies could confirm the theory of endothelial activation and hypercoagulability, but other mechanisms – still under discussion – should not be excluded.
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus SARS-CoV-2, is characterized by a wide range of symptoms, most of which cause acute respiratory distress syndrome [1, 2], associated with intensive care unit (ICU) admission and high mortality [3]. On March 11, 2020, the large global outbreak of the disease led the World Health Organization (WHO) to declare COVID-19 a pandemic, with 11,874,226 confirmed cases and 545,481 deaths worldwide (July 9, 2020) [4]. In many cases, the clinical manifestations of COVID-19 are characteristic of a mild disease that may, however, worsen to a critical lower respiratory infection [2]. At the onset of the disease, the most frequent symptoms are fever, dry cough, fatigue, and shortness of breath as the infection progresses may appear signs and symptoms of respiratory failure that require ICU admission [5, 6]. Although acute respiratory distress syndrome is the most important cause of ICU admission for COVID-19 patients, several studies have underlined the presence of neurological symptoms such as confusion, dizziness, impaired consciousness, ataxia, seizure, anosmia, ageusia, vision impairment, and stroke [7, 8]. In particular, the state of hypercoagulability in patients affected by COVID-19 favors the formation of small and/or large blood clots in multiple organs, including the brain, potentially leading to cerebrovascular disease (ischemic stroke but also intracranial hemorrhage) [9, 10 ].
We found an interesting case of stroke following a SARS-CoV-2 infection in a young patient. A 29-year-old woman, during her ICU hospitalization for COVID-19-related pneumonia, was diagnosed with ischemic stroke of the right hemisphere, without other cardiac/cerebrovascular risk factors except hypertension. The young age of the patient and the absence of higher cerebrovascular risk factors make the present case very interesting as it can help demonstrate that COVID-19 is an independent risk factor for acute ischemic stroke. In a case series of 214 patients with COVID-19 (mean [SD] age, 52.7 [15.5] years), neurologic symptoms were more common in patients with severe infection who were older than the others [ 11 ]. New-onset CVD was more common in COVID-19 patients who had underlying cerebrovascular risk factors, such as older age (>65 years) [ 12 ], and very few cases of stroke in patients younger than 50 years have been reported [ 12, 13 ]. Our case seems to be the only one younger than 30 years.
On the night between March 19 and 20, 2020, a 29-year-old woman was referred to our hospital “Policlinico Riuniti di Foggia” due to a progressive respiratory distress associated with a 2-day history of fever, nausea, and vomiting. At presentation, the heart rate was 128 bpm, the blood oxygen saturation measured by means of the pulse oximeter was 27%, the respiratory rate was 27 breaths per minute, and the blood pressure was 116/77 mm Hg. The arterial blood gas test showed a pH of 7.52, pO 2 20 mm Hg, and pCO 2 34 mm Hg. The patient was immediately transferred to the ICU where she underwent tracheostomy and endotracheal intubation for mechanical ventilation due to her severe clinical condition and deteriorated pulmonary gas exchange. The diagnosis of COVID-19 was confirmed by PCR on a nasopharyngeal swab.
The family medical history was normal, and the only known pre-existing medical conditions were polycystic ovary syndrome (diagnosed 3 years earlier), conversion disorder, and hypertension (both diagnosed 2 years earlier). Ramipril and nebivolol were prescribed for the high blood pressure treatment, and sertraline was prescribed for the conversion disorder treatment. Drug therapy adherence was inconstant. The patient had no history of diabetes, cardiac pathologies, strokes, transient ischemic attacks, thromboembolic, or other vascular pathologies.
Laboratory tests showed neutrophilic leukocytosis (white blood cell count 14.79 × 10 3 , neutrophil percentage 89.8%, and neutrophil count 13.29 × 10 3 ), a prolonged prothrombin time (15.3 s) with a slightly elevated international normalized ratio (1.38), and elevated D-dimer (6,912 ng/mL) and fibrinogen levels (766 mg/dL). Other findings are shown in Table 1 .
Laboratory test

This pharmacological therapy was set as follows: enoxaparin 6,000 U.I. once a day, piperacillin 4 g/tazobactam 0.5 g twice a day; Kaletra, a combination of lopinavir and ritonavir indicated for human immunodeficiency virus (HIV) infection treatment, 2 tablets twice a day; hydroxychloroquine 200 mg once a day; and furosemide 250 mg, calcium gluconate, and aminophylline 240 mg 3 times a day. No adverse events were reported.
On April 7, 2020, during her stay in the ICU and after suspension of the medications used for sedation, left hemiplegia was reported. The same day, the patient underwent a computed tomography examination of the head, which showed areas of hypodensity in the right hemisphere due to recent cerebral ischemia.
On April 16, 2020, the patient was oriented to time, place, and person. Central facial palsy on the left side, dysarthria, and facial drop were present, with complete paralysis of the ipsilateral upper and lower limbs. The power of all the muscles of the left limbs was grade 0 according to the Medical Research Council (MRC) scale. Deep tendon reflexes were reduced on the left upper limb but hyperactive on the ipsilateral lower limb, with a slight increase in the muscle tonus. The senses of touch, vibration, and pain were reduced on the left side of the face and body.
On the same day, the patient underwent magnetic resonance imaging (MRI) of the brain (Fig. 1 a), showing lesions on the right hemisphere affecting the territories of the anterior and middle cerebral arteries. On May 5, 2020, magnetic resonance angiography showed an early duplication of the sphenoidal segment of the right middle cerebral artery, the branches of which are irregular with rosary bead-like aspects (Fig. 1 d, e); on the same day, the second MRI (Fig. 1 b) confirmed the lesions. Computed tomography of the chest (Fig. 1 c) and abdomen (Fig. 1 f), performed 5 days after the MRI of the brain, showed not only multifocal bilateral ground-glass opacities but also a basal subpleural area of increased density within the left lung (4 × 4 × 3 cm), consistent with a pulmonary infarction. In addition, a vascular lesion, consistent with a splenic infarct, was found in the inferior pole of the spleen. Doppler echocardiography of the hearth showed regular right chambers and left atrium and a slightly hypertrophic left ventricle with normal size and kinetics (ejection fraction: 55%). The age of the patient and the absence of serious concomitant cardiovascular diseases place the emphasis on the capacity of SARS-CoV-2 infection to be an independent cerebrovascular risk factor.

