Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest Content
- BMJ Journals

You are here
- Volume 11, Issue 1
- Relationship between continuity of primary care and hospitalisation for patients with COPD: population-based cohort study from South Korea
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-6644-0808 Iyn-Hyang Lee 1 , 2 ,
- Eunjung Choo 3 ,
- Sejung Kim 4 ,
- http://orcid.org/0000-0002-0299-5131 Nam Kyung Je 5 ,
- Ae Jeong Jo 4 and
- Eun Jin Jang 4
- 1 College of Pharmacy , Yeungnam University , Gyeongsan , Korea (the Republic of)
- 2 Department of Health Sciences , University of York , York , UK
- 3 College of Pharmacy , Ajou University , Suwon , Korea (the Republic of)
- 4 Department of Data Science , Andong National University , Andong , Korea (the Republic of)
- 5 College of Pharmacy , Pusan National University , Busan , Korea (the Republic of)
- Correspondence to Dr Iyn-Hyang Lee; leeiynhyang{at}ynu.ac.kr ; Dr Eun Jin Jang; ejjang{at}anu.ac.kr
Objectives The existing evidence for the impacts of continuity of care (COC) in patients with chronic obstructive pulmonary disease (COPD) is low to moderate. This study aimed to investigate the associations between relational COC within primary care and COPD-related hospitalisations using a robust methodology.
Design Population-based cohort study.
Setting National Health Insurance Service database, South Korea.
Participants 92 977 adults (≥40 years) with COPD newly diagnosed between 2015 and 2016 were included. The propensity score (PS) matching approach was used. PSs were calculated from a multivariable logistic regression that included eight baseline characteristics.
Exposure COC within primary care.
Main outcome measures The primary outcome was the incidence of COPD-related hospitalisations. Cox proportional hazard models were used to estimate HRs and 95% CIs.
Results Out of 92 977 patients, 66 677 of whom were cared for continuously by primary doctors (the continuity group), while 26 300 were not (the non-continuity group). During a 4-year follow-up period, 2094 patients (2.25%) were hospitalised; 874 (1.31%) from the continuity group and 1220 (4.64%) from the non-continuity group. After adjusting for confounding covariates, patients in the non-continuity group exhibited a significantly higher risk of hospital admission (adjusted HR (aHR) 2.43 (95% CI 2.22 to 2.66)). This risk was marginally reduced to 2.21 (95% CI 1.99 to 2.46) after PS matching. The risk of emergency department (ED) visits, systemic corticosteroid use and costs were higher for patients in the non-continuity group (aHR 2.32 (95% CI 2.04 to 2.63), adjusted OR 1.25 (95% CI 1.19 to 1.31) and exp β =1.89 (95% CI 1.82 to 1.97), respectively). These findings remained consistent across the PS-matched cohort, as well as in the sensitivity and subgroup analyses.
Conclusions In patients with COPD aged over 40, increased continuity of primary care was found to be associated with less hospitalisation, fewer ED visits and lower healthcare expenditure.
- COPD epidemiology
Data availability statement
Data may be obtained from a third party and are not publicly available. The raw data that support the findings of this study are available only for authorised researchers in South Korea and for a limited period due to the information protection law for patient privacy. Study protocol is available from the corresponding author on request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjresp-2024-002472
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Clinical and economic outcomes improve with high levels of relational continuity between doctors and patients.
However, there is limited empirical evidence demonstrating the impact of continuous primary care on chronic obstructive pulmonary disease-related hospitalisations.
WHAT THIS STUDY ADDS
This population-based study, with internal validity, demonstrated that reduced continuity of care in primary settings correlated with a heightened risk of hospital admission.
The findings, investigated in a healthcare system with markedly different environments, would enhance the external validity of the evidence.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study highlights the need for further investigation into how factors influencing continuity levels vary according to different healthcare system environments.
Healthcare providers may need to prioritise strategies that enhance ongoing patient–provider relationships.
The evidence supports the development of policies that promote consistent primary care relationships.
Introduction
It is widely acknowledged that primary care quality efficiently improves overall health outcomes, 1 2 and continuity of care (COC) is considered a core element of primary care. 3–5 According to a systematic review by Huntley et al , COC is one of four identified systemic features that affect unscheduled secondary care utilisation, and the other three were access, practice features and quality of care. 6 COC has been characterised by informational, management and relational continuity. 2 7–9 Informational continuity involves the exchange of medical histories among current and previous healthcare providers. Management continuity entails coordinating and harmonising care according to shared treatment plans across providers. 1 2 7 9 Relational continuity (also called interpersonal continuity) concerns long-term therapeutic relationships between patients and their healthcare providers. 2 While informational and management continuity is an issue among service providers, relational continuity centres on the relationship between the service provider and the patient. Relational continuity places focus on the individual rather than on the illness, this concept reflects the value of primary care. 1 A long-term patient–provider relationship can improve communication and establish trust, 1 2 10 which results in a greater willingness to share crucial information with providers and increases the likelihood of patients adhering to treatment and preventive advice. 10 These relationships also help healthcare providers understand their patients better, improve the effectiveness of chronic condition management and the development of long-term disease-monitoring strategies. 1 10 Undoubtedly, high-quality primary care can facilitate planned end-of-life care in the community rather than in hospital. 11 As a result, studies show that high relational COC might lower the risk of premature mortality and prevent the exacerbation of chronic conditions. 12–16 Furthermore, patients with higher relational continuity tend to use secondary care less 16–20 and lower medical costs. 16 21 22 Conversely, low relational continuity negatively affects patient experience. 23 24
Although evidence is accumulating for a wide range of conditions, evidence linking the benefits of high relational continuity in patients with specific conditions is insufficient. A recent systematic review expressed concern that the current level of evidence is low to moderate for asthma and chronic obstructive pulmonary disease (COPD). 12 After this systematic literature review, two new studies on COC in patients with COPD have been published. 25 26 However, the Canadian study still uses a design that measures exposure and outcome simultaneously, leading to confusion about the temporal relationship between cause and effect. 25 Additionally, concerns have been expressed that it will become difficult to maintain patient–doctor relationships as complexity increases in terms of illnesses and medical organisations. 4 27
Approximately, 97% of the Korean population is covered by the National Health Insurance (NHI) while the remaining 3% are covered by a medical aid programme (MedAid) that provides more comprehensive coverage to low-income households. 28 The South Korean healthcare system is composed of primary, secondary and tertiary institutions. A mandatory referral document is required only for accessing tertiary hospitals. Tertiary hospitals are mostly university-affiliated institutions located in a few metropolitan cities. They serve as major centres for medical education and training, offering a wide range of specialised medical treatments. In the absence of legal restrictions, except for those involving tertiary hospitals, patients often prioritise proximity to medical facilities. Patients may receive primary care at clinics or hospital outpatient departments if these are within their catchment area. As a result, the country has been evaluated to have a weak gate-keeping primary care function. 29 In this regard, the relationship between COC and outcomes in the Korean healthcare system has implications for other countries concerned about the weakening of primary care systems.
Set against the presented background, this study aimed to investigate the impacts of relational COC between patients with COPD and primary care doctors on clinical and economic outcomes. COPD is an ambulatory care sensitive condition (ACSC), and its effective management and treatment within ambulatory settings obviate hospital admission. 30 31 We hypothesised that high relational care continuity with a primary care doctor results in better clinical results and lower costs.
Study design and data source
This population-based cohort study was performed according to the Strengthening the Reporting of Observational Studies in Epidemiology guideline. 32 Levels of patient relational COC with their doctors were explored, comparative cohorts based on COC levels were established and the associations between COCs and hospital admission rates and other outcome measures were investigated. Anonymised national insurance claims data between 2014 and 2021 provided by the Korean National Health Insurance Service (KNHIS) were analysed. The KNHIS database contains details of all claims made by Korean residents. These details include deidentified patient sociodemographic information, diagnoses, all medical services provided and medications dispensed and death records. 33
Study time frame
Figure 1 illustrates the study period from 2014 to 2021. Index dates were defined as the dates of the first diagnosis of COPD during 2015–2016. The 12-month period preceding the index date was defined as the preindex year. The exposure period was defined as the 2-year period following the index date, and the outcome period was defined as the period from the end of the exposure period to 31 December 2021. Patients were followed from the end of the exposure period until outcome determinations, death or the end of data collection, whichever came first.
- Download figure
- Open in new tab
- Download powerpoint
Defined study periods.
Study population
Patients with COPD newly diagnosed in 2015–2016 at primary clinics, aged ≥40 years on index date, and who made at least four ambulatory visits during the exposure period were enrolled in the study. 19 34 COPD was identified using the International Classification of Disease, 10th revision (ICD-10) as diagnosis codes J42–44 but excluding McLeod syndrome (a genetic disease coded J43.0). Patients with a claims record for COPD in the preindex year were excluded, and those who visited tertiary facilities, died or experienced hospitalisations or emergency department (ED) visits during the exposure period were also excluded. After exclusions, the final analytic cohort contained 92 977 unique patients.
Measuring continuity of primary care
Continuity of primary care is conceptualised as the extent to which a patient’s visits are concentrated among primary care doctors. Primary care is defined differently across countries. 35 We defined primary care as community-based care and included visits to small-sized and medium-sized facilities, excluding tertiary hospitals, in the COC measurement. To measure COC, we used the Bice & Boxerman Continuity of Care Index (COCI) and Usual Provider Continuity (UPC). 21 36 37 COCI is a dispersion index, and a useful metric for patients who may potentially visit different providers and is used chiefly for claims data analysis. 38 COCI is recommended in South Korea because patients are almost free to contact doctors of choice due to the weak gatekeeper role of primary care. 29 In this study, we also calculated UPC because it is one of the most popular continuity indices used in studies. 38 UPC is a density index that quantifies patients’ visit patterns by focusing on specific providers. 38
Bice and Boxerman COCI and UPC were calculated using the following formulae. 37–39
Where N is the total number of visits made by a patient to a doctor, n i is the number of patient visits with provider i and M is the number of potentially available providers. In the UPC equation, provider i is the provider patients usually visit.
Both indices have a value of unity (1) if a patient always visits one specific health provider and a value of 0 if the patient visits different providers at each visit.
Outcome measures
The primary outcome measure was the incidence of COPD-related hospitalisation during the outcome period because hospitalisation rates for ACSCs can indicate the quality of primary care. Secondary outcomes were the incidence of COPD-related ED visits and COPD-related costs. Patients using corticosteroids in oral or injection form, defined as systemic corticosteroid use, were also investigated to evaluate COPD exacerbation. COPD-related medical costs were defined as the average annual medical costs for each year during the outcome period. Data related to COPD were identified by the ICD-10 diagnosis codes mentioned above. All medical costs were standardised costs to 2020 KRW to remove any effects of inflation.
Covariates included individual characteristics such as age and sex. NHI contributions were classified as high, moderate or low and used as proxies of patients’ economic circumstances. Two types of health insurance programmes, that is, NHI and MedAid were included. Locations of residences at index dates were classified as large urban (metropolitan cities), small urban (other cities) or rural areas. Large and small urban, and rural areas were classified based on population densities. Elixhauser Comorbidity Indices (ECIs) were computed as proxies of patient health statuses 40 based on diagnoses obtained from outpatient and inpatient records during the preindex year. In addition, we considered whether patients had a diagnosis of asthma, allergic rhinitis, atopic dermatitis, hypertension, diabetes, dyslipidaemia, cancer, osteoarthritis or rheumatoid arthritis during the preindex year or were being prescribed inhalers for COPD treatment at index dates or systemic corticosteroids during the exposure period. Patients who made more than 14 outpatient clinic visits during the exposure period were defined as frequent doctor visitors (90th percentile or higher). Based on index dates, disability status was defined as none, moderate or severe, and smoking status as never-smoker, ex-smoker, smoker or no record. For reference purposes, we also collected average annual COPD-related medical costs during exposure periods.
Configuration of the continuity cohort
Interim analysis was conducted to determine continuity score distributions of primary care doctor visits during the exposure period. Interim analyses showed that patients with a COCI of 1 accounted for 71.7% of doctor visits and those with a UPC of 1 accounted for 71.7%. We allocated patients with a continuity score of 1 during the exposure period to the continuity group and the others to the non-continuity group. Propensity score (PS) matching was used to improve comparability between the continuity and non-continuity groups and to reduce the effect of known confounders on study outcomes. PS matching was performed using a logistic regression model containing age, sex, health insurance programme, insurance contributions, residence urbanisation level, disability, ECI score and asthma coexistence as covariates. The continuity group was matched 1:1 to the non-continuity group without replication using the nearest matching method with 0.2 times the logit of PS as a calliper.
Statistical analysis
Baseline characteristics and healthcare utilisation were summarised using means, SD, medians and IQR for continuous variables, and frequencies and proportions for categorical variables. Intergroup differences in baseline characteristics between the continuity and non-continuity groups were assessed using the absolute standardised difference method and when they exceeded 0.1, the intergroup difference was considered as a meaningful difference between the two groups existed. 41 COCIs, UPCs and annual health service utilisations during the exposure period in the two groups were compared by the independent t-test or the χ 2 test, based on type of data. The effects of COC on hospitalisation and ED visits due to COPD were assessed using a Fine-Gray subdistribution hazard model that considered death as a competing risk. 42 HRs and 95% CIs were provided. The use of systemic corticosteroids was assessed using a logistic regression model, and the results are presented as ORs. COPD-associated costs were estimated using a gamma regression model, and results are reported as exponential coefficients. In the analysis of the total cohort, sex, age, insurance programme, insurance contributions, urbanisation level of residence, disability, ECIs, asthma coexistence, COPD inhaler use, systemic corticosteroid use during the exposure period, smoking and frequent doctor visits were adjusted as covariates, and in the PS matched cohort, COPD inhaler use, corticosteroid use during the exposure period, smoking and frequent doctor visits were adjusted as covariates. Individuals whose information about insurance contribution, living area and smoking status was missing were not excluded, and missing values were considered as an independent category in the statistical models. A cumulative incidence graph was used to compare the continuity and non-continuity groups. For sensitivity analysis, we calculated COCIs every 2 years following the index date to construct longitudinal data. These data were then analysed using the generalised estimating equations method, adjusting for the same baseline characteristics and covariates as in the adjusted model for the total cohort. Subgroup analyses were performed on basic characteristics. The analysis was conducted by using SAS V.9.4 (SAS Institute), and statistical significance was accepted for p values <0.05.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Baseline characteristics of the study population
The selection process is shown in figure 2 . A total of 92 977 patients were eligible for analysis.
Identification of study participants and formation of study cohort. COCI, Continuity of Care Index; COPD, chronic obstructive pulmonary disease; ECI, Elixhauser Comorbidity Indices; PS, propensity score.
Table 1 shows the baseline characteristics of the study population. After classifying patients according to the COCI criteria, 66 677 were allocated to the continuity group and 26 300 to the non-continuity group. In the continuity group, there were more female patients than in the non-continuity group. Patients in the non-continuity group were older, had more beneficiaries, more with disabilities, more who were former or current smokers, more who had five or more chronic conditions and a higher copresence of asthma than those in the continuity group. In the non-continuity group, patients were less likely to live in large urban areas. 25 866 patients in the non-continuity group were matched to an equal number of patients in the continuity group by PS matching. The characteristics of matched cohorts were well balanced, showing no meaningful differences in major factors ( table 1 ). In the exposure period, the non-continuity group made more frequent doctor visits and had higher medical expenses compared with the continuity group ( table 1 ).
- View inline
Baseline characteristics of study population and health service utilisation during the exposure period
Changes in COC
The mean COCI of study patients was 0.87 during the exposure period, which slightly decreased to 0.84 during the 4-year study outcome period. By group, while the mean COCI decreased from 1.0 to 0.96 in the continuity group, it remained around 0.54 in the non-continuity group throughout the study period ( online supplemental figure 1 ). COCI changes were similar in the PS-matched cohorts ( online supplemental figure 1 ). The mean UPCs were slightly larger than those of COCIs, and the pattern of changes was similar to that of the COCI ( online supplemental figure 1 ). The average study periods for the continuity and non-continuity groups were 5.89 (SD 0.88) and 5.74 (1.05) years, respectively, and in the PS-matched cohort were 5.82 (0.96) and 5.74 (1.05), respectively.
Supplemental material
Risks of hospital admission.
874 patients (1.31%) were hospitalised in the continuity group, and 1220 patients (4.64%) in the non-continuity for COPD-related reasons ( table 2 ). After adjusting for key covariates, patients in the non-continuity group were found to be at significantly higher risk of hospitalisation (adjusted HR 2.43 (95% CI 2.22 to 2.66)). Figure 3 presents the cumulative incidence of hospitalisation during the outcome period from the primary analysis. The sensitivity analysis indicated a reduced but consistent result (adjusted HR 1.25 (95% CI 1.14 to 1.36)). In the PS-matched cohort, adjusted HR for hospital admission was 2.21 (95% CI 1.99 to 2.46). The demographic features that increased the risk of hospitalisation included being men, aged 65 or older, residing in small urban or rural areas, using a COPD inhaler at the index date, using systemic corticosteroids during the exposure period, having coexistence asthma, being a past or current smoker and being a frequent doctor visitor ( online supplemental table 1 ).
Cumulative incidence of hospital admission and ED visit by level of continuity. (A) Hospital admissions in the total cohort, (B) Hospital admissions in the PS-matched cohort. (C) ED visits in the total cohort, (D) ED visits in the PS-matched cohorts. ED, emergency department; PS, propensity score.
Descriptive summary of study outcomes and association of continuity of primary care with study outcomes
Risks of ED visits
Patients in the non-continuity group were more likely to visit an ED than those in the continuity group (adjusted HR 2.32 (95% CI 2.04 to 2.63)) ( table 2 ). However, the magnitude of the adjusted HR insignificantly decreased to 1.13 (95% CI 0.99 to 1.28) in the sensitivity analysis (p=0.061). In the PS-matched cohort, the adjusted HR for an ED visit was 2.06 (95% CI 1.78 to 2.38). The demographic features that increased the risk of an ED visit were similar to those of hospital admission ( online supplemental table 1 ).
Risk of systemic corticosteroid use
6461 (9.69%) patients in the continuity group and 3807 (14.48%) in the non-continuity group were newly prescribed systemic corticosteroids ( table 2 ). After adjusting for covariates, the non-continuity group had a 1.25-fold higher risk of being prescribed systemic corticosteroids (adjusted OR 1.25 (95% CI 1.19 to 1.31)). The adjusted OR of the PS-matched cohort was similar (1.29 (95% CI 1.22 to 1.37)).
COPD-related medical costs
Mean annual COPD-related medical cost was significantly higher in the non-continuity group ( table 2 ). After adjusting for major covariates, gamma regression modelling analysis demonstrated patients in the non-continuity group spent on them about 1.89 (95% CI 1.82 to 1.97) times more than patients in the continuity group. The intergroup difference was similar for the PS-matched cohort (1.79 (95% CI 1.70 to 1.87) times more).
Subgroup analyses on hospitalisation and ED visits
Primary analysis trends were similar for all subgroups of the total cohort or PS-matched cohort ( online supplemental figure 2 ).
This study analysed nationwide claims data to investigate the impact of continuity of primary care on the clinical and economic outcomes of patients with COPD in the Korean healthcare environment. Study patients with low-level COCs with doctors were twice as likely to be hospitalised or visit an ED and incurred 1.8 times higher healthcare costs. In addition, the risk of receiving systemic corticosteroids due to COPD exacerbation was 1.25 times greater in the non-continuity group than in the continuity group, and similar results were obtained after PS matching. Sensitivity and subgroup analyses produced consistent results. The study shows that high relational continuity of primary care is associated with better health outcomes and lower healthcare costs, which concurs with previous studies. 12 Recently, a study conducted in Ontario, Canada, reported that patients with COPD aged over 35 who received non-continuous primary care had a 2.81-fold higher risk of hospitalisation and a 2.12-fold higher risk of emergency room visits. 25 Additionally, a Norwegian study found that patients in the lowest 30% for UPC had a 3.3-fold increased risk of death compared with those with a UPC of 1. 26
This study contributes as the robust methodology employed enhances the internal validity of the findings from previous studies. We analysed nationwide claims data for the entire Korean population. PS matching increased comparability between cohorts. In order to avoid overestimating outcome risks, the period measuring continuity and outcomes was separated and sensitivity analysis was conducted. 12 34 The analysis conducted on the PS-matched groups and the sensitivity analysis consistently yielded the same results, which underscored the reliability and validity of the study. Nonetheless, several limitations should be considered when interpreting the results of this study. First, the COC measurements used for the analysis were related to visit patterns and concentration rather than the interpersonal nature of the continuity relationship. 7 Second, we used claims data for the analysis, which inherently introduces its shortcomings. For example, the claims data did not include information about individual doctors, which can be more problematic in a hospital outpatient setting than in a clinic. To address this, we operationally defined visits to the same institution and the same department in the hospital outpatient setting as visits to the same provider. In the Korean healthcare reservation system, patients are typically scheduled to see the same doctor if they visit the same medical department within the institution. Another important shortcoming is that we were unable to incorporate objective parameters reflecting patient health statuses (eg, laboratory results) into the analysis. However, the use of systemic corticosteroids was included as a secondary outcome measure to assess disease exacerbation. Third, the results of this study should be interpreted with the understanding that they pertain to patients with COPD in the early stages of the disease. Future research should also evaluate the impact of COC on end-of-life management in COPD. Lastly, care should be taken when generalising our results because the primary care facilities of this study were chosen to reflect the Korean situation. While the inclusion criteria were limited to primary clinics, visits to small-sized and medium-sized facilities, excluding tertiary hospitals, were considered in the measurement of COCI. This might limit the comparability of this study with previous research. However, our results were obtained from a different healthcare setting and are concordant with those of previous studies. In this regard, our analysis may contribute to improving the external validity of this theme.
Interestingly, despite the nearly free choice of healthcare providers in the Korean healthcare system, the proportion of study patients with a COCI of 1 was high at 72%. A COCI of 1 indicates that patients exclusively visited one doctor throughout the observation period, rendering UPC-based analyses meaningless, as a COCI of 1 is equivalent to a UPC of 1. The unexpectedly high COCI value among Korean patients with COPD contrasts with approximately 46% of Korean patients with newly developed dyslipidaemia 16 and 62% of Austrian patients with diabetes who had a COCI of 1. 43 However, direct comparisons of COC measurements across diseases or countries are challenging. For instance, in Norway, the proportion of patients with a UPC≥0.75 were lower in asthma (42%–48%) or COPD (49%–52%) than in diabetes (59%) or heart failure (62%–72%), 44 which is the opposite of what was presented earlier. A recent study reported that COCI among patients with COPD in Norway was observed to be lower than that in Germany, even though Norway has a mandated gatekeeping system. 45 This suggests that various factors, beyond health policy, could influence COC. Although there is growing awareness of a positive association between COC and clinical results, understanding of the factors that influence COC is limited.
In this regard, further research is needed to determine the reasons for the high COC exhibited by Korean patients with COPD. One possible explanation may lie in the nature of COPD, which is characterised by symptoms such as persistent, progressive airflow limitation. As compared with other chronic conditions like dyslipidaemia and early hypertension, COPD can cause immediate discomfort and increase patient desire for medication. Moreover, there is a high probability that a patient will return to the same doctor when symptoms are adequately managed using medication. In this regard, one US study reported that COCIs for COPD were slightly higher than those for congestive heart failure or diabetes. 21 However, this might not be the sole reason for the high COC, given that in Taiwan, only 46% of patients with COPD exhibited a COCI of 1, even though the inclusion criteria in the Taiwanese study were similar to those in the present study. 22 The high proportion of patients with a COCI of 1 in this study may reduce comparability with other studies. Due to this high proportion, classifying all cases with a COCI less than 1 as non-continuity suggests that our non-continuity group may actually have more continuity than those in other studies, potentially undervaluing the impact of primary care continuity.
This study’s findings offer crucial insights for policy-makers and primary care providers. Despite evolving patient–doctor dynamics, it is vital for primary care providers to sustain ongoing relationships with patients. In healthcare systems without traditional family doctor models, efforts to foster consistent connections between patients and their doctors are essential. Exploring options such as proactive information sharing can address challenges linked to multiple doctor involvement. Additionally, coordinated care among healthcare professionals may ease the complexities of fragmented encounters.
Ethics statements
Patient consent for publication.
Not applicable.
Acknowledgments
This study analysed claims data from the Korean National Health Insurance Service (research management number NHIS-2023-1-211).
- Freeman GK ,
- Hjortdahl P
- Haggerty JL ,
- Freeman GK , et al
- Tarrant C ,
- Mainous AG , et al
- Te Winkel M ,
- Schers H , et al
- Huntley A ,
- Lasserson D ,
- Wye L , et al
- Salisbury C ,
- Sampson F ,
- Ridd M , et al
- Schers HJ ,
- Schellevis FG , et al
- Sweeney K , et al
- Green H , et al
- Engström S ,
- Ekstedt M , et al
- Pereira Gray DJ ,
- Sidaway-Lee K ,
- White E , et al
- Yang HK , et al
- Cheng H-M ,
- Lu M-C , et al
- Choo E , et al
- Campbell D ,
- Elliott M , et al
- Williams H , et al
- Chen W-H , et al
- Zielinski A
- Hussey PS ,
- Schneider EC ,
- Rudin RS , et al
- Rodriguez HP ,
- Rogers WH ,
- Marshall RE , et al
- Pollack CE ,
- Asch DA , et al
- Woodhouse K , et al
- Pahlavanyali S ,
- Hetlevik Ø ,
- Baste V , et al
- Health Insurance Review and Assessment Service & National Health Insurance Service
- Bardsley M ,
- Davies S , et al
- Elm E von ,
- Altman DG ,
- Egger M , et al
- National Health Insurance Sharing Service
- Lee J , et al
- van Walraven C ,
- Jennings A , et al
- Boxerman SB
- Steinwachs DM
- Sundararajan V ,
- Halfon P , et al
- Lonjon G , et al
- Austin PC ,
- Latouche A ,
- Geroldinger A ,
- Sauter SK ,
- Heinze G , et al
- Blinkenberg J , et al
- Swanson JO ,
- Sundmacher L , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
I-HL and EJJ contributed equally.
Contributors I-HL, EJJ, NKJ and EC wrote the statistical analysis plan, analysed data and SK carried out data cleaning and statistical analyses. I-HL and EJJ wrote the first draft of the manuscript. AJJ, EC and SK prepared tables and figures. NKJ and AJJ critically reviewed and edited the first draft. EJJ supervised all statistical analysis processes. I-HL was a fund holder and supervised all process of this research. All authors read and approved the final manuscript and met the ICMJE criteria for authorship. I-HL is the guarantor.
Funding This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) grant number (2022R1F1A1073485).
Disclaimer The funding organisations had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Author note Transparency declaration: The lead author (I-HL) affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Caregivers Corner
When to transition from curative measures to palliative or hospice care.