Imaging. a April 16, 2020; MRI of the brain: lesions in the right hemisphere affecting the territories of the anterior and the middle cerebral arteries. b May 5, 2020; MRI of the brain: same lesions in the right hemisphere shown in the previous image. d , e May 5, 2020; MRA showed an early duplication of the sphenoidal segment of the right middle cerebral artery, the branches of which are irregular with rosary bead-like aspect and reduction of blood flow in the middle cerebral artery. c April 20, 2020; CT of the abdomen: vascular lesion, consistent with a splenic infarct, found in the inferior pole of the spleen. f April 20, 2020; CT of the chest: basal subpleural area of increased density within the left lung (4 × 4 × 3 cm), consistent with a pulmonary infarction. MRA, magnetic resonance angiography; CT, computed tomography; MRI, magnetic resonance imaging.
The pandemic outbreak of novel SARS-CoV-2 infection has caused great concern among the services and authorities responsible for public health due to not only the mortality rate but also the danger of filling up hospital capacities in terms of ICU beds and acute non-ICU beds. In this regard, the nonrespiratory complications of COVID-19 should also be taken into great consideration, especially those that threaten patients’ lives and extend hospitalization times. Stroke is one of these complications, since a greater incidence of stroke among patients with COVID-19 than the non-COVID-19 population has been reported, and a preliminary case-control study demonstrated that SARS-CoV-2 infection represents a risk factor for acute ischemic stroke [ 14 ].
We found that the reported case is extremely interesting, since the woman is only 29 years old and considering how stroke in a young patient without other known risk factors is uncommon. Not only elderly people have higher risk factors associated with acute ischemic stroke or embolization vascular events [ 15 ], but it is also true that advanced age is strongly associated with severe COVID-19 and death. The severity of the disease is directly linked to immune dysregulation, cytokine storm, and acute inflammation state, which in turn are more common in patients who present immunosenescence [6].
Inflammation plays an important role in the occurrence of cardiovascular and cerebrovascular diseases since it favors atherosclerosis and affects plaque stability [ 16 ]. The ischemic stroke of the 29-year-old woman does not appear to be imputable to emboli originating a pre-existing atheromatous plaque, both for the age of the patient and for the absence of plaques at the Doppler ultrasound study of the supra-aortic trunks.
Most likely, COVID-19-associated hypercoagulability and endothelial dysfunction are the causes of ischemic stroke, as suggested by other studies and case reports [ 10, 13, 17 ]. Although the mechanisms by which SARS-CoV-2 infection leads to hypercoagulability are still being studied, current knowledge suggests that cross talk between inflammation and thrombosis has a crucial role [ 18 ]. The release of inflammatory cytokines leads to the activation of epithelial cells, monocytes, and macrophages. Direct infection of endothelial cells through the ACE2 receptor also leads to endothelial activation and dysfunction, expression of tissue factor, and platelet activation and increased levels of VWF and FVIII, all of which contribute to thrombin generation and fibrin clot formation [ 17 ]. The 29-year-old patient showed an increased level of D-dimer, which is a degradation product of cross-linked fibrin, indicating a global activation of hemostasis and fibrinolysis and conforming to the hypothesis of COVID-19-associated hypercoagulability. Endothelial activation and hypercoagulability are also confirmed by positivity to β2 glycoprotein antibodies. Anticardiolipin antibody and/or β2 glycoprotein antibody positivity has been reported in a few studies [ 17, 19, 20 ]. In addition, widespread thrombosis in SARS-CoV-2 infection could also be caused by neutrophil extracellular traps (NETs). Neutrophilia [ 21 ] and an elevated neutrophil-lymphocyte ratio [ 22 ] have been reported by numerous studies as predictive of worse disease outcomes, and recently, the contribution of NETs in the pathophysiology of COVID-19 was reported [ 23 ]. Thrombogenic involvement of NETs has been described in various settings of thrombosis, including stroke, myocardial infarction, and deep vein thrombosis [ 24 ]. The high neutrophil count found in our case does not exclude the hypothesis that NETs are involved in the pathogenesis of ischemic stroke.
Ischemic stroke in young patients without pre-existing cerebrovascular risk factors is very unusual. In this regard, our case of an ischemic stroke, reported in a 29-year-old woman, is very interesting. Although it is not possible to determine precisely when the thromboembolic event occurred, our case of stroke during COVID-19-related pneumonia seems to confirm that COVID-19 is an independent risk factor for acute ischemic stroke. The mechanisms by which coronavirus disease leads to stroke are still under study, but it is clear that hypercoagulability and endothelial activation play a key role. Testing for SARS-CoV-2 infection should be considered for patients who develop neurologic symptoms, but it is equally important to monitor COVID-19 patients during their hospitalization to find any neurological sign or symptom in a timely manner. Our case suggests that discovering neurological deficits in sedated patients promptly can be very difficult; for this reason, sedation in mechanically ventilated patients has to be considered only if strictly necessary. Performing serial laboratory testing and waking up the patient as soon as clinical conditions allow are strategies that should be taken into account.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal.
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
No funding was received for the publication of this case report.
All authors agree with the contents of the manuscript and were fully involved in the study and preparation of the manuscript. All authors read and approved the final version of the manuscript. M.A. Mimmo, M.P. Lo Muzio, M. Delli Bergoli, and L. Amoruso collected the data. C. Avvantaggiato wrote the manuscript with support of N. Cinone, L. Santoro, and C. Ciritella. C. Avvantaggiato, A. Turitto, and L. Stuppiello researched and discussed the neurophysiological principles of this study. P. Fiore and A. Santamato supervised the project.

Email alerts
Citing articles via, suggested reading.
- Online ISSN 1662-680X
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account

What are you initial questions/concerns about this patient? What data do you want to focus on with your first head-to-toe assessment? What do you anticipate this patient needing based off this report? You fill out your report sheet, and it looks something like this:

You know you need more data, so you take a look through the chart. Namely, you want to see what his lab results are and want to check to see that the admitting MD ordered a lipid panel and HbA1c for this morning. No results yet, so it’s time to go see your patient…after all, he’s on q 30 min neuro checks until 10am. It’s going to be a busy morning!
Your initial assessment and AM rounds
0700: You head in to the room with the main goal of assessing Rick’s neurological status and BP. Because of his ETOH history, he’s at a higher risk for bleed than someone who doesn’t drink a case of beer a day. Speaking of this drinking habit…you want to confirm with the patient. If he really does drink that much, we’re going to have a problem in a day or two and we need to be prepared.
Your initial head-to-toe assessment reveals the following:
- NIHSS = 6…slowly improving!
- Pt is able to hold left arm up for 10 seconds with only slight drift noted…improvement! Pt states he can use urinal independently now. Yay!
- Facial droop is minor; speech only mildly slurred.
- VSS on nicardipine gtt. BP from the cuff is 150/74.
- Occasional PVCs on the monitor, but he’s holding his BP so you’re not too worried.
- Lungs clear, normoactive bowel sounds.
- Abrasion to right knee looks clean (thanks to the ED nurses!), open to air.
- No complaints of pain, no complaints of headache, blurred vision or worsening numbness in any extremity.
- Both IVs patent
- Pt confirms he does drink a case of beer a day.
- Pt states he is tired from being up all night, but understands need for frequent assessments. States he will try to sleep in between.
0715: You sit down to chart your initial assessment and look through the labs and orders. Your AM labs don’t show anything of surprise and you notice the lipid panel AND the HbA1c were ordered. That new resident is on top of her game!
0730: Back in to wake Rick and conduct a neuro assessment No changes. Whew! You get those SCDs on and help Rick brush his teeth. You didn’t finish your initial charting yet, so you do that now. You hope to get it done before your 0800 neuro check.
Your morning continues in this vein until 0900. You’ve been conducting neuro exams every 30 minutes and you’re happy to see that Rick’s NIHSS is now 3…he scores points for slight drift in left arm, left leg and slight slurred speech. Looks like he’s going to have a pretty impressive recovery.
During this time you also note that your AM labs are in:
- Na 140; K 3.2; Mg 1.6; Ca 8.2; Ph 1.1; Cr .90; BUN 12
- WBC 8.5; Hgb 9.5; Hct 37%; PLT 130; INR 1.2
- Total cholesterol: 260; HDL 26; LDL 210
- HbA1C: 12 (woah!)
0900: Time for morning meds! Because Rick is still NPO pending his swallow evaluation, you don’t have many meds to give…a protonix IV, keppra (prophylactic for seizure) and that’s about it! You know he’s going to do awesome on his swallow eval, so you call Speech Therapy to see when they might be by. If you can get him on some PO antihypertensives, you can get this nicardipine gtt off and stop taking his BP every 15 minutes (ouch!). Your 0900 neuro exam goes off without a hitch. You notice more frequent ectopy on the monitor and attribute it to his low K and Mag levels. Otherwise, Rick is doing awesome…and wouldn’t you know it, his wife is a lovely person who knows you are an RN who went to school for years and years and not a glorified waitress, so she never once asks you to bring her a cup of coffee. 😉
1000: Time for a blood sugar check. If this one is also elevated, you’re going to need to talk to the MD about coverage. And yes it is…240. All that stress must be causing an SNS response. Your last q 30 neuro check is stable and you let Rick know you’ll be spacing them out to hourly. He grunts in response and goes back to sleep. You let Jan, his wife, know to alert you immediately if he complains of any worsening stroke symptoms or a headache.
1010: Time for rounds! You update the medical team on Rick’s condition and ask for the following:
- A banana bag (an IV infusion of vitamins and thiamine that we give to ETOH patients)
- PO blood pressure meds (pending your swallow eval)
- A statin (his cholesterol is super high)
- Diabetic educator (based off his super high HbA1c)
- ETOH protocol (includes anti-anxiety meds and librium to help control DTs)
- K and Mag replacement
- BS coverage with an aggressive sliding scale
Just then, the speech therapist comes in to conduct an evaluation of Rick’s ability to swallow. She lets you know that he “did great” and will be on a chopped diet with thin liquids. This means his food will be cut up small, but he can have liquids that are NOT thickened…which is good, because thickened liquids are really weird. You make sure to ask her what she thinks of his ability to swallow pills and she states that he should do fine with average-sized pills…larger ones may need to be halved. Woohoo! Rick is making progress!
Before the MD leaves to hang out in the lounge, you alert her that the pt passed his swallow eval and has a recommendation from Speech Therapy. She puts in the orders for a carb-control, cardiac/reduced sodium diet and you are good to go!
1100: Another neuro exam…funny how those hours fly by, isn’t it? All is well in Rick’s world and he is performing components of the exam without even being asked. Is this cheating? 🙂 You hang his banana bag, give him his PO meds and check the compatibility of Mag with nicardipine. You decide not to run anything concurrent with this potent calcium channel blocker and realize you need to start another PIV (those banana bags are not compatible with ANYTHING and you want to get the Mag going now). You nail the IV on your first try and start the magnesium replacement. Your K replacement is ordered PO, so you want to get him a little lunch first so it doesn’t upset his stomach. Besides, when Mag is optimized, it makes it easier for the body to “hang on” to its potassium…so getting that Mag in there is a good idea!
1200: You check Rick’s blood sugar, see that it’s still high (232) and provide insulin coverage. You bring him his lunch tray AND a big ol’ potassium pill that you’ve kindly cut in half. You watch as he safely swallows the pill and you notice his BP is vastly improved. You cut the nicardipine gtt down to 2.5 and anticipate it being off within the hour. Good job, you! Rick’s neuro status is stable and you think his speech might be even more improved.
1245 : Jan comes rushing up to the nurse’s station saying, “He said he had a horrible headache and now I can’t get him to wakeup.” You hear a voice inside your head saying, “ooooooooohhhhh shoooooooooooot” Only you don’t say “shoot.” As you rush into his room questions run through your head:
- Did we overshoot his blood sugar control? Is this hypoglycemia?
- Did I turn the nicardipine down too much? Is he having a hypertensive bleed?
- Is he having a “hemorrhagic conversion” a common complication of stroke (especially after TPA)
- Is he able to protect his airway?
- How fast can we get to CT scan?
You grab a glucometer on your way in and ask one of your nurse pals to come in with you. You hand him the glucometer as you try to wake your pt. Your efforts are unsuccessful. You hear a gurgling in Rick’s throat and watch the monitor to see his O2 sat dropping to the mid 80s. We’ve got problems.
You immediately lower the head of the bed and ask your pal for the BVM (bag valve mask). You reposition the airway and notice slight improvement, but the sats still don’t climb above 90. You start bagging the patient and ask your nurse friend to call RT and the MD. Rick needs to be intubated STAT for airway protection and then you gotta go to CT.
As reinforcements enter the room, you ask someone to finish the blood sugar (which never got done) and RT takes over bagging the pt. With manual ventilation, Rick’s sats come up to 98%, but he is still non-responsive. The blood sugar reads 140, so that’s not the problem. You run a BP and see it’s well within parameters. Just then, the MD walks in as another RT shows up with the intubation try and a vent. Good teamwork, guys!
Doc Waters quickly assesses the situation, gets into place at the head of the bed and deftly intubates Rick as his wife cries in the corner. You notice your awesome charge nurse consoling her as she explains what is happening.
As CXR technician shows up to confirm endotracheal tube (ETT) placement. While they are setting up the machine, you quickly place an OGT tube so you can get confirmation of it at the same time as your ETT. They shoot the film and you then whisk Rick off to CT scan with your RT buddy at your side. Your heart sinks as you fear the worst has happened to your patient.
1315: CT scan shows Rick has now bled into his brain. It is one of the known risks of TPA and you are crushed that it has happened to your patient, especially one so young and who was doing so well. You let Doc Waters know of the results and page the neurologist on call as well. When you speak to the neurologist, he tells you he’s consulted neurosurgery and ordered an MRI.
1400: You’ve spoken to the neurologist and received orders for tighter BP control (goal < 140) so you titrate the nicardipine gtt and also ask for an arterial line for closer monitoring. You get Rick settled after his emergent intubation, place a Foley catheter, update Jan and note that his neuro exam is awful…GCS is 3 (no eye opening, no verbal and decorticate posturing). The art line gets placed and MRI calls to tell you they’re ready for your patient. Off you go!
1530: You’re back from MRI and you call the neurosurgeon to let her know the results are available to view.
1545: Neurosurgeon calls you back to ask that you get an EVD kit ready for her. She’s on her way up to place a drain at the bedside. You look at the clock and realize you still have four more hours to go. It’s been a tough day. You find the cranial access kit (which includes a DRILL!) and get the EVD system set up.

1630: Your EVD is in place (thanks to a fast-acting neurosurgeon) and you notice that your intracranial pressure (ICP) is elevated. The neurosurgeon orders prophylactic Ancef (after all, she just performed a neurosurgical procedure!) mannitol q6 hours prn for elevated ICP as well as orders to keep the EVD at continuous drainage. Because you’ve recently reviewed the management of elevated ICP , you know that mannitol requires you to monitor your patient’s serum sodium and serum osmolality. You send a stat chemistry vial and do what you can to keep the ICP within parameters (decreased stimulation, positioning, sedation). With the sedation on board, you’re able to turn off your nicardipine gtt, but you want to keep a close eye and make sure that your CPP (cerebral perfusion pressure) is maintained above 70 (CPP = MAP – ICP). You may need levophed in order to keep CPP up while your patient is sedated. A stark contrast from how you started the day.
1700: You give your mannitol, clear your pumps and spend the next hour catching up on charting. You’re happy to see that the mannitol works quickly to reduce ICP and you make a mental note to keep an eye on urine output as you expect it to drastically increase due to the osmotic diuresis effect of this medication. You’ll want to keep a close eye on those electrolytes (especially K and Mag) Oh, speaking of K and Mag…did you remember to recheck those after you replaced them? You had a busy day and you had to prioritize…but better do that now, just to be safe! Chances are you can “add-on” the K and Mag test to the blood vial you sent earlier for the serum sodium. You spot Doc Waters and ask her to please review the x-ray for OGT placement so you can use it. She does and it’s fine…she writes an order to start Glucerna 1.2 at 20ml/hr.
1800: Your hourly neuro exam shows no changes as your EVD continues to drain drain drain. You do your end-of-shift charting, ensure your IV bags are not going to run dry during your NOC shift buddy’s first hour, replace suction tubing, tidy up the room, get Rick repositioned and start the tube feeding. Though Rick didn’t fare well, you did everything you could, followed protocol and kept the team updated. You can be proud of the work you did today.
Your report sheet, after everything that happened, now looks like this:

You are now ready to give an awesome end-of-shift report. Now go home and sleep!
____________________________________________________________
The information, including but not limited to, audio, video, text, and graphics contained on this website are for educational purposes only. No content on this website is intended to guide nursing practice and does not supersede any individual healthcare provider’s scope of practice or any nursing school curriculum. Additionally, no content on this website is intended to be a substitute for professional medical advice, diagnosis or treatment.
Want more resources to help you through nursing school? Grab the study guide right here !
Looking for a neuro study sheet? How about this one?
Want to review this on the run? Tune in to episode 135 of the Straight A Nursing podcast to study on the go!
Last Updated on December 9, 2022 by SupportVA
20 Secrets of Successful Nursing Students
Being a successful nursing student is more than just study tips and test strategies. It’s a way of life.
you may also like...