Deciding when to stop seeking curative measures and transition to palliative or hospice care is a deeply personal and often challenging decision. Understanding the differences between these types of care and recognizing the appropriate time for each can help families make informed choices.
Curative Measures vs. Palliative and Hospice Care Curative measures aim to cure or significantly prolong life by treating the underlying disease. These treatments can include surgeries, chemotherapy, radiation, and other aggressive interventions. While these measures can be effective, they may also come with significant side effects and a reduced quality of life.
Palliative care focuses on providing relief from the symptoms and stress of a serious illness. It is appropriate at any stage of a serious illness and can be provided alongside curative treatments. The goal of palliative care is to improve the quality of life for both the patient and their family by addressing physical, emotional, and spiritual needs.
Hospice care is a type of palliative care specifically for patients who are nearing the end of life, typically with a prognosis of six months or less if the disease follows its natural course. Hospice care prioritizes comfort and quality of life, rather than attempting to cure the illness. It often involves a team of healthcare professionals who provide medical, emotional, and spiritual support to the patient and their family.
When to Consider Palliative or Hospice Care
Frequent Hospitalizations: If a patient is experiencing frequent hospitalizations or emergency room visits without significant improvement, it may be time to consider palliative or hospice care.
Declining Health: When a patient's health is steadily declining despite aggressive treatments, and the treatments are causing more harm than benefit, transitioning to palliative or hospice care can provide better quality of life.
Symptom Management: If managing symptoms such as pain, breathlessness, or fatigue becomes the primary focus, palliative care can offer specialized support to alleviate these issues.
Patient and Family Wishes: Respecting the wishes of the patient and their family is crucial. If the patient expresses a desire to focus on comfort rather than curative treatments, it is important to honor their preferences.
Making the decision to transition from curative measures to palliative or hospice care is never easy, but understanding the options and recognizing the signs can help families provide the best possible care for their loved ones. By prioritizing comfort and quality of life, palliative and hospice care offer compassionate support during a challenging time.
If you need support in your caregiving journey reach out to the Duke Caregiver Support Program for free resources and support. Also, please watch our weekly caregiver educations segments every Monday on Eyewitness News 10-11am.