#356: All About Ascites

Ascites Nursing Review

#350: Two Types of Calcium Channel Blockers

#349: Assessing Skin Signs Case Study
Complete Your CE
Course case studies, external link, this link leads outside of the netce site to:.
While we have selected sites that we believe offer good, reliable information, we are not responsible for the content provided. Furthermore, these links do not constitute an endorsement of these organizations or their programs by NetCE, and none should be inferred.
Ischemic Stroke
Course #90284 - $60-
#90284: Ischemic Stroke
Your certificate(s) of completion have been emailed to
- Back to Course Home
- Review the course material online or in print.
- Complete the course evaluation.
- Review your Transcript to view and print your Certificate of Completion. Your date of completion will be the date (Pacific Time) the course was electronically submitted for credit, with no exceptions. Partial credit is not available.
Patient M is an active woman, 70 years of age, who lost consciousness and collapsed at home. Her daughter, who was visiting her at the time, did not witness the collapse but found her mother on the floor, awake, confused, and slightly short of breath. The daughter estimated that she called EMS within 5 minutes after the collapse, and EMS responded within 15 minutes. EMS evaluated Patient M, drew blood for a glucose level, and determined that she may have had a stroke. They notified the nearest designated comprehensive stroke center that they would be arriving with the patient in approximately 20 minutes. Patient M's daughter accompanied her.
The triage and transportation of an individual with suspected stroke should be similar to that for an individual with serious trauma, and treatment is recommended within 3 hours after the onset of stroke. Because of the limited time available for assessment and diagnosis before optimal treatment, the EMS dispatcher should notify EMS personnel immediately and coordinate transport of the individual to the closest emergency facility, preferably one that is a designated primary (or comprehensive) stroke care center.
On presentation in the emergency department, Patient M is immediately triaged. Because Patient M is still somewhat confused, her daughter is asked to provide information on the patient's history. The daughter reports that her mother had had an episode of sudden-onset numbness and tingling in the right limb, with slight confusion and slurred speech, 3 days previously. The episode lasted only 5 minutes, and Patient M had not called her primary care physician. Additional information provided by the daughter indicates that Patient M has been treated for hypertension for 10 years but notes that she is often not compliant with her antihypertensive medicine, a diuretic. The patient has never smoked, drinks occasionally, and is of normal weight.
Patient M has two significant risk factors for stroke; one is a long history of hypertension. More than two-thirds of individuals older than 65 years of age are hypertensive, and it is important for individuals with hypertension to have regular blood pressure screening and to maintain a blood pressure of less than 140/90 mm Hg. Antihypertension therapy has been found to reduce the incidence of stroke by 30% to 40%. Patient M's noncompliance with her antihypertension medicine likely includes her among the 65% of known hypertensive individuals in whom blood pressure is not controlled.
Patient M's previous episode of numbness, confusion, and slurred speech appears to be evidence of a TIA, another substantial risk factor for stroke. Research has shown that approximately 5% of patients will have an ischemic stroke within 7 days after a TIA. In addition, the risk of stroke within 7 days is doubled for patients with TIAs who did not seek treatment. As is the case for many individuals who have a TIA, Patient M did not seek medical attention because the clinical symptoms resolved quickly. However, research findings indicate that urgent treatment should be provided for TIAs, as early treatment for TIA and minor stroke has been shown to reduce the risk of early recurrent stroke by 80%.
On physical examination, Patient M's blood pressure is 150/95 mm Hg. She has pain in her left arm and a slight headache. There is a focal carotid bruit on the right. She is assessed with use of the NIHSS and found to have 1/5 weakness in the left upper and lower extremities and left visual/spatial neglect. The results of laboratory tests, including a complete blood count, prothrombin time, serum electrolyte levels, cardiac biomarkers, and renal function studies, are all within normal limits. CT of the head obtained about 45 minutes into her ED evaluation (1.5 to 2 hours since last well) indicates an occlusion in a branch of the right internal carotid artery with 50% narrowing due to atherosclerosis. An area of ischemia/infarction is visible in the right anterior cerebral hemisphere. There is no evidence of subarachnoid hemorrhage. Approximately 2.5 to 3 hours after Patient M collapsed at home, she is treated with IV rt-PA at a dose of 0.9 mg/kg. Twenty-four hours later, aspirin antiplatelet therapy is started at an initial dose of 325 mg, and a maintenance dose of 75 mg per day.
Many of the patient's symptoms, including her loss of consciousness, shortness of breath, pain, and headache, are nontraditional symptoms of stroke. Studies have demonstrated that nontraditional symptoms are more prevalent among women, often leading to a delay in the evaluation for stroke. EMS personnel and clinicians should be aware of the potential for nontraditional symptoms in women and carry out a diagnostic evaluation addressing a suspicion of stroke.
Patient M is eligible for thrombolytic therapy with rt-PA according to evidence-based guidelines developed by the AHA/ASA: her blood pressure is lower than 185/110 mm Hg, the onset of symptoms is less than 3 hours prior to the start of treatment, and the laboratory values are within normal limits. Antiplatelet therapy with aspirin 325 mg daily (versus anticoagulant therapy with warfarin) is recommended for treatment of patients with stroke or TIA due to intracranial atherosclerosis with 50% to 99% occlusion. Antiplatelet therapy is not recommended as an adjunctive therapy within 24 hours of thrombolytic therapy.
When Patient M's condition is stabilized, her primary care physician and consultant neurologist provide a referral for stroke rehabilitation, and a multidisciplinary rehabilitation team is formed to assess her rehabilitative needs, recommend the proper rehabilitation setting, and develop a treatment strategy tailored to her specific needs that includes daily antiplatelet therapy. Patient M is again assessed with the NIHSS, and the score is 12. The patient's cognitive and communication skills are intact on evaluation with the FIM, with the exception of the previously documented left visual/spatial neglect. The assessment also includes evaluation of the patient's risk for complications. Because of her spatial neglect, she is screened with the Berg Balance Scale and the Stops Walking When Talking test. The score on the Berg Balance Scale is 43, and Patient M does stop walking to engage in conversation. Psychosocial assessment includes screening with the Center for Epidemiologic Studies Depression (CES-D) Scale, as well as review of the medical history and conversations with the patient and her children; no signs of depression are present.
Patient M's score of 12 on the NIHSS falls within the range (6 to 15) that indicates she is likely to benefit from rehabilitation. Evaluating a stroke survivor's risk of complications is an important component of the overall assessment, and among the most common complications are falls, deep vein thrombosis, pressure ulcers, swallowing dysfunction, bladder and bowel dysfunction, and depressive symptoms. In assessing the risk of complications, the Berg Balance Scale appears to be the most appropriate screen for patients who are likely to fall, and a score of less than 45 is associated with a likelihood of falling. The risk of a fall is also increased when a patient stops walking to talk, as Patient M did, during the Stops Talking When Walking test.
Screening for signs of depression is also essential, as depression affects approximately 33% of stroke survivors. Signs of depression are subtle and may be vague. Several screening tools are available, but there is no universally accepted tool for use in the post-stroke setting. The CES-D was chosen in this case because it is easy to administer, is useful in older individuals, and has been found to be effective for screening in the stroke population, except for individuals who have aphasia. The diagnosis of depression in stroke survivors should be based on sources in addition to a formal screening tool, such as a medical evaluation, patient self-report, observation of patient behavior, patient history, and staff reports of changes in behavior and motivation.
The rehabilitation team discusses the results of the assessment with Patient M's daughter and son, both of whom live about 45 minutes away from the patient. Together, the team and the family members explore options to determine the best approach to rehabilitation. A decision is made to transfer Patient M to an inpatient stroke unit, and a rehabilitation program is developed. The nurse on the team discusses the program with Patient M and her children and explains the course of rehabilitation and the expectations. Rehabilitation will focus on an exercise program consisting of aerobic exercise, strength training, stretching, and coordination and balance activities.
Early initiation of rehabilitation is a particularly strong predictor of improved outcome, and rehabilitation in a stroke unit has been associated with improved quality of life, survival, and functional status at 5 years compared with a general healthcare facility. No studies have demonstrated the superiority of one rehabilitation setting over another, and the inpatient setting was chosen primarily to ensure consistent care, given how far away Patient M's children live, and the limited support she otherwise has for healthcare needs. Decisions about the setting and program for rehabilitation should be shared with family members, and family and other caregivers should be provided with educational resources about the rehabilitation process.
The exercise program developed for Patient M is designed to help her regain the ability to independently carry out activities of daily living safely and to regain a functional level of ambulation. The benefits of an exercise program include increasing fitness, strength, and flexibility; improving function; preventing injuries and falls; and reducing the risk of recurrent stroke.
Patient M gradually resumes the ability to function independently, and after more than 2 weeks in the stroke rehabilitation unit, the score on the NIHSS has improved to 5. Before she is discharged to her home, the rehabilitation team provides instructions for exercises to continue at home and recommends moderate physical activity as a secondary prevention measure. The team also educates Patient M about the importance of maintaining a normal blood pressure through use of her antihypertension medication and lifestyle modifications. At a follow-up visit with her primary care clinician at 3 months, Patient M's blood pressure is 135/80 mm Hg, and she reports that she has been compliant with her antihypertension medicine and antiplatelet therapy and is functioning well at home.
- About NetCE
- Do Not Sell My Personal Information
Copyright © 2024 NetCE · Contact Us
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Case Rep

Case report
Clinical course of a 66-year-old man with an acute ischaemic stroke in the setting of a covid-19 infection, saajan basi.
1 Department of Stroke and Acute Medicine, King's Mill Hospital, Sutton-in-Ashfield, UK
2 Department of Acute Medicine, University Hospitals of Derby and Burton, Derby, UK
Mohammad Hamdan
Shuja punekar.
A 66-year-old man was admitted to hospital with a right frontal cerebral infarct producing left-sided weakness and a deterioration in his speech pattern. The cerebral infarct was confirmed with CT imaging. The only evidence of respiratory symptoms on admission was a 2 L oxygen requirement, maintaining oxygen saturations between 88% and 92%. In a matter of hours this patient developed a greater oxygen requirement, alongside reduced levels of consciousness. A positive COVID-19 throat swab, in addition to bilateral pneumonia on chest X-ray and lymphopaenia in his blood tests, confirmed a diagnosis of COVID-19 pneumonia. A proactive decision was made involving the patients’ family, ward and intensive care healthcare staff, to not escalate care above a ward-based ceiling of care. The patient died 5 days following admission under the palliative care provided by the medical team.
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is a new strain of coronavirus that is thought to have originated in December 2019 in Wuhan, China. In a matter of months, it has erupted from non-existence to perhaps the greatest challenge to healthcare in modern times, grinding most societies globally to a sudden halt. Consequently, the study and research into SARS-CoV-2 is invaluable. Although coronaviruses are common, SARS-CoV-2 appears to be considerably more contagious. The WHO figures into the 2003 SARS-CoV-1 outbreak, from November 2002 to July 2003, indicate a total of 8439 confirmed cases globally. 1 In comparison, during a period of 4 months from December 2019 to July 2020, the number of global cases of COVID-19 reached 10 357 662, increasing exponentially, illustrating how much more contagious SARS-CoV-2 has been. 2
Previous literature has indicated infections, and influenza-like illness have been associated with an overall increase in the odds of stroke development. 3 There appears to be a growing correlation between COVID-19 positive patients presenting to hospital with ischaemic stroke; however, studies investigating this are in progress, with new data emerging daily. This patient report comments on and further characterises the link between COVID-19 pneumonia and the development of ischaemic stroke. At the time of this patients’ admission, there were 95 positive cases from 604 COVID-19 tests conducted in the local community, with a predicted population of 108 000. 4 Only 4 days later, when this patient died, the figure increased to 172 positive cases (81% increase), illustrating the rapid escalation towards the peak of the pandemic, and widespread transmission within the local community ( figure 1 ). As more cases of ischaemic stroke in COVID-19 pneumonia patients arise, the recognition and understanding of its presentation and aetiology can be deciphered. Considering the virulence of SARS-CoV-2 it is crucial as a global healthcare community, we develop this understanding, in order to intervene and reduce significant morbidity and mortality in stroke patients.

A graph showing the number of patients with COVID-19 in the hospital and in the community over time.
Case presentation
A 66-year-old man presented to the hospital with signs of left-sided weakness. The patient had a background of chronic obstructive pulmonary disease (COPD), atrial fibrillation and had one previous ischaemic stroke, producing left-sided haemiparesis, which had completely resolved. He was a non-smoker and lived in a house. The patient was found slumped over on the sofa at home on 1 April 2020, by a relative at approximately 01:00, having been seen to have no acute medical illness at 22:00. The patients’ relative initially described disorientation and agitation with weakness noted in the left upper limb and dysarthria. At the time of presentation, neither the patient nor his relative identified any history of fever, cough, shortness of breath, loss of taste, smell or any other symptoms; however, the patient did have a prior admission 9 days earlier with shortness of breath.
The vague nature of symptoms, entwined with considerable concern over approaching the hospital, due to the risk of contracting COVID-19, created a delay in the patients’ attendance to the accident and emergency department. His primary survey conducted at 09:20 on 1 April 2020 demonstrated a patent airway, with spontaneous breathing and good perfusion. His Glasgow Coma Scale (GCS) score was 15 (a score of 15 is the highest level of consciousness), his blood glucose was 7.2, and he did not exhibit any signs of trauma. His abbreviated mental test score was 7 out of 10, indicating a degree of altered cognition. An ECG demonstrated atrial fibrillation with a normal heart rate. His admission weight measured 107 kg. At 09:57 the patient required 2 L of nasal cannula oxygen to maintain his oxygen saturations between 88% and 92%. He started to develop agitation associated with an increased respiratory rate at 36 breaths per minute. On auscultation of his chest, he demonstrated widespread coarse crepitation and bilateral wheeze. Throughout he was haemodynamically stable, with a systolic blood pressure between 143 mm Hg and 144 mm Hg and heart rate between 86 beats/min and 95 beats/min. From a neurological standpoint, he had a mild left facial droop, 2/5 power in both lower limbs, 2/5 power in his left upper limb and 5/5 power in his right upper limb. Tone in his left upper limb had increased. This patient was suspected of having COVID-19 pneumonia alongside an ischaemic stroke.
Investigations
A CT of his brain conducted at 11:38 on 1 April 2020 ( figure 2 ) illustrated an ill-defined hypodensity in the right frontal lobe medially, with sulcal effacement and loss of grey-white matter. This was highly likely to represent acute anterior cerebral artery territory infarction. Furthermore an oval low-density area in the right cerebellar hemisphere, that was also suspicious of an acute infarction. These vascular territories did not entirely correlate with his clinical picture, as limb weakness is not as prominent in anterior cerebral artery territory ischaemia. Therefore this left-sided weakness may have been an amalgamation of residual weakness from his previous stroke, in addition to his acute cerebral infarction. An erect AP chest X-ray with portable equipment ( figure 3 ) conducted on the same day demonstrated patchy peripheral consolidation bilaterally, with no evidence of significant pleural effusion. The pattern of lung involvement raised suspicion of COVID-19 infection, which at this stage was thought to have provoked the acute cerebral infarct. Clinically significant blood results from 1 April 2020 demonstrated a raised C-reactive protein (CRP) at 215 mg/L (normal 0–5 mg/L) and lymphopaenia at 0.5×10 9 (normal 1×10 9 to 3×10 9 ). Other routine blood results are provided in table 1 .

CT imaging of this patients’ brain demonstrating a wedge-shaped infarction of the anterior cerebral artery territory.

Chest X-ray demonstrating the bilateral COVID-19 pneumonia of this patient on admission.
Clinical biochemistry and haematology blood results of the patient
| Hb | 157 g/L |
| WCC | 5.8 |
| Platelet count | 195 |
| MCV | 86 |
| Neutrophils | 4.9 |
| Lymphocytes | 0.5 |
| INR | 1.3 |
| PT | 16.7 |
| APTT | 28 |
| Sodium | 137 |
| Potassium | Sample haemolysed |
| Urea | 9.5 |
| Creatinine | 84 |
| eGFR | 83 |
| Bilirubin | 26 |
| Albumin | 29 |
| CRP | 215 |
| Total protein | 69 |
APTT, activated partial thromboplastin time; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; INR, international normalised ratio; MCV, mean corpuscular volume; PT, prothrombin time; WCC, white cell count.
Interestingly the patient, in this case, was clinically assessed in the accident and emergency department on 23 March 2020, 9 days prior to admission, with symptoms of shortness of breath. His blood results from this day showed a CRP of 22 mg/L and a greater lymphopaenia at 0.3×10 9 . He had a chest X-ray ( figure 4 ), which indicated mild radiopacification in the left mid zone. He was initially treated with intravenous co-amoxiclav and ciprofloxacin. The following day he had minimal symptoms (CURB 65 score 1 for being over 65 years). Given improving blood results (declining CRP), he was discharged home with a course of oral amoxicillin and clarithromycin. As national governmental restrictions due to COVID-19 had not been formally announced until 23 March 2020, and inconsistencies regarding personal protective equipment training and usage existed during the earlier stages of this rapidly evolving pandemic, it is possible that this patient contracted COVID-19 within the local community, or during his prior hospital admission. It could be argued that the patient had early COVID-19 signs and symptoms, having presented with shortness of breath, lymphopaenia, and having had subtle infective chest X-ray changes. The patient explained he developed a stagnant productive cough, which began 5 days prior to his attendance to hospital on 23 March 2020. He responded to antibiotics, making a full recovery following 7 days of treatment. This information does not assimilate with the typical features of a COVID-19 infection. A diagnosis of community-acquired pneumonia or infective exacerbation of COPD seem more likely. However, given the high incidence of COVID-19 infections during this patients’ illness, an exposure and early COVID-19 illness, prior to the 23 March 2020, cannot be completely ruled out.