Related Topics
- HEALTH & FITNESS
- CAREGIVERS CORNER
- ABC11 TOGETHER

Helping an Aging Loved One with a Legacy Project
When caregiving clouds gather: a guide to finding your sunshine., harmonizing care: a guide for family caregivers to communicate., finding solace after loss: the road to recovery for family caregivers.
You must have javascript enabled to use this website.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Palliative care in patients with hepatocellular carcinoma: Results from a survey among hepatologists and palliative care physicians
Affiliations.
- 1 Division of Gastroenterology and Hepatology, Foundation IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy.
- 2 Department of Pathophysiology and Transplantation, CRC "A. M. and A. Migliavacca" Center for Liver Disease, University of Milan, Milan, Italy.
- 3 Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
- 4 Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy.
- 5 Division of Internal Medicine and Hepatology, Humanitas Research Hospital IRCCS, Rozzano, Italy.
- 6 Department of Oncology, ASST Lodi, Lodi, Italy.
- 7 UO Palliative Care, Department of Primary Care, APSS Trento, Italy.
- 8 Gastroenterology & Hepatology Unit, Department of Health Promotion, Mother & Child Care, Internal Medicine & Medical Specialties (PROMISE), University of Palermo, Palermo, Italy.
- 9 Fondazione FARO ETS, Turin, Italy.
- 10 Italian Journal of Palliative Care, Italian Society of Palliative Care, Milan, Italy.
- 11 Department of Medical and Surgical Sciences, Alma Mater Studiorum - University of Bologna, Bologna, Italy.
- 12 Unit of Semeiotics, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
- PMID: 39193728
- DOI: 10.1177/02692163241269794
Background: Delays and limitations of palliative care in patients with liver transplantation- ineligible end-stage hepatocellular carcinoma according to Barcelona Clinic Liver Cancer staging system may be explained by different perceptions between hepatologists and palliative care physicians in the absence of shared guidelines.
Aim: To assess physicians' attitudes toward palliative care in end-stage hepatocellular carcinoma and to understand what the obstacles are to more effective management and co-shared between palliative care physicians and hepatologists.
Design: Members of the Italian Association for the Study of Liver Disease and the Italian Society of Palliative Care were invited to a web-based survey to investigate practical management attitude for patients with liver transplant- ineligible end-stage hepatocellular carcinoma.
Participants: Physician members of the of the two associations, representing several hospitals and services in the country.
Results: Ninety-seven hepatologists and 70 palliative care physicians completed the survey: >80% regularly follow 1-19 patients; 58% of hepatologists collaborate with palliative care physicians in the management of patients, 55% of palliative care physicians take care of patients without the aid of hepatologists. Management of cirrhosis differed significantly between the two groups in terms of prescription of albumin, esophagogastroduodenoscopy, anti-viral treatment, anticoagulation, indication to paracentesis and management of encephalopathy. Full-dose acetaminophen is widely used among hepatologists, while opioids are commonly used by both categories, at full dosage, regardless of liver function.
Conclusions: This survey highlights significant differences in the approach to patients with liver transplantation- ineligible end-stage hepatocellular carcinoma, reinforcing the need for shared guidelines and further studies on palliative care in the setting.
Keywords: Liver cancer; albumin; cirrhosis; liver transplantation; pain.
PubMed Disclaimer
Conflict of interest statement
Declaration of conflicting interestThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M. Iavarone: Advisory Board/Speaker Bureau for Bayer, Gilead Sciences, BMS, Janssen, Ipsen, MSD, BTG-Boston Scientific, AbbVie, Guerbet, EISAI, Roche, Astra-Zeneca; A. Aghemo: Advisory Board/Speaker Bureau for GILEAD SCIENCES, ABBVIE, MSD, MYLAN, ALFASIGMA, SOBI, INTERCEPT; P. Lampertico: Advisory Board/Speaker Bureau for BMS, ROCHE, GILEAD SCIENCES, GSK, ABBVIE, MSD, ARROWHEAD, ALNYLAM, JANSSEN, SBRING BANK, MYR, EIGER, ALIGOS, ANTIOS, VIR.
Related information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Spotify is currently not available in your country.
Follow us online to find out when we launch., spotify gives you instant access to millions of songs – from old favorites to the latest hits. just hit play to stream anything you like..

Listen everywhere
Spotify works on your computer, mobile, tablet and TV.

Unlimited, ad-free music
No ads. No interruptions. Just music.

Download music & listen offline
Keep playing, even when you don't have a connection.

Premium sounds better
Get ready for incredible sound quality.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The prevalence and prognosis of cachexia in patients with non-sarcopenic dysphagia: a retrospective cohort study.