Chest X-ray conducted on prior admission illustrating mild radiopacification in the left mid zone.
On the current admission, this patient was managed with nasal cannula oxygen at 2 L. By the end of the day, this had progressed to a venturi mask, requiring 8 L of oxygen to maintain oxygen saturation. He had also become increasingly drowsy and confused, his GCS declined from 15 to 12. However, the patient was still haemodynamically stable, as he had been in the morning. An arterial blood gas demonstrated a respiratory alkalosis (pH 7.55, pCO 2 3.1, pO 2 6.7 and HCO 3 24.9, lactate 1.8, base excess 0.5). He was commenced on intravenous co-amoxiclav and ciprofloxacin, to treat a potential exacerbation of COPD. This patient had a COVID-19 throat swab on 1 April 2020. Before the result of this swab, an early discussion was held with the intensive care unit staff, who decided at 17:00 on 1 April 2020 that given the patients presentation, rapid deterioration, comorbidities and likely COVID-19 diagnosis he would not be for escalation to the intensive care unit, and if he were to deteriorate further the end of life pathway would be most appropriate. The discussion was reiterated to the patients’ family, who were in agreement with this. Although he had evidence of an ischaemic stroke on CT of his brain, it was agreed by all clinicians that intervention for this was not as much of a priority as providing optimal palliative care, therefore, a minimally invasive method of treatment was advocated by the stroke team. The patient was given 300 mg of aspirin and was not a candidate for fibrinolysis.
Outcome and follow-up
The following day, before the throat swab result, had appeared the patient deteriorated further, requiring 15 L of oxygen through a non-rebreather face mask at 60% FiO 2 to maintain his oxygen saturation, at a maximum of 88% overnight. At this point, he was unresponsive to voice, with a GCS of 5. Although, he was still haemodynamically stable, with a blood pressure of 126/74 mm Hg and a heart rate of 98 beats/min. His respiratory rate was 30 breaths/min. His worsening respiratory condition, combined with his declining level of consciousness made it impossible to clinically assess progression of the neurological deficit generated by his cerebral infarction. Moreover, the patient was declining sharply while receiving the maximal ward-based treatment available. The senior respiratory physician overseeing the patients’ care decided that a palliative approach was in this his best interest, which was agreed on by all parties. The respiratory team completed the ‘recognising dying’ documentation, which signified that priorities of care had shifted from curative treatment to palliative care. Although the palliative team was not formally involved in the care of the patient, the patient received comfort measures without further attempts at supporting oxygenation, or conduction of regular clinical observations. The COVID-19 throat swab confirmed a positive result on 2 April 2020. The patient was treated by the medical team under jurisdiction of the hospital palliative care team. This included the prescribing of anticipatory medications and a syringe driver, which was established on 3 April 2020. His antibiotic treatment, non-essential medication and intravenous fluid treatment were discontinued. His comatose condition persisted throughout the admission. Once the patients’ GCS was 5, it did not improve. The patient was pronounced dead by doctors at 08:40 on 5 April 2020.
SARS-CoV-2 is a type of coronavirus that was first reported to have caused pneumonia-like infection in humans on 3 December 2019. 5 As a group, coronaviruses are a common cause of upper and lower respiratory tract infections (especially in children) and have been researched extensively since they were first characterised in the 1960s. 6 To date, there are seven coronaviruses that are known to cause infection in humans, including SARS-CoV-1, the first known zoonotic coronavirus outbreak in November 2002. 7 Coronavirus infections pass through communities during the winter months, causing small outbreaks in local communities, that do not cause significant mortality or morbidity.
SARS-CoV-2 strain of coronavirus is classed as a zoonotic coronavirus, meaning the virus pathogen is transmitted from non-humans to cause disease in humans. However the rapid spread of SARS-CoV-2 indicates human to human transmission is present. From previous research on the transmission of coronaviruses and that of SARS-CoV-2 it can be inferred that SARS-CoV-2 spreads via respiratory droplets, either from direct inhalation, or indirectly touching surfaces with the virus and exposing the eyes, nose or mouth. 8 Common signs and symptoms of the COVID-19 infection identified in patients include high fevers, severe fatigue, dry cough, acute breathing difficulties, bilateral pneumonia on radiological imaging and lymphopaenia. 9 Most of these features were identified in this case study. The significance of COVID-19 is illustrated by the speed of its global spread and the potential to cause severe clinical presentations, which as of April 2020 can only be treated symptomatically. In Italy, as of mid-March 2020, it was reported that 12% of the entire COVID-19 positive population and 16% of all hospitalised patients had an admission to the intensive care unit. 10
The patient, in this case, illustrates the clinical relevance of understanding COVID-19, as he presented with an ischaemic stroke underlined by minimal respiratory symptoms, which progressed expeditiously, resulting in acute respiratory distress syndrome and subsequent death.
Our case is an example of a new and ever-evolving clinical correlation, between patients who present with a radiological confirmed ischaemic stroke and severe COVID-19 pneumonia. As of April 2020, no comprehensive data of the relationship between ischaemic stroke and COVID-19 has been published, however early retrospective case series from three hospitals in Wuhan, China have indicated that up to 36% of COVID-19 patients had neurological manifestations, including stroke. 11 These studies have not yet undergone peer review, but they tell us a great deal about the relationship between COVID-19 and ischaemic stroke, and have been used to influence the American Heart Associations ‘Temporary Emergency Guidance to US Stroke Centres During the COVID-19 Pandemic’. 12
The relationship between similar coronaviruses and other viruses, such as influenza in the development of ischaemic stroke has previously been researched and provide a basis for further investigation, into the prominence of COVID-19 and its relation to ischaemic stroke. 3 Studies of SARS-CoV-2 indicate its receptor-binding region for entry into the host cell is the same as ACE2, which is present on endothelial cells throughout the body. It may be the case that SARS-CoV-2 alters the conventional ability of ACE2 to protect endothelial function in blood vessels, promoting atherosclerotic plaque displacement by producing an inflammatory response, thus increasing the risk of ischaemic stroke development. 13
Other hypothesised reasons for stroke development in COVID-19 patients are the development of hypercoagulability, as a result of critical illness or new onset of arrhythmias, caused by severe infection. Some case studies in Wuhan described immense inflammatory responses to COVID-19, including elevated acute phase reactants, such as CRP and D-dimer. Raised D-dimers are a non-specific marker of a prothrombotic state and have been associated with greater morbidity and mortality relating to stroke and other neurological features. 14
Arrhythmias such as atrial fibrillation had been identified in 17% of 138 COVID-19 patients, in a study conducted in Wuhan, China. 15 In this report, the patient was known to have atrial fibrillation and was treated with rivaroxaban. The acute inflammatory state COVID-19 is known to produce had the potential to create a prothrombotic environment, culminating in an ischaemic stroke.
Some early case studies produced in Wuhan describe patients in the sixth decade of life that had not been previously noted to have antiphospholipid antibodies, contain the antibodies in blood results. They are antibodies signify antiphospholipid syndrome; a prothrombotic condition. 16 This raises the hypothesis concerning the ability of COVID-19 to evoke the creation of these antibodies and potentiate thrombotic events, such as ischaemic stroke.
No peer-reviewed studies on the effects of COVID-19 and mechanism of stroke are published as of April 2020; therefore, it is difficult to evidence a specific reason as to why COVID-19 patients are developing neurological signs. It is suspected that a mixture of the factors mentioned above influence the development of ischaemic stroke.
If we delve further into this patients’ comorbid state exclusive to COVID-19 infection, it can be argued that this patient was already at a relatively higher risk of stroke development compared with the general population. The fact this patient had previously had an ischaemic stroke illustrates a prior susceptibility. This patient had a known background of hypertension and atrial fibrillation, which as mentioned previously, can influence blood clot or plaque propagation in the development of an acute ischaemic event. 15 Although the patient was prescribed rivaroxaban as an anticoagulant, true consistent compliance to rivaroxaban or other medications such as amlodipine, clopidogrel, candesartan and atorvastatin cannot be confirmed; all of which can contribute to the reduction of influential factors in the development of ischaemic stroke. Furthermore, the fear of contracting COVID-19, in addition to his vague symptoms, unlike his prior ischaemic stroke, which demonstrated dense left-sided haemiparesis, led to a delay in presentation to hospital. This made treatment options like fibrinolysis unachievable, although it can be argued that if he was already infected with COVID-19, he would have still developed life-threatening COVID-19 pneumonia, regardless of whether he underwent fibrinolysis. It is therefore important to consider that if this patient did not contract COVID-19 pneumonia, he still had many risk factors that made him prone to ischaemic stroke formation. Thus, we must consider whether similar patients would suffer from ischaemic stroke, regardless of COVID-19 infection and whether COVID-19 impacts on the severity of the stroke as an entity.
Having said this, the management of these patients is dependent on the likelihood of a positive outcome from the COVID-19 infection. Establishing the ceiling of care is crucial, as it prevents incredibly unwell or unfit patients’ from going through futile treatments, ensuring respect and dignity in death, if this is the likely outcome. It also allows for the provision of limited or intensive resources, such as intensive care beds or endotracheal intubation during the COVID-19 pandemic, to those who are assessed by the multidisciplinary team to benefit the most from their use. The way to establish this ceiling of care is through an early multidisciplinary discussion. In this case, the patient did not convey his wishes regarding his care to the medical team or his family; therefore it was decided among intensive care specialists, respiratory physicians, stroke physicians and the patients’ relatives. The patient was discussed with the intensive care team, who decided that as the patient sustained two acute life-threatening illnesses simultaneously and had rapidly deteriorated, ward-based care with a view to palliate if the further deterioration was in the patients’ best interests. These decisions were not easy to make, especially as it was on the first day of presentation. This decision was made in the context of the patients’ comorbidities, including COPD, the patients’ age, and the availability of intensive care beds during the steep rise in intensive care admissions, in the midst of the COVID-19 pandemic ( figure 1 ). Furthermore, the patients’ rapid and permanent decline in GCS, entwined with the severe stroke on CT imaging of the brain made it more unlikely that significant and permanent recovery could be achieved from mechanical intubation, especially as the damage caused by the stroke could not be significantly reversed. As hospitals manage patients with COVID-19 in many parts of the world, there may be tension between the need to provide higher levels of care for an individual patient and the need to preserve finite resources to maximise the benefits for most patients. This patient presented during a steep rise in intensive care admissions, which may have influenced the early decision not to treat the patient in an intensive care setting. Retrospective studies from Wuhan investigating mortality in patients with multiple organ failure, in the setting of COVID-19, requiring intubation have demonstrated mortality can be up to 61.5%. 17 The mortality risk is even higher in those over 65 years of age with respiratory comorbidities, indicating why this patient was unlikely to survive an admission to the intensive care unit. 18
Regularly updating the patients’ family ensured cooperation, empathy and sympathy. The patients’ stroke was not seen as a priority given the severity of his COVID-19 pneumonia, therefore the least invasive, but most appropriate treatment was provided for his stroke. The British Association of Stroke Physicians advocate this approach and also request the notification to their organisation of COVID-19-related stroke cases, in the UK. 19
Learning points
- SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is one of seven known coronaviruses that commonly cause upper and lower respiratory tract infections. It is the cause of the 2019–2020 global coronavirus pandemic.
- The significance of COVID-19 is illustrated by the rapid speed of its spread globally and the potential to cause severe clinical presentations, such as ischaemic stroke.
- Early retrospective data has indicated that up to 36% of COVID-19 patients had neurological manifestations, including stroke.
- Potential mechanisms behind stroke in COVID-19 patients include a plethora of hypercoagulability secondary to critical illness and systemic inflammation, the development of arrhythmia, alteration to the vascular endothelium resulting in atherosclerotic plaque displacement and dehydration.
- It is vital that effective, open communication between the multidisciplinary team, patient and patients relatives is conducted early in order to firmly establish the most appropriate ceiling of care for the patient.
Contributors: SB was involved in the collecting of information for the case, the initial written draft of the case and researching existing data on acute stroke and COVID-19. He also edited drafts of the report. MH was involved in reviewing and editing drafts of the report and contributing new data. SP oversaw the conduction of the project and contributed addition research papers.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Academic Support for Nursing Students
No notifications.
Disclaimer: This case study has been written by a student and not our expert nursing writers. View professional sample case studys here.
View full disclaimer
Any opinions, findings, conclusions, or recommendations expressed in this case study are those of the author and do not necessarily reflect the views of NursingAnswers.net. This case study should not be treated as an authoritative source of information when forming medical opinions as information may be inaccurate or out-of-date.
PBL - Stroke Case Study
Info: 3486 words (14 pages) Nursing Case Study Published: 11th Feb 2020
Reference this
Tagged: stroke aphasia
If you need assistance with writing your nursing case study, our professional nursing case study writing service is here to help!
- Describe the blood supply of the brain.
- Explore the epidemiology of strokes.
- Explain the value of CT with and without contrast in this PBL scenario.
- Consider the treatment and prognosis of the patient.
- Amlodipine : A calcium channel blocker. It works by blocking calcium influx into smooth muscles cells of the wall
- Aphasia [2] : Difficulty in using language. It is categorised into four main types:
- Expressive aphasia – patients know what to say, but are having trouble saying what they mean.
- Receptive aphasia – patients are having difficulty making sense of the words or diagrams.
- Anomic aphasia – patients are facing problems recalling words, names or numbers. (“speaking in a
- Global aphasia – patients cannot speak, understand speech, read, or write. It is the combination of
- Pack year : unit for measuring the smoking history of a person as to be used in risk factor estimation.

- Equivocal plantar response : normal and consistent plantar reflex of both legs. Plantar reflex is a reflex elicited

- Haemorrhagic stroke : Aneurism of blood vessels in the brain that burst.
- Ischemic stroke : Blood vessels in the brain are either clog by local atherosclerosis or thromboembolism.
- Transient ischemic attack (TIA) : Same pathophysiology as ischemic stroke, but occurrence last less than 24 hours. Therefore, it is always a retrospective diagnosis.
|
|
|
| Smoking | Age > 75 |
| Diabetes | Men |
| Being overweight/obese | Family history |
| Alcohol Use | Genetic predisposition |
- Explain the basic principle of CT with and without contrast in this PBL scenario.
- Thrombolytic (within golden 3-4.5 hours): Tissues plasminogen activator (tPA); Alteplase; Urokinase
- Intravenous fibrinolytic therapy
- Surgery: carotid endoterectomy/ angioplasty
- Ultrasound-enhanced thrombolysis
- Speech & language therapy helps people who have problems producing or understanding speech.
- Physiotherapy helps with relearning movement and co-ordination of muscles.
- Psychological care helps with common mental health problems such as depression.
- Occupational therapy helps with assessing patients’ home and improving their abilities to carry out daily activities such as dressing and eating.
- Adams HP Jr, et al. Guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1994; 25: 1901-1914.
- Brierley JB. Experimental hypoxic brain damage. Journal of Clinical Pathology 1977s3-11: 181-187.
- Bryan RN, et al. Diagnosis of acute cerebral infarction: Comparison of CT and MR imaging. AJNR Am J Neuroradiol 1991; 12: 611-620.
- Clarke DD, Sokoloff L. Regulation of Cerebral Metabolic Rate. Basic Neurochemistry: Molecular, Cellular and Medical Aspects , 6 th edition. Philadelphia: Lippincott-Raven; 1999.
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest . 2000;106(6):723-731.
- MedlinePlus. Aphasia . Available at: http://www.nlm.nih.gov/medlineplus/aphasia.html . [Accessed 24 th March 2015].
- Michael-Titus A, Revest P, Shortland P. STROKE AND HEAD INJURY. The Nervous System , 2 nd edition: Elsevier Limited; 2010. pp. 200-209.
- NICE. Alteplase for treating acute ischaemic stroke (review of technology appraisal guidance 122) . Available at: http://www. nice.org.uk/guidance/ta264/chapter/1-guidance. [Accessed 11 April 2015].
- Rull G. Thrombolytic Treatment of Acute Ischaemic Stroke . Available at http://www.patient.co.uk/doctor/thrombolytic-treatment-of-acute-ischaemic-stroke . [Accessed 11 April 2015].
- Stoke Association. State of the Nation Stroke Statistics-January 2015 . Available at: http://www.stroke.org.uk/resource-sheet/state-nation-stroke-statistics . [Accessed 11 April 2015].
- Townsend N, et al. Coronary heart disease statistics 2012 edition . British Heart Foundation: London
- Xavier AR, et al. Neuroimaging of Stroke: A Review. South Med J . 2003;96(4). Available at: http://www.medscape.com/viewarticle/452843_2 . [Accessed 11 April 2015]
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services

- Case Study Service

- Nursing Essay Writing Service

- Reflective Writing Service
Related Content
Content relating to: "aphasia"
Aphasia has many etiologies, such as stroke, infection, brain abscess, brain tumour, nutritional deficiencies, or toxemia. However, the most common cause of aphasia in adults is cerebrovascular accident, which includes several types and classifications for aphasia.
Related Articles

Framework for Speech Enhancement and Recognition
A Generalized Framework for Speech Enhancement and Recognition with Special Focus On Patients with Speech Disorders Literature Review Kumara Sharma et.al. have proposed Harmonics-to-Noise Ratio and ...
Stroke The Causes And Effects Health And Social Care Essay
In this assignment I discuss Paul who is a 65 year old male and has suffered a stroke. My aim is to explain what a stroke is, the causes and effects, and give you my understanding of how I as a Healt...
Written by: Yung Bing Yong Introduction The brain is the primary organ of the centre nervous system that controls our body and houses our mind. It is metabolically active and dependent on a continuou...
DMCA / Removal Request
If you are the original writer of this case study and no longer wish to have your work published on the NursingAnswers.net website then please:
Our academic writing and marking services can help you!
- Marking Service
- Samples of our Work
- Full Service Portfolio
Related Lectures
Study for free with our range of nursing lectures!
- Drug Classification
- Emergency Care
- Health Observation
- Palliative Care
- Professional Values