1. Introduction
2. materials and methods, 2.1. study design and patients, 2.2. diagnosis of sarcopenic dysphagia, 2.3. cachexia diagnostic criteria.
- Subjective symptom: loss of appetite;
- Objective measure: reduced grip strength (less than 28 kg in men and less than 18 kg in women);
- Biomarker: increased C-reactive protein (CRP) levels (greater than 0.5 mg/dL).
2.4. Outcomes and Other Data
2.5. statistical analysis, 4. discussion, 5. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Rech, R.S.; de Goulart, B.N.G.; Dos Santos, K.W.; Marcolino, M.A.Z.; Hilgert, J.B. Frequency and associated factors for swallowing impairment in community-dwelling older persons: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2022 , 34 , 2945–2961. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rajati, F.; Ahmadi, N.; Naghibzadeh, Z.A.; Kazeminia, M. The global prevalence of oropharyngeal dysphagia in different populations: A systematic review and meta-analysis. J. Transl. Med. 2022 , 20 , 175. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ribeiro, M.; Miquilussi, P.A.; Gonçalves, F.M.; Taveira, K.V.M.; Stechman-Neto, J.; Nascimento, W.V.; de Araujo, C.M.; Schroder, A.G.D.; Massi, G.; Santos, R.S. The Prevalence of Oropharyngeal Dysphagia in Adults: A Systematic Review and Meta-analysis. Dysphagia 2024 , 39 , 163–176. [ Google Scholar ] [ CrossRef ]
- Blanař, V.; Hödl, M.; Lohrmann, C.; Amir, Y.; Eglseer, D. Dysphagia and factors associated with malnutrition risk: A 5-year multicentre study. J. Adv. Nurs. 2019 , 75 , 3566–3576. [ Google Scholar ] [ CrossRef ]
- Xue, W.; He, X.; Su, J.; Li, S.; Zhang, H. Association between dysphagia and activities of daily living in older adults: A systematic review and meta-analysis. Eur. Geriatr. Med. 2024 , 1–17. [ Google Scholar ] [ CrossRef ]
- Roberts, H.; Lambert, K.; Walton, K. The Prevalence of Dysphagia in Individuals Living in Residential Aged Care Facilities: A Systematic Review and Meta-Analysis. Healthcare 2024 , 12 , 649. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Almirall, J.; Rofes, L.; Serra-Prat, M.; Icart, R.; Palomera, E.; Arreola, V.; Clavé, P. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur. Respir. J. 2013 , 41 , 923–928. [ Google Scholar ] [ CrossRef ]
- Patel, D.A.; Krishnaswami, S.; Steger, E.; Conover, E.; Vaezi, M.F.; Ciucci, M.R.; Francis, D.O. Economic and survival burden of dysphagia among inpatients in the United States. Dis. Esophagus 2018 , 31 , dox131. [ Google Scholar ] [ CrossRef ]
- Márquez-Sixto, A.; Navarro-Esteva, J.; Batista-Guerra, L.Y.; Simón-Bautista, D.; Rodríguez-de Castro, F. Prevalence of Oropharyngeal Dysphagia and Its Value as a Prognostic Factor in Community-Acquired Pneumonia: A Prospective Case-Control Study. Cureus 2024 , 16 , e55310. [ Google Scholar ] [ CrossRef ]
- Kakehi, S.; Isono, E.; Wakabayashi, H.; Shioya, M.; Ninomiya, J.; Aoyama, Y.; Murai, R.; Sato, Y.; Takemura, R.; Mori, A.; et al. Sarcopenic Dysphagia and Simplified Rehabilitation Nutrition Care Process: An Update. Ann. Rehabil. Med. 2023 , 47 , 337–347. [ Google Scholar ] [ CrossRef ]
- Arai, H.; Maeda, K.; Wakabayashi, H.; Naito, T.; Konishi, M.; Assantachai, P.; Auyeung, W.T.; Chalermsri, C.; Chen, W.; Chew, J.; et al. Diagnosis and outcomes of cachexia in Asia: Working Consensus Report from the Asian Working Group for Cachexia. J. Cachexia Sarcopenia Muscle 2023 , 14 , 1949–1958. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pandey, S.; Bradley, L.; Del Fabbro, E. Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions. Cancers 2024 , 16 , 1696. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bertocchi, E.; Frigo, F.; Buonaccorso, L.; Venturelli, F.; Bassi, M.C.; Tanzi, S. Cancer cachexia: A scoping review on non-pharmacological interventions. Asia Pac. J. Oncol. Nurs. 2024 , 11 , 100438. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sakaguchi, T.; Maeda, K.; Takeuchi, T.; Mizuno, A.; Kato, R.; Ishida, Y.; Ueshima, J.; Shimizu, A.; Amano, K.; Mori, N. Validity of the diagnostic criteria from the Asian Working Group for Cachexia in advanced cancer. J. Cachexia Sarcopenia Muscle 2024 , 15 , 370–379. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, F.M.; Zhuang, C.L.; Dong, Q.T.; Yu, Z.; Cheng, J.; Shen, X.; Wang, S.L. Characteristics and prognostic impact of cancer cachexia defined by the Asian Working Group for Cachexia consensus in patients with curable gastric cancer. Clin. Nutr. 2024 , 43 , 1524–1531. [ Google Scholar ] [ CrossRef ]
- Ida, S.; Imataka, K.; Morii, S.; Katsuki, K.; Murata, K. Frequency and Overlap of Cachexia, Malnutrition, and Sarcopenia in Elderly Patients with Diabetes Mellitus: A Study Using AWGC, GLIM, and AWGS2019. Nutrients 2024 , 16 , 236. [ Google Scholar ] [ CrossRef ]
- Yoshikoshi, S.; Imamura, K.; Yamamoto, S.; Suzuki, Y.; Harada, M.; Osada, S.; Matsuzawa, R.; Matsunaga, A. Prevalence and relevance of cachexia as diagnosed by two different definitions in patients undergoing hemodialysis: A retrospective and exploratory study. Arch. Gerontol. Geriatr. 2024 , 124 , 105447. [ Google Scholar ] [ CrossRef ]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021 , 6 , 100092. [ Google Scholar ] [ CrossRef ]
- Buccheri, E.; Dell’Aquila, D.; Russo, M.; Chiaramonte, R.; Vecchio, M. Appendicular Skeletal Muscle Mass in Older Adults Can Be Estimated with a Simple Equation Using a Few Zero-Cost Variables. J. Geriatr. Phys. Ther. 2024 . [ Google Scholar ] [ CrossRef ]
- Huang, L.; Shu, X.; Ge, N.; Gao, L.; Xu, P.; Zhang, Y.; Chen, Y.; Yue, J.; Wu, C. The accuracy of screening instruments for sarcopenia: A diagnostic systematic review and meta-analysis. Age Ageing 2023 , 52 , afad152. [ Google Scholar ] [ CrossRef ]
- Wakabayashi, H.; Kakehi, S.; Mizuno, S.; Kinoshita, T.; Toga, S.; Ohtsu, M.; Nishioka, S.; Momosaki, R. Prevalence and prognosis of cachexia according to the Asian Working Group for Cachexia criteria in sarcopenic dysphagia: A retrospective cohort study. Nutrition 2024 , 122 , 112385. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yamanaka, M.; Wakabayashi, H.; Nishioka, S.; Momosaki, R. Malnutrition and cachexia may affect death but not functional improvement in patients with sarcopenic dysphagia. Eur. Geriatr. Med. 2024 , 15 , 777–785. [ Google Scholar ] [ CrossRef ]
- Mizuno, S.; Wakabayashi, H.; Fujishima, I.; Kishima, M.; Itoda, M.; Yamakawa, M.; Wada, F.; Kato, R.; Furiya, Y.; Nishioka, S.; et al. Construction and Quality Evaluation of the Japanese Sarcopenic Dysphagia Database. J. Nutr. Health Aging 2021 , 25 , 926–932. [ Google Scholar ] [ CrossRef ]
- Miyai, I.; Sonoda, S.; Nagai, S.; Takayama, Y.; Inoue, Y.; Kakehi, A.; Kurihara, M.; Ishikawa, M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural Repair 2011 , 25 , 540–547. [ Google Scholar ] [ CrossRef ]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019 , 19 , 91–97. [ Google Scholar ] [ CrossRef ]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Kayashita, J.; Nishioka, S.; Sonoda, A.; Kuroda, Y.; et al. Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clin. Rep. 2017 , 2 , 1–10. [ Google Scholar ] [ CrossRef ]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020 , 21 , 300–307.e2. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Maeda, K.; Akagi, J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia 2015 , 30 , 80–87. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013 , 46 , 201–206. [ Google Scholar ] [ CrossRef ]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989 , 42 , 703–709. [ Google Scholar ] [ CrossRef ]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019 , 38 , 1–9. [ Google Scholar ] [ CrossRef ]
- Ueshima, J.; Inoue, T.; Saino, Y.; Kobayashi, H.; Murotani, K.; Mori, N.; Maeda, K. Diagnosis and prevalence of cachexia in Asians: A scoping review. Nutrition 2024 , 119 , 112301. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020 , 18 , 646–654. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Geppert, J.; Rohm, M. Cancer cachexia: Biomarkers and the influence of age. Mol. Oncol. 2024 , 1–17. [ Google Scholar ] [ CrossRef ]
- Permuth, J.B.; Park, M.A.; Chen, D.T.; Basinski, T.; Powers, B.D.; Gwede, C.K.; Dezsi, K.B.; Gomez, M.; Vyas, S.L.; Biachi, T.; et al. Leveraging real-world data to predict cancer cachexia stage, quality of life, and survival in a racially and ethnically diverse multi-institutional cohort of treatment-naïve patients with pancreatic ductal adenocarcinoma. Front. Oncol. 2024 , 14 , 1362244. [ Google Scholar ] [ CrossRef ]
- Han, J.; Liu, X.; Wang, J.; Tang, M.; Xu, J.; Tan, S.; Wu, G. Prognostic value of body composition in patients with digestive tract cancers: A prospective cohort study of 8267 adults from China. Clin. Nutr. ESPEN 2024 , 62 , 192–198. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019 , 10 , 43. [ Google Scholar ] [ CrossRef ]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: Role of Activin. J. Cachexia Sarcopenia Muscle 2022 , 13 , 2146–2161. [ Google Scholar ] [ CrossRef ]
- Bonomi, P.D.; Crawford, J.; Dunne, R.F.; Roeland, E.J.; Smoyer, K.E.; Siddiqui, M.K.; McRae, T.D.; Rossulek, M.I.; Revkin, J.H.; Tarasenko, L.C. Mortality burden of pre-treatment weight loss in patients with non-small-cell lung cancer: A systematic literature review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024 , 15 , 1226–1239. [ Google Scholar ] [ CrossRef ]
- Amano, K.; Okamura, S.; Baracos, V.E.; Mori, N.; Sakaguchi, T.; Uneno, Y.; Hiratsuka, Y.; Hamano, J.; Miura, T.; Ishiki, H.; et al. Impacts of fluid retention on prognostic abilities of cachexia diagnostic criteria in cancer patients with refractory cachexia. Clin. Nutr. ESPEN 2024 , 60 , 373–381. [ Google Scholar ] [ CrossRef ]
- Papadopoulou, S.K.; Tsintavis, P.; Potsaki, P.; Papandreou, D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020 , 24 , 83–90. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Churilov, I.; Churilov, L.; MacIsaac, R.J.; Ekinci, E.I. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos. Int. 2018 , 29 , 805–812. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wan, S.N.; Thiam, C.N.; Ang, Q.X.; Engkasan, J.; Ong, T. Incident sarcopenia in hospitalized older people: A systematic review. PLoS ONE 2023 , 18 , e0289379. [ Google Scholar ] [ CrossRef ]
- Petermann-Rocha, F.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Frailty, sarcopenia, cachexia and malnutrition as comorbid conditions and their associations with mortality: A prospective study from UK Biobank. J. Public Health 2022 , 44 , e172–e180. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wakabayashi, H. Hospital-associated sarcopenia, acute sarcopenia, and iatrogenic sarcopenia: Prevention of sarcopenia during hospitalization. J. Gen. Fam. Med. 2023 , 24 , 146–147. [ Google Scholar ] [ CrossRef ]
- Liposits, G.; Singhal, S.; Krok-Schoen, J.L. Interventions to improve nutritional status for older patients with cancer—A holistic approach is needed. Curr. Opin. Support. Palliat. Care 2023 , 17 , 15–21. [ Google Scholar ] [ CrossRef ]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients 2023 , 15 , 1802. [ Google Scholar ] [ CrossRef ]
- Mititelu, M.; Licu, M.; Neacșu, S.M.; Călin, M.F.; Matei, S.R.; Scafa-Udriște, A.; Stanciu, T.I.; Busnatu, Ș.; Olteanu, G.; Măru, N.; et al. An Assessment of Behavioral Risk Factors in Oncology Patients. Nutrients 2024 , 16 , 2527. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mangano, G.R.A.; Avola, M.; Blatti, C.; Caldaci, A.; Sapienza, M.; Chiaramonte, R.; Vecchio, M.; Pavone, V.; Testa, G. Non-Adherence to Anti-Osteoporosis Medication: Factors Influencing and Strategies to Overcome It. A Narrative Review. J. Clin. Med. 2022 , 12 , 14. [ Google Scholar ] [ CrossRef ]
- Finch, A.; Benham, A. Patient attitudes and experiences towards exercise during oncological treatment. A qualitative systematic review. Support. Care Cancer 2024 , 32 , 509. [ Google Scholar ] [ CrossRef ]
- Gautam, P.; Shankar, A. Management of cancer cachexia towards optimizing care delivery and patient outcomes. Asia Pac. J. Oncol. Nurs. 2023 , 10 , 100322. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Park, M.A.; Whelan, C.J.; Ahmed, S.; Boeringer, T.; Brown, J.; Crowder, S.L.; Gage, K.; Gregg, C.; Jeong, D.K.; Jim, H.S.L.; et al. Defining and Addressing Research Priorities in Cancer Cachexia through Transdisciplinary Collaboration. Cancers 2024 , 16 , 2364. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cheung, C.; Boocock, E.; Grande, A.J.; Maddocks, M. Exercise-based interventions for cancer cachexia: A systematic review of randomised and non-randomised controlled trials. Asia Pac. J. Oncol. Nurs. 2023 , 10 , 100335. [ Google Scholar ] [ CrossRef ]
- Slee, A.; Reid, J. Exercise and nutrition interventions for renal cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2024 , 27 , 219–225. [ Google Scholar ] [ CrossRef ]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021 , 13 , 761. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aprile, G.; Basile, D.; Giaretta, R.; Schiavo, G.; La Verde, N.; Corradi, E.; Monge, T.; Agustoni, F.; Stragliotto, S. The Clinical Value of Nutritional Care before and during Active Cancer Treatment. Nutrients 2021 , 13 , 1196. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Total n = 175 | Cachexia (+) n = 30 | Cachexia (−) n = 145 | p-Value | |
|---|---|---|---|---|
| Age, years, mean ± SD * | 77 ± 11 | 78 ± 9 | 77 ± 11 | 0.437 |
| Sex, n (%) | 0.103 | |||
| Men | 103 (59%) | 22 (73%) | 81 (56%) | |
| Women | 72 (41%) | 8 (27%) | 64 (44%) | |
| Setting, n (%) | 0.001 | |||
| Acute care hospitals | 78 (45%) | 23 (77%) | 55 (38%) | |
| Rehabilitation hospitals | 76 (43%) | 4 (13%) | 72 (50%) | |
| Others | 21 (12%) | 3 (10%) | 18 (12%) | |
| Main causative diseases of dysphagia, n (%) | ||||
| Cerebral infarction | 77 (44%) | 9 (30%) | 68 (47%) | 0.113 |
| Cerebral hemorrhage | 19 (11%) | 2 (7%) | 17 (12%) | 0.536 |
| Subarachnoid hemorrhage | 9 (5%) | 0 (0%) | 9 (6%) | 0.361 |
| Parkinsonism | 15 (9%) | 5 (17%) | 10 (7%) | 0.142 |
| Dementia | 12 (7%) | 3 (10%) | 9 (6%) | 0.435 |
| Cancer | 8 (5%) | 7 (23%) | 1 (1%) | <0.001 |
| Others | 35 (20%) | 4 (13%) | 31 (21%) | 0.453 |
| Comorbidities, n (%) | ||||
| Chronic heart failure | 21 (12%) | 9 (30%) | 12 (8%) | <0.001 |
| Cancer | 13 (7%) | 15 (50%) | 8 (6%) | <0.001 |
| Chronic renal failure | 8 (5%) | 3 (10%) | 5 (3%) | 0.139 |
| Chronic obstructive pulmonary disease | 8 (5%) | 4 (13%) | 4 (3%) | 0.030 |
| Chronic respiratory failure | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Progressive worsening or uncontrolled chronic infections | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Chronic liver failure | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Rheumatoid arthritis and other collagen diseases | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Sarcopenia, n (%) | 133 (76%) | 26 (87%) | 107 (74%) | 0.133 |
| Malnutrition, n (%) | 92 (53%) | 19 (63%) | 73 (50%) | 0.181 |
| Body mass index, kg/m , mean ± SD * | 21.0 ± 4.0 | 18.9 ± 3.0 | 21.4 ± 4.0 | <0.001 |
| Body weight change in 6 months, %, median (IQR **) | 3.3 (−1.9, 12.7) | 11.0 (3.3, 19.7) | 1.5 (−3.5, 10.5) | 0.074 |
| Handgrip strength, kg, mean ± SD * | 16.5 ± 10.9 | 15.2 ± 10.2 | 16.8 ± 11.1 | 0.467 |
| C-reactive protein, mg/dL, median (IQR **) | 0.5 (0.1, 1.9) | 3.4 (0.8, 11.6) | 0.3 (0.1, 1.0) | <0.001 |
| FILS *** initial, median (IQR **) | 7 (1, 7) | 2 (1, 7) | 7 (1, 7) | 0.160 |
| FILS follow-up, median (IQR **) | 8 (7, 8) | 7 (5.5, 8) | 8 (7, 8) | 0.585 |
| Barthel Index initial, median (IQR **) | 25 (10, 50) | 25 (5, 67.5) | 30 (10, 50) | 0.406 |
| Barthel Index follow-up, median (IQR **) | 50 (20, 85) | 35 (12.5, 75) | 50 (20, 85) | 0.469 |
| Death, n (%) | 7 (4%) | 5 (17%) | 2 (1%) | 0.002 |
| No | Sex | Causative Disease of Admission | Cause of Death | Sarcopenia | Malnutrition | Cachexia |
|---|---|---|---|---|---|---|
| 1 | Woman | Urinary tract infection | Senility | + | + | + |
| 2 | Man | Gastric cancer | Systemic metastatic cancer | + | − | + |
| 3 | Man | Colorectal cancer | Colorectal cancer | + | + | + |
| 4 | Man | Urinary tract infection | Pyothorax | + | + | + |
| 5 | Man | Cardiogenic cerebral embolism | Unknown | + | + | + |
| 6 | Woman | Cerebral infarction | Stroke | + | + | − |
| 7 | Woman | Multiple system atrophy | Septic shock | + | + | − |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Kakehi, S.; Wakabayashi, H.; Nagai, T.; Nishioka, S.; Isono, E.; Otsuka, Y.; Ninomiya, J.; Momosaki, R. The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study. Nutrients 2024 , 16 , 2917. https://doi.org/10.3390/nu16172917
Kakehi S, Wakabayashi H, Nagai T, Nishioka S, Isono E, Otsuka Y, Ninomiya J, Momosaki R. The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study. Nutrients . 2024; 16(17):2917. https://doi.org/10.3390/nu16172917
Kakehi, Shingo, Hidetaka Wakabayashi, Takako Nagai, Shinta Nishioka, Eri Isono, Yukiko Otsuka, Junki Ninomiya, and Ryo Momosaki. 2024. "The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study" Nutrients 16, no. 17: 2917. https://doi.org/10.3390/nu16172917
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Advertise with us
- Wednesday, September 04, 2024
Most Widely Read Newspaper
PunchNG Menu:
- Special Features
- Sex & Relationship
ID) . '?utm_source=news-flash&utm_medium=web"> Download Punch Lite App
Gombe extends palliative distribution to security agencies

Kindly share this story:

- Northern govs vow to tackle insecurity
- Gombe to inaugurate medical emergency agency
- Gombe gov charges promoted police officers on dedication

- Haruna Abdullahi
- Joshua Danmalam
- Muhammadu Yahaya
All rights reserved. This material, and other digital content on this website, may not be reproduced, published, broadcast, rewritten or redistributed in whole or in part without prior express written permission from PUNCH.
Contact: [email protected]
Stay informed and ahead of the curve! Follow The Punch Newspaper on WhatsApp for real-time updates, breaking news, and exclusive content. Don't miss a headline – join now!
VERIFIED NEWS: As a Nigerian, you can earn US Dollars with REGULAR domains, buy for as low as $24, resell for up to $1000. Earn $15,000 monthly. Click here to start.

Latest News
Emir sanusi seeks fg’s mandate to resolve fulani crisis, paralympics: ogunleke nigeria’s last table tennis hope, court remands ‘whistleblower’ pidom in kuje prison, knocks for s’africa over junior d’tigers visa denial, abure, loyalists to boycott otti’s stakeholders’ meeting today.

Hunger protest: Wanted Briton alleges invasion, police quiz staff
Lorem ipsum dolor sit amet, conse adipiscing elit.

- Subscriptions
- Advanced search

Advanced Search

Palliative and end-of-life care for patients with severe COPD
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Figures & Data
- Info & Metrics
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. For many patients, maximal therapy for COPD produces only modest or incomplete relief of disabling symptoms and these symptoms result in a significantly reduced quality of life.
Despite the high morbidity and mortality associated with severe COPD, many patients receive inadequate palliative care. There are several reasons for this. First, patient–physician communication about palliative and end-of-life care is infrequent and often of poor quality. Secondly, the uncertainty in predicting prognosis for patients with COPD makes communication about end-of-life care more difficult. Consequently, patients and their families frequently do not understand that severe COPD is often a progressive and terminal illness.
The purpose of the present review is to summarise recent research regarding palliative and end-of-life care for patients with COPD. Recent studies provide insight and guidance into ways to improve communication about end-of-life care and thereby improve the quality of palliative and end-of-life care the patients receive. Two areas that may influence the quality of care are also highlighted: 1) the role of anxiety and depression, common problems for patients with COPD; and 2) the importance of advance care planning.
Improving communication represents an important opportunity for the improvement of the quality of palliative and end-of-life care received by these patients.
- Chronic bronchitis
- chronic obstructive pulmonary disease
- communication
- end-of-life care
- palliative care
SERIES “COMPREHENSIVE MANAGEMENT OF END-STAGE COPD”
Edited by N. Ambrosino and R. Goldstein
Number 6 in this Series
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of mortality and the 12th leading cause of disability worldwide 1 , 2 . By the year 2020, COPD will be the third leading cause of mortality and the fifth leading cause of disability worldwide 3 – 5 . For many patients, maximal therapy for COPD produces only modest relief of symptoms, leaving patients with significantly reduced health-related quality of life. Many patients with COPD receive inadequate palliative care. The purpose of the present review is to examine problems in the delivery of high-quality palliative care to patients with severe COPD and to identify ways in which to address these problems. Since other articles in the series have discussed treatment of dyspnoea and other symptoms and improving quality of life, the current review will focus on communication about palliative and end-of-life care.
- THE DEFINITION OF PALLIATIVE AND END-OF-LIFE CARE
The goal of palliative care is to prevent and relieve suffering and to support the best possible quality of life for patients and their families, regardless of the stage of disease or the need for other therapies 6 . The World Health Organization adopted the following definition of palliative care: “Palliative care means patient and family-centered care that optimizes quality of life by anticipating, preventing, and treating suffering. Palliative care throughout the continuum of illness involves addressing physical, intellectual, emotional, social and spiritual needs and to facilitate patient autonomy, access to information and choice” 7 . As such, palliative care expands traditional treatment goals to include: enhancing quality of life; helping with medical decision making and identifying the goals of care; addressing the needs of family and other informal caregivers; and providing opportunities for personal growth 6 . In contrast, the term “end-of-life care” usually refers to care concerning the final stage of life and focuses on care of the dying person and their family. The time period for end-of-life care is arbitrary and should be considered variable depending on the patient's trajectory of illness 8 , 9 . Using these definitions, palliative care includes end-of-life care, but is broader and also includes care focused on improving quality of life and minimising symptoms before the end-of-life period, as depicted in figure 1 ⇓ . Although end-of-life care usually refers to care in the final months, weeks or days, there is growing evidence that communication with patients and families about their preferences for end-of-life care should occur early in the course of a chronic life-limiting illness, in order to facilitate high-quality palliative and end-of-life care. The present review will summarise some of this evidence, particularly as it pertains to patients with severe COPD.
- Download figure
- Open in new tab
- Download powerpoint
Schematic diagram for use of the terms “palliative care” and “end-of-life care”.
- POOR PALLIATIVE CARE IN COPD AND THE LINK TO POOR COMMUNICATION
The Study to Understand Prognosis and Preferences for Outcomes and Treatments (SUPPORT) enrolled seriously ill, hospitalised patients in one of five hospitals in the USA with one of nine life-limiting illnesses, including COPD 10 . Compared with patients with lung cancer, patients with COPD were much more likely to die in the intensive care unit (ICU), on mechanical ventilation, and with dyspnoea 11 . These differences occurred despite most patients with COPD preferring treatment focused on comfort rather than on prolonging life. In fact, SUPPORT found that patients with lung cancer and patients with COPD were equally likely to prefer not to be intubated and not to receive cardiopulmonary resuscitation (CPR), yet patients with COPD were much more likely to receive these therapies 11 . A study in the UK also found that patients with COPD are much less likely to die at home and to receive palliative care services than patients with lung cancer 12 . Additional studies have documented the poor quality of palliative care and significant burden of symptoms among patients with COPD 13 . Healthcare for these patients is often initiated in response to acute exacerbations rather than being initiated proactively based on a previously developed plan for managing their disease 14 . A recent study of patients with COPD or lung cancer in the US Veterans Affairs Health System also found that patients with COPD were much more likely to be admitted to an ICU, and have greater lengths of stay in the ICU during their terminal hospitalisation, than patients with lung cancer. In the same study, significant geographic variation in ICU utilisation was found for patients with COPD 15 . Although variation in care may be influenced by many factors including availability, access and reimbursement issues, such geographic variation suggests a lack of consensus concerning the best approach to palliative and end-of-life care for patients with COPD. In summary, there are important opportunities for research and quality improvement if better palliative and end-of-life care is to be provided for patients with severe COPD.
- CHALLENGES IN PROGNOSTICATION FOR INDIVIDUALS WITH COPD
In COPD, it may be difficult to identify those patients who are likely to die within 6 months. The prognostic models used in SUPPORT, which were based on the Acute Physiology and Chronic Health Evaluation II, documented this difficulty. These models showed that, at 5 days prior to death, patients with lung cancer were predicted to have <10% chance of surviving for 6 months, while patients with COPD were predicted to have >50% chance 11 . Recent efforts to identify disease-specific prognostic models for patients with COPD do improve prognostic accuracy, but do not predict individual short-term survival as well as can be done for many patients with cancer 16 – 18 .
Therefore, uncertainty concerning prognosis plays a more prominent role in discussions of prognosis and end-of-life care for patients with COPD than for patients with cancer. As physicians have difficulty discussing prognosis in the setting of uncertainty 19 , it is not surprising that they also struggle with knowing when to raise issues about end-of-life care. Nevertheless, it remains the responsibility of physicians caring for patients with severe COPD to educate them about end-of-life care and to ensure that they receive care consistent with their informed preferences for care at the end of life.
Despite the uncertainty, a profile emerges of the types of patients at high risk of mortality or morbidity over the next 6 months. Physicians should be encouraged to identify patients with COPD for whom discussions about treatment preferences or end-of-life care are especially important 20 . This profile could include an assessment of: forced expiratory volume in one second (FEV 1 ) <30% predicted; oxygen dependence; one or more hospital admissions in the previous year for an acute exacerbation of COPD; left heart failure or other comorbidities; weight loss or cachexia; decreased functional status; increasing dependence on others; and age >70 yrs (table 1 ⇓ ). The presence of two or more of these prognostic indicators should be a reason for such discussions. Multiple criteria increase the relevance and urgency for the discussion, as they increase the risk for a life-threatening exacerbation and for receiving care at the end of life that is not consistent with their informed treatment preferences.
- View inline
Characteristics that should trigger a discussion on preferences about end-of-life care
It is also important that clinicians understand patient preferences for end-of-life care and provide them with an opportunity to discuss these issues. Most patients with life-limiting illness prefer to discuss their preferences for end-of-life care relatively early in the course of their illness 21 , as these discussions are less stressful when the patient is feeling relatively well. Although most patients with COPD will prefer a trial of mechanical ventilation for acute respiratory failure, raising these issues in advance of the requirement makes it easier to revisit these discussions when patients are unwell 21 , 22 .
- CURRENT STATE OF COMMUNICATION ABOUT END-OF-LIFE CARE
Studies have shown that only a minority of patients with moderate-to-severe COPD have discussed treatment preferences and end-of-life care issues with their physicians 23 – 25 and most believe that their physicians do not know their preferences for end-of-life care 24 , 25 . The current author’s group recently found that only a third of patients with oxygen-dependent COPD had discussed end-of-life care with their physicians and <25% of physicians had discussed some important aspects of end-of-life care with their patients, including talking about how long the patient might live and what dying might be like for the patient and the family 23 . The study also examined the items that patients with COPD rated as being performed most poorly, which included talking about prognosis. Therefore, improved communication regarding end-of-life care, prognosis and dying needs to be targeted.
A recent study by McNeely et al. 26 examined the perspectives of Canadian respirologists on communication about end-of-life care for patients with COPD. When respirologists discussed mechanical ventilation for end-stage COPD, the discussions occurred late in the disease trajectory, most commonly taking place in the ICU, with only 23% occurring in the clinic or office 26 . It was found that 84% of physicians waited until dyspnoea was severe and 75% waited until the FEV 1 was <30% pred. A survey of 214 general practitioners in the UK found that, although 82% felt that general practitioners should discuss the prognosis with patients with COPD, only 41% reported “often” or “always” discussing prognosis with these patients 27 . Furthermore, two thirds of these physicians who reported infrequently discussing end-of-life care reported feeling inadequately prepared to have such discussions. These studies suggest that communication about end-of-life care is an important area for improvement in the care of patients with COPD.
- DIRECTION FOR IMPROVING COMMUNICATION ABOUT PROGNOSIS AND ABOUT END-OF-LIFE CARE
There are a number of studies that provide some specific direction for physicians in their communication about end-of-life care for patients with severe COPD. One qualitative study compared patients with severe COPD to patients with metastatic cancer or advanced AIDS, noting that patients with COPD were more likely to express concern about the lack of education that they received about their disease 28 . Patients with COPD wanted more information in the following five specific areas: diagnosis and disease process, treatment, prognosis, what dying might be like, and advance care planning (table 2 ⇓ ).
Components of end-of-life care that patients would like to discuss with their physician 28
A qualitative study of patients with severe COPD in the UK also showed that most, but not all patients wanted more information about their prognosis 29 . In a survey of older patients with serious illnesses and limited life expectancy, just over half (56%) of participants with COPD wanted to know their life expectancy 30 . Often, family members may want this information even when patients do not 31 . Therefore, it is important to determine how much information about prognosis patients and family members want.
Expert recommendations suggest numeric expressions of risk ( e.g. “eight out of 10 people will have side-effects from this drug”) generally lead to better comprehension than do qualitative expressions of risk ( e.g. “this drug is very likely to cause side-effects”) 32 . Moreover, since prognostic information applies to outcomes of groups of patients, experts recommend that prognostic information be phrased in terms of outcomes for populations rather than in terms of individual outcomes ( e.g. “out of a group of 100 patients like your mother, I would expect about 10 to survive this”) 33 . In addition, because some individuals do not wish to receive prognostic information, experts recommend the “ask-tell-ask” approach to discussing prognosis 34 – 36 . For this approach, physicians first seek permission to discuss prognosis prior to doing so and afterwards ask patients and families their understanding of the prognosis to ensure comprehension. Table 3 ⇓ summarises some of these recommendations for discussing prognosis.
Tips for talking about end-of-life care, prognosis and advance care planning
Another area of communication that was rated as poorly performed by patients with severe COPD was asking about the patients' spiritual or religious beliefs 23 . Several studies suggest that the majority of patients want to discuss spiritual or religious issues with their physicians, although some patients do not have a strong preference and others have a preference to not have such discussions with physicians 37 – 39 . Studies from Europe suggest that physicians' religion may be an important determinant of the care patients receive 40 , 41 . Several reviews suggest approaches that physicians can take in determining whether patients want to discuss the patient's religion and spirituality and having these discussions with patients 42 , 43 , although empirical data about which specific techniques improve patient experience are limited.
- OVERCOMING THE BARRIERS TO COMMUNICATION ABOUT END-OF-LIFE CARE FOR PATIENTS WITH COPD
An important step towards improving communication about end-of-life care is to identify the barriers to this communication and implement strategies to overcome them. A recent study of patients with oxygen-dependent COPD examined the barriers to communication about end-of-life care and noted them to be many, diverse and patient-specific 22 . Although 15 barriers were identified, only two were endorsed by >50% of patients, suggesting that an individualised process is required.
The two most commonly mentioned barriers were: “I'd rather concentrate on staying alive than talk about death” and “I'm not sure which physician will be taking care of me if I get very sick.” The former suggests the need for skill in talking about a difficult subject with patients who would rather not discuss it. Although many patients do not wish to discuss dying and death, these same patients often have strong opinions about CPR and mechanical ventilation 44 . Among patients in SUPPORT who did not want to discuss CPR, 25% did not want to have CPR and among those who did not want to discuss mechanical ventilation, 80% did not want to have prolonged mechanical ventilation. Therefore, it may be important to have these discussions even with patients who are reluctant to do so. It may be helpful in these situations to acknowledge that discussions about dying can be difficult, but are nonetheless important for patients, families and physicians to undertake, and may provide an opportunity to decrease the burden of decision making on family members. It can also be useful to explicitly state that such a discussion will not diminish the physicians' focus on finding therapies to improve survival or quality of life, as some patients worry that such a discussion might cause a physician to “give up” on them 45 . Another useful tool for raising these discussions may be to frame them in the context of “hoping for the best, while preparing for the worst” 46 .
The other most common barrier, “I'm not sure which physician will be taking care of me if I get very sick,” suggests that patients may fear abandonment by their physicians at the end of life and that discussions of continuity of care between physicians and patients may be an important component of discussions about dying and death. In a qualitative study of patients with severe COPD, metastatic cancer or advanced AIDS, concerns about continuity of care and abandonment emerged as important for patients with each of these diseases 21 , 47 .
The most common physician-endorsed barriers to communication about end-of-life care 22 include: “There is too little time during our appointments to discuss everything we should”; “I worry that discussing end-of-life care will take away his/her hope”; and “The patient is not ready to talk about the care s/he wants if s/he gets sick.” These barriers suggest that interventions also need to target physicians and address systems-level issues, such as ensuring adequate time for discussions about end-of-life care. Some patients prefer a direct approach to discussing prognosis, while others prefer a more indirect approach 31 . There is no relationship between the number of barriers endorsed by patients and the number endorsed by their physicians 22 . This lack of an association suggests that encouraging interventions may be more successful if they target both patients and physicians.
- THE ROLE OF DEPRESSION AND HEALTH STATUS IN COMMUNICATION ABOUT END-OF-LIFE CARE
Although anxiety and depression are common in COPD, they are not well recognised or treated 48 – 52 . In a recent study, only a third of patients with COPD and clinically significant depression or anxiety were being treated and only half of those with severe depression or anxiety were being treated 53 . Antidepressants can significantly improve mood among patients with COPD and depression 54 . Furthermore, in patients with COPD and depression, antidepressants can also decrease ratings of dyspnoea and other physical symptoms 54 .
Anxiety and depression may modify patients' treatment preferences concerning end-of-life care. Patients with COPD and a higher burden of depressive symptoms have a stronger preference against CPR 55 . This association is similar to findings of two prior investigations, one from SUPPORT 10 and one by Blank et al. 56 , which assessed preferences for CPR, but not specifically for patients with COPD. Patients with depression are more likely to change their treatment preferences after depression resolves 57 . They also rate the quality of patient–physician communication about end-of-life care significantly lower than those without depression 23 . It is unclear whether this finding reflects an effect of depression on patients' ratings of the quality of communication or whether the quality of communication is negatively influenced by depression but, regardless of the reason, it is important for physicians to be aware of this relationship between depression and quality of communication about end-of-life care. Simply put, the presence of anxiety and depression may influence the patient's ability to understand the impact of decisions that they make and therefore, if possible, these symptoms should be treated prior to end-of-life decision making.
Another noteworthy finding about treatment preferences was that health status, as measured by the Saint George's Respiratory Questionnaire, was not associated with end-of-life treatment preferences in a population of patients with severe COPD 55 . These data confirm and extend the results of two prior studies performed in elderly outpatients without severe comorbidities 58 and in seriously ill, hospitalised patients with several different primary diagnoses 59 . Healthcare providers should not assume that poorer health status is necessarily associated with a higher likelihood of refusing life-sustaining treatment. Since physicians consistently underestimate patients' quality of life compared with the patients' own assessments 60 , 61 and since there is evidence that physicians' estimates of patients' treatment preferences are influenced by the physicians' estimates of patients' quality of life 62 , it is important that physicians caring for patients with severe COPD examine their own assumptions and directly obtain treatment preferences from patients. A recent survey from Europe showed that physicians and nurses placed more value on quality of life and less value on prolonging life than patients or family members 63 . Clinicians should keep this difference in mind and avoid inappropriately imposing their values on patients and their families.
- THE ROLE OF ADVANCE DIRECTIVES AND ADVANCE CARE PLANNING IN PATIENTS WITH SEVERE COPD
There has been considerable dissatisfaction with the lack of effects of advance directives on end-of-life care 64 . A number of studies suggest that advance directives do not influence the treatments that patients receive 65 – 67 and do not change end-of-life decision making 68 – 70 . It is clear that advance directives in the absence of good communication about end-of-life care do not improve the quality of end-of-life care. However, in the context of good communication between patients, families and physicians, advance directives may be an important component to end-of-life care. In addition, some studies suggest that the presence of advance directives are associated with decreased levels of stress and increased ratings of the quality of care among family members of dying patients 71 , 72 .
Advance directives may be especially useful among patients with COPD because of their likely trajectory of illness, with unpredictable exacerbations that may make patients suddenly critically ill 8 . In addition, some patients with COPD have strong feelings about the situations in which they would want to forego CPR or, particularly relevant for COPD, mechanical ventilation for acute respiratory failure. Other authors have reported on the development of COPD-specific advance directives for this purpose, although there have not been studies showing that these advance directives improve the quality of end-of-life care 73 , 74 . Nonetheless, advance directives are a topic that should be discussed with all patients with moderate or severe COPD as part of advance care planning and other aspects of communication about end-of-life care. For patients who have experienced noninvasive or invasive ventilation in the past, physicians have the opportunity to use patients' prior experiences as a reference point for discussing potential future episodes of acute respiratory failure and those circumstances under which they would not want these types of ventilation. Patients' prior experiences with life support, or with relatives or friends who have required life support, can be important facilitators to patient–physician communication about treatment preferences and end-of-life care 22 .
- CARE OF THE PATIENT DYING FROM COPD AND THE ROLE OF THE HOSPICE
Some patients with COPD will die from lung cancer or cardiovascular disease 2 , 75 , whereas others die from progressive respiratory dysfunction, or a systemic complication of it 14 . Physicians caring for patients dying with severe COPD may need to consider the value of treating their comorbidities in the setting of advanced and terminal COPD. For example, indications for treatment of hypertension may change as COPD advances. Physicians should consider physiological changes as patients become progressively closer to death and reassess the risks and benefits of the treatments for these comorbidities 76 .
Improvements in care of the dying have been largely confined to patients in hospice care and specifically to patients with cancer 77 . Patients dying of progressive COPD are less likely to be offered the opportunity to benefit from hospice services when compared with patients with cancer 12 . The illness trajectory among patients with COPD is more likely to be a gradual slow decline interspersed with unpredictable acute exacerbations, which are often followed by an improvement back towards, but not quite reaching, their pre-exacerbation baseline 9 . This trajectory does not fit the traditional hospice model well and may be part of the reason that patients with COPD are less likely to receive hospice and palliative care services. However, understanding this trajectory and discussing it with patients and their families may offer an opportunity to adapt hospice and palliative care services to meet the needs of these patients and their families 8 . Accomplishing this goal represents one of the most important challenges to improving end-of-life care for patients with severe COPD.