Write for Us
Do you have a 2:1 degree or higher in nursing or healthcare?
Study Resources
Free resources to assist you with your nursing studies!
- APA Citation Tool
- Example Nursing Essays
- Example Nursing Assignments
- Example Nursing Case Studies
- Reflective Nursing Essays
- Nursing Literature Reviews
- Free Resources
- Reflective Model Guides
- Nursing and Healthcare Pay 2021
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Cardioembolic Stroke: A Case Study
Affiliation.
- 1 Lisa A. Babkair holds an academic appointment at King AbdulAziz University, College of Nursing, Jeddah, Saudi Arabia, and is currently a doctoral candidate at New York University, New York, New York. [email protected].
- PMID: 28148612
- DOI: 10.4037/ccn2017127
Cardioembolic stroke is a critical health condition that requires immediate intervention. Cardiac emboli are the most common type of embolism and account for 14% to 30% of all ischemic strokes. Atrial fibrillation is the most common cause of cardioembolic strokes, and its prevalence increases substantially with age. Other factors that increase the risk for cardioembolic stroke include hypertension, diabetes mellitus, hyperlipidemia, cardiac disease, and lifestyle choices. General supportive care and treatment of the acute phase and subsequent complications should be started immediately. Nurses must play an active role in screening patients for stroke subtypes, using appropriate diagnostic tools, and providing medical and nursing interventions. Nurses also play a crucial role in prevention by providing education to patients and patients' families on how to recognize stroke signs and symptoms. This case study discusses the course of illness, treatment, and prevention strategies for patients who have suffered cardioembolic stroke due to atrial fibrillation.
©2017 American Association of Critical-Care Nurses.
PubMed Disclaimer
Similar articles
- Brain salvage for cardiac cerebral embolism following myocardial infarction. Kawarada O, Yokoi Y. Kawarada O, et al. Catheter Cardiovasc Interv. 2010 Apr 1;75(5):679-83. doi: 10.1002/ccd.22330. Catheter Cardiovasc Interv. 2010. PMID: 20020521
- Left Atrial Spinning Ball Thrombus in a Patient with Cardioembolic Stroke: A Case Report. Machida A, Kawana Y, Soejima I, Bo T, Amano E. Machida A, et al. J Stroke Cerebrovasc Dis. 2020 Dec;29(12):105355. doi: 10.1016/j.jstrokecerebrovasdis.2020.105355. Epub 2020 Oct 3. J Stroke Cerebrovasc Dis. 2020. PMID: 33022584
- Pulmonary vein spontaneous echocontrast and stroke after pulmonary lobectomy. Gual-Capllonch F, Teis A, Palomeras E. Gual-Capllonch F, et al. J Clin Ultrasound. 2013 Jun;41(5):321-2. doi: 10.1002/jcu.21913. Epub 2012 Mar 27. J Clin Ultrasound. 2013. PMID: 22457267
- Acute cardioembolic cerebral infarction: answers to clinical questions. Arboix A, Alio J. Arboix A, et al. Curr Cardiol Rev. 2012 Feb;8(1):54-67. doi: 10.2174/157340312801215791. Curr Cardiol Rev. 2012. PMID: 22845816 Free PMC article. Review.
- Atrial fibrillation and cardioembolic stroke. Ferro JM. Ferro JM. Minerva Cardioangiol. 2004 Apr;52(2):111-24. Minerva Cardioangiol. 2004. PMID: 15194993 Review.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Silverchair Information Systems
Other Literature Sources
- scite Smart Citations
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

COMMENTS
Nurse Laura started an 18 gauge IV in Randall's left AC and started him on a bolus of 500 mL of NS. A blood sample was collected and quickly sent to the lab. Nurse Laura called the Emergency Department Tech to obtain a 12 lead EKG. Pertinent Lab Results for Randall. The physician and the nurse review the labs: WBC 7.3 x 10^9/L. RBC 4.6 x 10^12/L.
ESTUDIO DE CASO 1 Y 2
Darrell Jackson, 81 year old male, came to the Emergency Department at Los Robles Hospital by ambulance after he collapsed in a coffee shop. Upon arrival he presented with left sided weakness, facial drooping, and aphasia. He was diagnosed with an ischemic stroke, right humerus head fracture, and right wrist fracture.
Cerebrovascular Accident (Stroke) Nursing Care and ...
A 66-year-old man was admitted to hospital with a right frontal cerebral infarct producing left-sided weakness and a deterioration in his speech pattern. The cerebral infarct was confirmed with CT imaging. The only evidence of respiratory symptoms on admission was a 2 L oxygen requirement, maintaining oxygen saturations between 88% and 92%. In a matter of hours this patient developed a greater ...
Read chapter 7 of Patient Management in the Telemetry/Cardiac Step-Down Unit: A Case-Based Approach online now, exclusively on AccessMedicine. AccessMedicine is a subscription-based resource from McGraw Hill that features trusted medical content from the best minds in medicine.
Day 1: Critical Care (99291) A 67-year-old woman with hypertension and diabetes presents to the emergency department with abrupt onset of left hemiparesis 45 minutes ago. Pre-evaluation: Discussed presentation and vital signs with ED provider (3 mins). Face-to-face evaluation: Performed medically appropriate history and exam.
General Submissions: Presentations (Oral and Poster) Ischemic stroke: A case study. Rudolf Cymorr Kirby P. Martinez, PhD, MA, RN, CAA, LMT, CSTP, FRIN. Item Link - Use this link for citations and online mentions. This presents an analysis of a case of Ischemic stroke in terms of possible etiology, pathophysiology, drug analysis and nursing care.
Our reason for choosing this disorder. Stroke is the leading cause of disability in the United States. As advanced practice nurses, we anticipate caring for those impacted by strokes in many healthcare settings including emergency rooms, acute care, rehab settings, extended care facilities, and in primary care.
Nurse Darius works on a neurology unit and is caring for Calvin, a 67-year-old male with a history of hyperlipidemia who was recently admitted following a stroke. After settling Calvin in his room, Nurse Darius goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Calvin's care by recognizing and ...
Stroke case study 2. Here is the second stroke case study nursing: Stroke case study 2 initial presentation. A 40-year-old individual with a history of obesity and a sedentary lifestyle arrives at the emergency department with sudden-onset confusion, right-sided weakness, and difficulty understanding speech.
Abstract. Increasing evidence reports a greater incidence of stroke among patients with Coronavirus disease 2019 (COVID-19) than the non-COVID-19 population and suggests that SARS-CoV-2 infection represents a risk factor for thromboembolic and acute ischemic stroke. Elderly people have higher risk factors associated with acute ischemic stroke or embolization vascular events, and advanced age ...
Rick's neuro status is stable and you think his speech might be even more improved. 1245: Jan comes rushing up to the nurse's station saying, "He said he had a horrible headache and now I can't get him to wakeup.". You hear a voice inside your head saying, "ooooooooohhhhh shoooooooooooot" Only you don't say "shoot.".
Nursing's Role in Successful Stroke Care Transitions ...
Dr. Schwamm: The hospital-based response to acute stroke in this case began with the administration of intravenous t-PA, which according to established guidelines, 9 is a class 1, level of ...
To our knowledge, the adoption of Learning Health System (LHS) concepts or approaches for improving stroke care, patient outcomes, and value have not previously been summarized. This topical review provides a summary of the published evidence about LHSs applied to stroke, and case examples applied to different aspects of stroke care from high and low-to-middle income countries. Our attempt to ...
Research has shown that approximately 5% of patients will have an ischemic stroke within 7 days after a TIA. In addition, the risk of stroke within 7 days is doubled for patients with TIAs who did not seek treatment. As is the case for many individuals who have a TIA, Patient M did not seek medical attention because the clinical symptoms ...
SCENARIO 1: Suspected Ischemic Stroke without Large Vessel Occlusion. PREHOSPITAL. ACTORS: Patient: 68 y/o female. Husband: approximately same age. 911 Call Taker. Engine Company (3): Firefighter/EMT, Driver Engineer, Lieutenant. ALS Ambulance crew (2): paramedics. A sixty-eight year-old female patient has a sudden onset of left-sided weakness ...
Due to the multidimensional presentations (size/location of ICH, presence or absence of IVH, etc.) of those with hemorrhagic stroke, we presented this case study in order to pose that a single surgical approach may not be the answer to improving outcomes in comparison to medical management but actually a transition to a multimodal manner that ...
Other hypothesised reasons for stroke development in COVID-19 patients are the development of hypercoagulability, as a result of critical illness or new onset of arrhythmias, caused by severe infection. Some case studies in Wuhan described immense inflammatory responses to COVID-19, including elevated acute phase reactants, such as CRP and D-dimer.
In this PBL scenario, a 67-year-old Caucasian woman who has a 15 pack-year smoking history arrived in A&E with ipsilateral weakness of both her right upper and lower limb, difficulty speaking and is known with atrial fibrillation and hypertension. Her current medication is warfarin and amlodipine.
Cardioembolic Stroke: A Case Study Crit Care Nurse. 2017 Feb;37(1):27-39. doi: 10.4037/ccn2017127. ... College of Nursing, Jeddah, Saudi Arabia, and is currently a doctoral candidate at New York University, New York, New York. [email protected]. PMID: 28148612 DOI: 10.4037/ccn2017127 Abstract Cardioembolic stroke is a critical health condition ...