Patients with COPD are at risk for respiratory failure and recent advances in the use of noninvasive ventilation raise questions about the use of this technology in the palliative care setting. The use of noninvasive ventilation for patients who choose to forego invasive mechanical ventilation is relatively common in Europe, with approximately one third of patients who receive end-of-life care in the hospital receiving noninvasive ventilation as the most aggressive form of ventilation before death 78 . A recent Society of Critical Care Medicine Task Force provided a systematic approach to considering noninvasive ventilation in palliative care settings, and concluded that although noninvasive ventilation may be appropriate as a form of life support for patients who choose to forego invasive ventilation, current literature does not support the routine use of noninvasive ventilation for the sole purpose of palliation of symptoms 79 . Furthermore, most hospice settings do not have familiarity with noninvasive ventilation and this lack of familiarity increases the risk for harm and decreases the chance of benefit from noninvasive ventilation.
- CONCLUSIONS
In summary, palliative care is an important component in the treatment of patients with severe chronic obstructive pulmonary disease. There is strong evidence to suggest that patients with chronic obstructive pulmonary disease receive poor-quality palliative care compared with patients with cancer 11 , 12 , 14 . One reason these patients may receive poor-quality palliative care is that patient–physician communication about end-of-life care is unlikely to occur, or only occurs late in the illness 23 – 25 . The quality of this communication is likely to be poor and current models for training physicians in communication about end-of-life care are inadequate 23 , 27 , 80 . Understanding the barriers to this communication may be an important step to improving it 22 . Furthermore, recognising the importance of anxiety and depression will also be important when discussing palliative and end-of-life care 55 . Advance directives and good advance care planning offer an opportunity for improving the quality of palliative care received by patients with severe chronic obstructive pulmonary disease. Finally, hospice and palliative care services represent an important opportunity for improving end-of-life care, but clinicians caring for patients with chronic obstructive pulmonary disease must find more effective ways to utilise these services.
- Statement of interest
None declared.
Previous articles in this series: No. 1: Viegi G, Pistelli F, Sherill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J 2007; 30: 993–1013. No. 2: Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J 2008; 31: 204–212. No. 3: Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J 2008; 31: 667–677. No. 4: Albert P, Calverley PMA. Drugs (including oxygen) in severe COPD. Eur Respir J 2008; 31: 1114–1124. No. 5: Clini EM, Ambrosino N. Nonpharmacological treatment and relief of symptoms in COPD. Eur Respir J 2008; 32: 218–228.
- Received September 24, 2007.
- Accepted October 20, 2007.
- © ERS Journals Ltd
- ↵ Pauwels RA, Buist AS, Calverley CR, Hurd SS. on behalf of the GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease, NHLBI/WHO Workshop Summary. Am J Respir Crit Care Med 2001 ; 163 : 1256 –1276. OpenUrl CrossRef PubMed
- ↵ Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 2002 ; 121 : Suppl. 5 121S –126S. OpenUrl CrossRef PubMed Web of Science
- ↵ Michaud CM, Murray CJ, Bloom BR. Burden of disease – implications for future research. JAMA 2001 ; 285 : 535 –539. OpenUrl CrossRef PubMed Web of Science
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997 ; 349 : 1498 –1504. OpenUrl CrossRef PubMed Web of Science
- ↵ Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000 ; 117 : Suppl. 2 5S –9S. OpenUrl CrossRef PubMed
- ↵ National Consensus Project for Quality Palliative Care: Clinical Practice Guidelines for quality palliative care, executive summary. J Palliat Med 2004 ; 7 : 611 –627. OpenUrl CrossRef PubMed
- ↵ The Hospice and Palliative Medicine National Consensus Guidelines, 2004. www.nationalconsensusproject.org Date last accessed: June 17, 2007. Last updated: 2004
- ↵ Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ 2005 ; 330 : 1007 –1011. OpenUrl FREE Full Text
- ↵ Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA 2003 ; 289 : 2387 –2392. OpenUrl CrossRef PubMed Web of Science
- ↵ The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: The Study to Understand Prognoses and Preferences for Outcomes and Risks of treatments (SUPPORT). JAMA 1995 ; 274 : 1591 –1598. OpenUrl CrossRef PubMed Web of Science
- ↵ Claessens MT, Lynn J, Zhong Z, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. J Am Geriatr Soc 2000 ; 48 : Suppl. 5 S146 –S153. OpenUrl PubMed Web of Science
- ↵ Gore JM, Brophy CJ, Greenstone MA. How do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000 ; 55 : 1000 –1006. OpenUrl Abstract / FREE Full Text
- ↵ Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med 2004 ; 98 : 439 –445. OpenUrl CrossRef PubMed Web of Science
- ↵ Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med 2006 ; 166 : 326 –331. OpenUrl CrossRef PubMed Web of Science
- ↵ Skilbeck J, Mott L, Page H, Smith D, Hjelmeland-Ahmedzai S, Clark D. Palliative care in chronic obstructive airways disease: a needs assessment. Palliat Med 1998 ; 12 : 245 –254. OpenUrl Abstract / FREE Full Text
- ↵ Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004 ; 350 : 1005 –1012. OpenUrl CrossRef PubMed Web of Science
- Ramirez-Venegas A, Sansores RH, Perez-Padilla R, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med 2006 ; 173 : 393 –397. OpenUrl CrossRef PubMed Web of Science
- ↵ Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007 ; 176 : 454 –459. OpenUrl CrossRef PubMed Web of Science
- ↵ Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med 1998 ; 158 : 2389 –2395. OpenUrl CrossRef PubMed Web of Science
- ↵ Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care 2004 ; 49 : 90 –97. OpenUrl PubMed
- ↵ Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians' skills at providing end-of-life care: perspectives of patients, families, and health care workers. J Gen Intern Med 2001 ; 16 : 41 –49. OpenUrl CrossRef PubMed Web of Science
- ↵ Knauft ME, Nielsen EL, Engelberg RA, Patrick DL, Curtis JR. Barriers and facilitators to communication about end-of-life care for patients with COPD. Chest 2005 ; 127 : 2188 –2196. OpenUrl CrossRef PubMed Web of Science
- ↵ Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient–physician communication about end-of-life care for patients with severe COPD. Eur Respir J 2004 ; 24 : 200 –205. OpenUrl Abstract / FREE Full Text
- ↵ Heffner JE, Fahy B, Hilling L, Barbieri C. Outcomes of advance directive education of pulmonary rehabilitation patients. Am J Respir Crit Care Med 1997 ; 155 : 1055 –1059. OpenUrl PubMed Web of Science
- ↵ Heffner JE, Fahy B, Hilling L, Barbieri C. Attitudes regarding advance directives among patients in pulmonary rehabilitation. Am J Respir Crit Care Med 1996 ; 154 : 1735 –1740. OpenUrl CrossRef PubMed Web of Science
- ↵ McNeely PD, Hébert PC, Dales RE, et al. Deciding about mechanical ventilation in end-stage chronic obstructive pulmonary disease: how respirologists perceive their role. CMAJ 1997 ; 156 : 177 –183. OpenUrl Abstract / FREE Full Text
- ↵ Elkington H, White P, Higgs R, Pettinari CJ. GPs' views of discussions of prognosis in severe COPD. Fam Pract 2001 ; 18 : 440 –444. OpenUrl Abstract / FREE Full Text
- ↵ Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Patients' perspectives on physicians' skills at end-of-life care: differences between patients with COPD, cancer, and AIDS. Chest 2002 ; 122 : 356 –362. OpenUrl CrossRef PubMed Web of Science
- ↵ Jones I, Kirby A, Ormiston P, et al. The needs of patients dying of chronic obstructive pulmonary disease in the community. Fam Pract 2004 ; 21 : 310 –313. OpenUrl Abstract / FREE Full Text
- ↵ Fried TR, Bradley EH, O'Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc 2003 ; 51 : 1398 –1403. OpenUrl CrossRef PubMed Web of Science
- ↵ Curtis JR, Engelberg RA, Young PJ, et al. An approach to understanding the interaction of hope and desire for explicit prognostic information among individuals with severe chronic obstructive pulmonary disease or advanced cancer. J Palliat Med 2008 ; 11 : 610 –620. OpenUrl CrossRef PubMed Web of Science
- ↵ Paling J. Strategies to help patients understand risks. BMJ 2003 ; 327 : 745 –748. OpenUrl FREE Full Text
- ↵ Thomson R, Edwards A, Grey J. Risk communication in the clinical consultation. Clin Med 2005 ; 5 : 465 –469. OpenUrl PubMed Web of Science
- ↵ Buckman R. How to Break Bad News. Baltimore, Johns Hopkins University Press, 1992
- Back AL, Arnold RM, Baile WF, Tulsky JA, Fryer-Edwards K. Approaching difficult communication tasks in oncology. CA Cancer J Clin 2005 ; 55 : 164 –177. OpenUrl CrossRef PubMed Web of Science
- ↵ White DB. Braddock CH 3rd, Bereknyei S, Curtis JR.. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med 2007 ; 167 : 461 –467. OpenUrl CrossRef PubMed Web of Science
- ↵ Ehman JW, Ott BB, Short TH, Ciampa RC, Hansen-Flaschen J. Do patients want physicians to inquire about their spiritual or religious beliefs if they become gravely ill?. Arch Intern Med 1999 ; 159 : 1803 –1806. OpenUrl CrossRef PubMed Web of Science
- King DE, Bushwick B. Beliefs and attitudes of hospital inpatients about faith healing and prayer. J Fam Pract 1994 ; 39 : 349 –352. OpenUrl PubMed Web of Science
- ↵ Anderson JM, Anderson LJ, Felsenthal G. Pastoral needs for support within an inpatient rehabilitation unit. Arch Phys Rehab Med 1993 ; 74 : 574 –578. OpenUrl CrossRef
- ↵ Sprung CL, Cohen SL, Sjokvist P, et al. End-of-life practices in European intensive care units: the Ethicus Study. JAMA 2003 ; 290 : 790 –797. OpenUrl CrossRef PubMed Web of Science
- ↵ Sprung CL, Maia P, Bulow HH, et al. The importance of religious affiliation and culture on end-of-life decisions in European intensive care units. Intensive Care Med 2007 ; 33 : 1732 –1739. OpenUrl CrossRef PubMed Web of Science
- ↵ Post SG Puchalski CM, Larson DB. Physicians and patient spirituality: professional boundaries, competency, and ethics. Ann Intern Med 2000 ; 132 : 578 –583. OpenUrl CrossRef PubMed Web of Science
- ↵ Lo B, Ruston D, Kates LW, et al. Discussing religious and spiritual issues at the end of life: a practical guide for physicians. JAMA 2002 ; 287 : 749 –754. OpenUrl CrossRef PubMed Web of Science
- ↵ Hofmann JC, Wenger NS, Davis RB, et al. Patients' preferences for communication with physicians about end-of-life decisions. Ann Intern Med 1997 ; 127 : 1 –12. OpenUrl PubMed Web of Science
- ↵ Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med 2001 ; 161 : 868 –874. OpenUrl CrossRef PubMed Web of Science
- ↵ Back AL, Arnold RM, Quill TE. Hope for the best, and prepare for the worst. Ann Intern Med 2003 ; 138 : 439 –443. OpenUrl CrossRef PubMed
- ↵ Carline JD, Curtis JR, Wenrich MD, Shannon SE, Ambrozy DM, Ramsey PG. Physicians' interactions with health care teams and systems in the care of dying patients: perspectives of dying patients, family members, and health care professionals. J Pain Symptom Manage 2003 ; 25 : 19 –28. OpenUrl CrossRef PubMed Web of Science
- ↵ McSweeny A, Heaton R, Grant I, Cugell D, Solliday N, Timms T. Chronic obstructive pulmonary disease; socioemotional adjustment and life quality. Chest 1980 ; 77 : 309 –311. OpenUrl CrossRef PubMed
- Prigatano GP, Wright EC, Levin D. Quality of life and its predictors in patients with mild hypoxemia and chronic obstructive pulmonary disease. Arch Intern Med 1984 ; 144 : 1613 –1619. OpenUrl CrossRef PubMed Web of Science
- Light RW, Merrill EJ, Despars JA, Gordon GH, Mutalipassi LR. Prevalence of depression and anxiety in patients with COPD. Chest 1985 ; 87 : 35 –38. OpenUrl CrossRef PubMed Web of Science
- Engstrom CP, Persson LO, Larsson S, Ryden A, Sullivan M. Functional status and well being in chronic obstructive pulmonary disease with regard to clinical parameters and smoking: a descriptive and comparative study. Thorax 1996 ; 51 : 825 –830. OpenUrl Abstract / FREE Full Text
- ↵ van Ede L, Yzermans CJ, Brouwer HJ. Prevalence of depression in patients with chronic obstructive pulmonary disease: a systematic review. Thorax 1999 ; 54 : 688 –692. OpenUrl Abstract / FREE Full Text
- ↵ Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005 ; 127 : 1205 –1211. OpenUrl CrossRef PubMed Web of Science
- ↵ Borson S, McDonald GJ, Gayle T, Deffebach M, Lakshminarayan S, Van Tuinen C. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics 1992 ; 33 : 190 –201. OpenUrl CrossRef PubMed Web of Science
- ↵ Stapleton RD, Nielsen EL, Engelberg RA, Patrick DL, Curtis JR. Association of depression and life-sustaining treatment preferences in patients with COPD. Chest 2005 ; 127 : 328 –334. OpenUrl CrossRef PubMed Web of Science
- ↵ Blank K, Robison J, Doherty E, Prigerson H, Duffy J, Schwartz HI. Life-sustaining treatment and assisted death choices in depressed older patients. J Am Geriatr Soc 2001 ; 49 : 153 –161. OpenUrl CrossRef PubMed Web of Science
- ↵ Rosenfeld KE, Wenger NS, Phillips RS, et al. Factors associated with change in resuscitation preferences of seriously ill patients. Arch Intern Med 1996 ; 156 : 1558 –1564. OpenUrl CrossRef PubMed Web of Science
- ↵ Uhlmann RF, Pearlman RA. Perceived quality of life and preferences for life-sustaining treatment in older adults. Arch Intern Med 1991 ; 151 : 495 –497. OpenUrl CrossRef PubMed Web of Science
- ↵ Levenson JW, McCarthy EP, Lynn J, Davis RB, Phillips RS. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc 2000 ; 48 : Suppl. 5 S101 –S109. OpenUrl PubMed
- ↵ Sprangers MA, Aaronson NK. The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease: a review. J Clin Epidemiol 1992 ; 45 : 743 –760. OpenUrl CrossRef PubMed Web of Science
- ↵ Wilson KA, Dowling AJ, Abdolell M, Tannock IF. Perception of quality of life by patients, partners and treating physicians. Qual Life Res 2000 ; 9 : 1041 –1052. OpenUrl CrossRef PubMed Web of Science
- ↵ Schneiderman LJ, Kaplan RM, Pearlman RA, Teetzel H. Do physicians' own preferences for life-sustaining treatment influence their perceptions of patients' preferences. J Clin Ethics 1993 ; 4 : 28 –33. OpenUrl PubMed Web of Science
- ↵ Sprung CL, Carmel S, Sjokvist P, et al. Attitudes of European physicians, nurses, patients, and families regarding end-of-life decisions: the ETHICATT study. Intensive Care Med 2007 ; 33 : 104 –110. OpenUrl CrossRef PubMed Web of Science
- ↵ Tonelli MR. Pulling the plug on living wills – a critical analysis of advance directives. Chest 1996 ; 110 : 816 –822. OpenUrl CrossRef PubMed Web of Science
- ↵ Schneiderman LJ, Kronick R, Kaplan RM, Anderson JP, Langer RD. Effects of offering advance directives on medical treatment and costs. Ann Intern Med 1992 ; 117 : 599 –606. OpenUrl CrossRef PubMed Web of Science
- Danis M, Mutran E, Garrett JM. A prospective study of the impact of patient preferences on life-sustaining treatment and hospital cost. Crit Care Med 1996 ; 24 : 1811 –1817. OpenUrl CrossRef PubMed Web of Science
- ↵ Danis M, Southerland LI, Garrett JM, et al. A prospective study of advance directives for life-sustaining care. N Engl J Med 1991 ; 324 : 882 –888. OpenUrl CrossRef PubMed Web of Science
- ↵ Teno JM, Lynn J, Connors AFJ, et al. The illusion of end-of-life savings with advance directives. J Am Geriatr Soc 1997 ; 45 : 513 –518. OpenUrl PubMed Web of Science
- Teno JM, Lynn J, Wegner N, et al. Advance directives for seriously ill hospitalized patients: effectiveness with the Patient Self-Determination Act and the SUPPORT Intervention. J Am Geriatr Soc 1997 ; 45 : 500 –507. OpenUrl PubMed Web of Science
- ↵ Teno JM, Licks S, Lynn J, et al. Do advance directives provide instructions that direct care?. J Am Geriatr Soc 1997 ; 45 : 508 –512. OpenUrl PubMed Web of Science
- ↵ Tilden VP, Tolle SW, Drach LL, Perrin NA. Out-of-hospital death: advance care planning, decedent symptoms, and caregiver burden. J Am Geriatr Soc 2004 ; 52 : 532 –539. OpenUrl CrossRef PubMed Web of Science
- ↵ Norris K, Merriman MP, Curtis JR, Asp C, Tuholske L, Byock IR. Next of kin perspectives on the experience of end of life care in the community setting. J Palliat Med 2007 ; 10 : 1101 –1115. OpenUrl CrossRef PubMed
- ↵ Dales RE, O'Connor A, Hebert P, Sullivan K, McKim D, Llewellyn-Thomas H. Intubation and mechanical ventilation for COPD: development of an instrument to elicit patient preferences. Chest 1999 ; 116 : 792 –800. OpenUrl CrossRef PubMed Web of Science
- ↵ Singer P. Advance directives in COPD. Monaldi Arch Chest Dis 1995 ; 50 : 62 –63. OpenUrl PubMed
- ↵ Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007 ; 356 : 775 –789. OpenUrl CrossRef PubMed Web of Science
- ↵ Stevenson J, Abernethy AP, Miller C, Currow DC. Managing comorbidities in patients at the end of life. BMJ 2004 ; 329 : 909 –912. OpenUrl FREE Full Text
- ↵ Fordham S, Dowrick C. Is care of the dying improving? The contribution of specialist and non-specialist to palliative care. Fam Pract 1999 ; 16 : 573 –579. OpenUrl Abstract / FREE Full Text
- ↵ Nava S, Sturani C, Hartl S, et al. End-of-life decision-making in respiratory intermediate care units: a European survey. Eur Respir J 2007 ; 30 : 156 –164. OpenUrl Abstract / FREE Full Text
- ↵ Curtis JR, Cook DJ, Sinuff T, et al. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med 2007 ; 35 : 932 –939. OpenUrl CrossRef PubMed Web of Science
- ↵ Block SD. Medical education in end-of-life care: the status of reform. J Palliat Med 2002 ; 5 : 243 –248. OpenUrl CrossRef PubMed
- Table of Contents
- Index by author
Thank you for your interest in spreading the word on European Respiratory Society .
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
More in this TOC Section
- Asthma remission: what is it and how can it be achieved?
- Asthma management in low and middle income countries
- Calcilytics for the management of asthma
Related Articles
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
The Role of Palliative Care in COPD
Anand s. iyer.
a Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL
b Lung Health Center, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL
c Center for Palliative and Supportive Care, Division of Gerontology, Geriatrics and Palliative Care, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL
Donald R. Sullivan
d Division of Pulmonary and Critical Medicine, Oregon Health and Sciences University, Portland, OR
e Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, OR
Kathleen O. Lindell
f College of Nursing, Medical University of South Carolina, Charleston, SC
Lynn F. Reinke
g Department of Biobehavioral Nursing and Health Informatics, School of Nursing, University of WA, Seattle, WA
h VA Puget Sound Health Care System, Seattle, WA
COPD is the fourth leading cause of death in the United States and is a serious respiratory illness characterized by years of progressively debilitating breathlessness, high prevalence of associated depression and anxiety, frequent hospitalizations, and diminished well-being. Despite the potential to confer significant quality-of-life benefits for patients and their care partners and to improve end-of-life (EOL) care, specialist palliative care is rarely implemented in COPD, and when initiated, it often occurs only at the very EOL. Primary palliative care delivered by frontline clinicians is a feasible model, but is not integrated routinely in COPD. In this review, we discuss the following: (1) the role of specialist and primary palliative care for patients with COPD and the case for earlier integration into routine practice; (2) the domains of the National Consensus Project Guidelines for Quality Palliative Care applied to people living with COPD and their care partners; and (3) triggers for initiating palliative care and practical ways to implement palliative care using case-based examples. This review solidifies that palliative care is much more than hospice and EOL care and demonstrates that early palliative care is appropriate at any point during the COPD trajectory. We emphasize that palliative care should be integrated long before the EOL to provide comprehensive support for patients and their care partners and to prepare them better for the EOL.
COPD is a serious respiratory illness and the fourth leading cause of the death in the United States. 1 COPD also has remained an important cause of disability, especially among older adults. 2 Patients often experience declining functional status, progressively debilitating breathlessness, unrecognized and undertreated psychological symptoms, and frequent hospitalizations near the end of life (EOL) that contribute to poor quality of life (QOL) and social isolation. 3 Patients frequently express difficulty coping with COPD’s unpredictable illness trajectory, and their care partners also experience significant burden. 4 , 5 In these situations, palliative care initiated early in the COPD trajectory has the potential to provide significant QOL benefits for patients and their care partners to prepare them for the EOL. This is supported by Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines discussing a role for palliative care in COPD, 3 calls for palliative care from multiple national organizations, 6 , 7 , 8 and real-world examples of successful palliative care implementation for those living with COPD. 9 However, significant barriers exist that prevent routine uptake of palliative care in COPD, making its use exceedingly rare and preventing its proactive integration.
Purpose of Review
The purpose of this review is to discuss the role of palliative care in COPD, with a specific focus on primary palliative care delivered by frontline clinicians. First, we define terms and review data on palliative care in COPD, including barriers to its early integration and success stories. Then, we offer practical recommendations to integrate early palliative care into routine COPD practice informed by domains of the National Consensus Project (NCP) Guidelines for Quality Palliative Care, including triggers for initiation and case-based examples. We hope that readers will recognize that palliative care is more than hospice and EOL care and will understand that early palliative care is appropriate at any point during the trajectory of COPD.
Brief Methods
Our team included experts (A. S. I., D. R. S., K. O. L., L. F. R.) who integrate palliative care into pulmonary-critical care medicine through health policy advocacy and clinical and research endeavors. Our team met virtually from 2020 through 2021 to discuss the current state of palliative care in COPD and the structure of this review. We developed an outline and list of potential topics informed by the NCP Guidelines for Quality Palliative Care 10 and achieved consensus through group discussion of the final topics to emphasize. We cross-referenced COPD with the following search terms in PubMed to guide this review: palliative care , supportive care , end-of-life care , and hospice . We organized overall themes into two main sections: the role of palliative care in COPD and practical recommendations for integrating palliative care into routine COPD practice.
Palliative Care Is More Than Hospice
Terminology.
Palliative care provides comprehensive support for several domains of physical, emotional, spiritual, social, and respite care needs experienced by patients who live with a serious illness like COPD. 10 An interprofessional team supports the broad needs of patients and their care partners and helps them to cope with living with a serious illness while proactively planning for the EOL. 11 The misconceptions that palliative care is only appropriate at the very EOL in the ICU, that it encompasses only goals of care discussions, and that it can function only as hospice care are significant barriers to its early integration in COPD. 12 Many pulmonary clinicians even interchange the terms palliative care and hospice because of misconceptions regarding the fundamental differences. To be clear, palliative care is appropriate from the time of diagnosis of a serious illness and can continue concurrently with illness-directed therapies ( Fig 1 ). 6 Palliative care should begin long before a person reaches end-stage COPD, which typically is defined as having very severe airflow obstruction on spirometry (FEV 1 < 30%; GOLD stage IV) and receiving maximum medical therapies and is ideally suited before someone has experienced a significant decline in their well-being and functional status. In contrast, hospice care in the United States is appropriate when curative options for a terminal serious illness are no longer deemed appropriate and when a patient is estimated to have only 6 months to live. 10 Hospice care provides support for symptoms such as pain and refractory breathlessness near the EOL, prepares a patient and their families for death, and supports family with respite care and bereavement.

Diagram showing early palliative care and trajectories of decline in serious illness. This figure illustrates the declining trajectories of well-being and function (y-axis) in serious illness over time (x-axis). Compare COPD and heart failure (red) with advanced cancer and idiopathic pulmonary fibrosis (blue), and visualize how hospitalizations (+) detrimentally impact well-being and function. Early palliative care (gray) starts proactively in the disease trajectory and increases in intensity over time as a patient experiences worsening symptoms, needs, and hospitalizations approaching the end of life (EOL). This approach seamlessly transitions from life-prolonging COPD therapies to a focus on comfort through hospice, EOL care, respite care, and bereavement.
As illustrated in Figure 1 and prior models of Murray et al 13 and Maddocks et al, 14 COPD generally has a variable and slow progression, often over years and punctuated largely by unpredictable exacerbations that accelerate declines in well-being and functional status. This illness trajectory in COPD can be heterogenous, and some patients may not experience a variability or decline toward end-stage COPD. This is in contrast to the relative clinical stability and precipitous decline near the EOL in patients with other serious illnesses such as advanced cancer and idiopathic pulmonary fibrosis. Notably, recent therapeutics and antifibrotics have made the trajectories of these serious illnesses also heterogenous.
Addressing the challenges of integrating palliative care too late in a serious illness, a movement has existed for some time to bring proactive palliative care in the trajectory of serious illness, that is, early palliative care ( Fig 1 ). Patients with moderate to very severe COPD and their care partners support early palliative care that begins in less severe COPD stages (FEV 1 < 80%; GOLD stage II) and increases in intensity as symptoms, care needs, and hospitalizations worsen approaching the EOL ( Fig 1 ). 4 During this time, a person living with COPD who is receiving early palliative care can and should continue to receive all COPD-directed therapies and can be referred for advanced COPD therapies such as endobronchial valves or concurrent lung transplantation evaluation, if appropriate. When the disease reaches its terminal stage, the focus then shifts to comfort, EOL care, bereavement, survivorship, and respite care, which early palliative care has been preparing for all along. Unfortunately, palliative care historically is consulted only late in the COPD trajectory near the very EOL ( Fig 1 ), which does a great disservice for patients and their families.
Specialist and Primary Palliative Care
Palliative care can be delivered by specialists and by clinicians trained in primary palliative care who are not formally board certified in hospice and palliative care. Specialist palliative care is provided by interprofessional teams of physicians, nurse practitioners, nurses, chaplains, social workers, and others as part of inpatient palliative care consultation services and outpatient palliative care clinics. Despite the overall growth in the number of hospitals that have access to specialist palliative care services in the past few decades, ambulatory palliative care programs are rare, and the number of subspecialty palliative care clinicians and fellowship training programs is insufficient to meet the growing demands of the aging population. 15 Furthermore, this scarcity of palliative care specialists results in significant racial and geographic disparities, with Black and rural Americans having very limited access. 16
Approximately 15 million Americans have received a diagnosis of COPD, and more than half of them will be older than 75 years in the coming decade. 17 As Americans grow older with chronic and debilitating serious illnesses like COPD, an estimated 10,000 or more palliative care clinicians will be needed to meet the rising demands. 15 , 18 Congressional bills such as the Palliative Care Hospice and Education Training Act (S.2080) respond to this challenge by expanding the palliative care workforce through training opportunities for clinicians who are not palliative care specialists. 19 This primary palliative care model in COPD could be delivered by primary care and pulmonary clinicians who receive training to integrate the principles of palliative care into their routine practices. 20 Accredited palliative care training programs exist at several universities across the country ( Table 1 ). 21 Some programs also offer online training in values-based and serious illness conversations, for example, the Center to Advance Palliative Care, VitalTalk, and Ariadne Labs. Although serious illness conversations are an important Accreditation Council for Graduate Medical Education milestone for pulmonary-critical care fellows, broader primary palliative care training that is inclusive of all the essential elements of palliative care, such as comprehensive symptom recognition and management and care partner support, is not a universal component of pulmonary-critical care fellowship training nor is it widely available for practicing clinicians. 22 , 23
Table 1
Primary Palliative Care Training Programs
| Program Name (Location) | Brief Description |
|---|---|
| University of Washington Graduate Certificate in Palliative Care (Seattle, WA) | Interprofessional curriculum designed for practicing clinicians from nursing, medicine, social work, spiritual care, and other disciplines seeking training in palliative care. The program focuses on skills for delivering integrated, person-centered palliative care using a team-based approach, emphasizing individual and team communication skills. |
| University of Pennsylvania Mid-Career Fellowship (Philadelphia, PA) | ACGME-certified pilot midcareer fellowship program allowing practicing University of Pennsylvania physicians to complete an accredited palliative care fellowship in a flexible format. The program is designed individually to build on each fellow’s existing skills and structured in conjunction with the clinical responsibilities. |
| University of Colorado Interprofessional Palliative Care Graduate Certificate and Master of Science in Palliative Care (Denver, CO) | Program designed to prepare clinicians as palliative care community specialists using a hybrid online and live learning environment. |
| University of Maryland Master of Science and Graduate Certificate in Palliative Care (Baltimore, MD) | Interprofessional masters for practicing clinicians who want further training in palliative care. The graduate certificate can be tailored to five different domains of palliative care. |
| Medical University of South Carolina Palliative Care Doctorate in Nursing Practice (Charleston, SC) | Program follows a master of science in nursing and provides a plan of study for nurses to gain advanced training in palliative care principles and clinical experiences. |
| Harvard Medical School Center for Palliative Care Courses (Boston, MA) | Short courses in “Palliative Care Education and Practice,” “Practical Aspects of Palliative Care,” “Palliative Care for Hospitalists and Intensivists,” and “Art & Science of Palliative Nursing.” |
ACGME = Accreditation Council for Graduate Medical Education.
A Brief Review on the State of the Science of Palliative Care in COPD
It has been more than a decade since Temel et al 24 demonstrated that early specialist palliative care concurrent with oncologic care at the time of diagnosis for patients with metastatic non-small cell lung cancer was associated with improved QOL, better mood, less aggressive EOL care, and increased survival. Recent data have corroborated these benefits, 25 and others have shown that nurse-led and telehealth models in advanced cancer improve outcomes for patients and their care partners. 26 , 27 The benefits of palliative care also extend to patients with nonmalignant diseases such as heart failure, where specialist palliative care is associated with reduced health-care use, more home deaths, and more cost-effective care, among other positive outcomes. 28 , 29 , 30 , 31
Patients with COPD, however, rarely receive palliative care, and if they do, it is often only very late in the disease course compared with patients with cancer and other advanced lung diseases. 32 , 33 , 34 This is perplexing because patients with COPD can have more prevalent anxiety, depression, and refractory breathlessness than those with advanced cancer. 35 In older adults with COPD in particular, patients receive hospice care on average only in the last month of life, and significant geographic disparities exist in its use. 36 This late referral to hospice care in COPD reflects the rare implementation of early palliative care in this population and may limit its ability to improve the quality of EOL care if enacted earlier.
Several educational, clinical, and operational barriers to specialist palliative care in COPD have been discovered. Educational barriers include limited knowledge of palliative care on the part of patients, their care partners, and clinicians. 4 , 12 , 37 In one study, only 30% of patients with COPD and their care partners had heard of palliative care, and pulmonologists also frequently mix the terms palliative care and hospice , as discussed earlier. 4 Clinical barriers include a lack of consensus referral criteria and a misplaced fear from pulmonologists that palliative care clinicians will overprescribe opioids and benzodiazepines that could lead to respiratory suppression in patients with COPD. 12 , 38 Finally, operational barriers include limited time to integrate palliative care or to have lengthy advance care planning discussions in busy pulmonary practices and a shortage in the specialist palliative care workforce, especially in rural areas. 12 Without more specialist palliative care clinicians, this workforce shortage in particular raises significant concerns about the potential bandwidth of palliative care specialists to handle a large influx of patients with COPD and emphasizes the need for more pulmonary clinicians to have access to and engage in primary palliative care training.
These barriers may be discouraging, but success stories do exist in COPD. Although the literature is missing large, multi-site randomized controlled trials of palliative care in COPD, observational data demonstrate that specialist palliative care in COPD among European populations has been associated with fewer hospital deaths, reduced costs, and use of high-intensity care at the EOL. 39 In Canada, specialist palliative care has been associated with a greater number of patients with advanced COPD dying at home, a preference of most of the population. 40 Finally, interprofessional programs that use respiratory therapists and spiritual care professionals, such as INSPIRED COPD and Breathlessness Services, have led to increased home deaths, reduced length-of-stay during terminal hospitalizations, and increased mastery of breathlessness. 9 , 41
Practical Recommendations for Implementing Palliative Care Into Routine COPD Practice
National consensus project guidelines for quality palliative care.
In this section, we recommend ways to incorporate principles of palliative care seamlessly into practice for patients living with COPD and their care partners. We highlight key domains of palliative care in COPD informed by the NCP Clinical Practice Guidelines for Quality Palliative Care (fourth edition), 10 which set “expectations for excellence among clinicians treating patients with serious illness” across eight domains ( Fig 2 ). Central to this framework is maximizing QOL and function while living with COPD.

Diagram showing the eight National Consensus Project Domains for Quality Palliative Care applied to COPD. We provide recommendations within each domain on how to integrate key aspects into routine COPD practice using interprofessional collaboration, a balance between primary and specialist palliative care, and a focus on maximizing quality of life and function. ADL = activities of daily living; BODE = Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Tolerance; IADL = instrumental activities of daily living; IMV = invasive mechanical ventilation; NIV = noninvasive ventilation.
Domain 1: Structure and Process of Care
Domain 1 focuses on interprofessional collaboration and care coordination across the continuum to reach patients with COPD in hospitals, clinics, community settings, and homes. 42 Integrating interprofessional palliative-focused teams into routine COPD care across the continuum is vital for patients who are hospitalized frequently and face complex transitions to home after discharge. Establishing an interprofessional model may be complex and challenging, especially in smaller community hospitals with limited resources and few palliative care specialists. However, interprofessional clinics in Australia and England that use pulmonary, palliative care, and psychology clinicians to care for people with advanced COPD have reduced health-care use and have increased home deaths. 43 , 44 We support this team-based approach to providing quality palliative care for patients with COPD by frontline clinicians and also recognize the need for early referral to social services, nutrition, and pulmonary rehabilitation to help address patient and family needs. 45 In the meantime, health systems should invest resources to bolster primary palliative care training for frontline clinicians and other team members such as lay navigators, care-transitions nurses, and case managers who can help patients with COPD to navigate complex health systems, to identify severe physical and psychological symptoms, and to ensure values and wishes about EOL are addressed proactively.
Domain 2: Physical Aspects of Care
Domain 2 focuses on easing the suffering experienced by patients with COPD as a result of physical symptoms. An ideal place for integration of palliative care in COPD is in the management of refractory breathlessness, the most frequently reported symptom in advanced COPD. 46 A palliative care approach to breathlessness management in COPD is multidimensional and interprofessional, inclusive of many of the following treatment strategies: (1) maximizing COPD-directed therapies and addressing treatable traits (eg, bronchoconstriction and hyperinflation), 47 (2) incorporating nonpharmacologic management (eg, cardiopulmonary rehabilitation, tobacco cessation counseling, relaxation techniques, fans, and energy conservation), 48 (3) prescribing low-dose opioids, and (4) developing a breathlessness action plan for acute exacerbations. 49
For patients with refractory breathlessness despite optimal treatment of underlying COPD and comorbidities, nonpharmacologic options such as fans are safe, inexpensive, and easy to use. 50 Noninvasive ventilation also helps patients with COPD who are experiencing acute hypercapnic respiratory failure and may be appropriate in select patients to prevent intubation, to facilitate recovery, to relieve refractory breathlessness, and in certain populations for chronic use. 51 Finally, international guidelines recommend the use of low-dose opioids for refractory breathlessness, 6 and several studies support their use. 52 However, pulmonary clinicians can be reluctant to prescribe opioids in COPD owing to concerns about hypoventilation, even though low-dose opioids have not been associated with increased mortality in COPD and a recent randomized controlled trial found no evidence for respiratory depression when clinicians used lower doses of sustained-release morphine. 53 It is important to note that opioids always should be accompanied by laxatives to prevent constipation.
Other important physical aspects of care that are common in patients with COPD, are associated with poor QOL, and could benefit from a palliative care approach include pain, disease-related malnutrition and unintentional weight loss, and fatigue. Pain is multidimensional, interconnected with breathlessness and depressive symptoms, and should be addressed interprofessionally. 54 , 55 Likewise, fatigue is commonly reported in COPD and impacts health-care use. Fatigue may be managed by integrating pulmonary rehabilitation and investigating comorbidities such as sleep apnea. 56 Finally, unintentional weight loss and malnutrition in COPD should raise red flags in the clinic about potential deterioration. 57 Malnutrition is amenable to treatment, and improvements in nutritional status have been associated with improved functional capacity, respiratory muscle strength, and QOL. 58 Nutritional requirements for patients with COPD should be assessed individually, 59 and nutritionists, who often are members of the interprofessional palliative care team, could be consulted from a primary palliative care standpoint.
Domain 3: Psychological and Psychiatric Aspects of Care
Domain 3 focuses on systematically assessing and addressing psychological symptoms such as anxiety and depression, which are reported in up to one-third to one-half of patients living with COPD and often go untreated. 60 Anxiety and depression result in considerable QOL impairment and are associated with increased risk of exacerbations and death. 61 , 62 These symptoms may be related to refractory breathlessness and would benefit from comprehensive management; however, other factors influence anxiety and depression such as hospitalizations and smoking. 63 Pulmonary rehabilitation is one of the most successful interventions for improving psychological symptoms. 64 However, data on pharmacologic options in COPD, such as antidepressants, are limited. 65 A team-based approach with palliative care clinicians and a patient’s primary care clinician could help to facilitate safe dosing and side-effect monitoring in patients who may need antidepressants and anxiolytics. Nonpharmacologic interventions such as cognitive behavioral therapy, acupuncture, breathing strategies, music therapy, and mindfulness lack robust evidence in COPD, but are a natural component of the interprofessional approach to palliative care and could be safe to integrate proactively for patients with COPD. 63 , 66
Domains 4, 5, and 6: Social, Spiritual, and Cultural Aspects of Care
A palliative care approach to COPD recognizes that patients and their care partners are a team and that patients living with serious illness are influenced greatly by their family and social situations. Training in how to integrate care partners fully into the care team and to assess and manage aspects of care partner burden are limited in pulmonary programs, whereas these are essential elements of palliative care. Domains 4 through 6 focus on support systems, social determinants of health, spiritual care needs, and how cultural background influences care. 67 Such an approach recognizes that the burden of COPD extends beyond the patient to their loved ones. A palliative care interprofessional team with case managers or social workers can explore the needs of care partners and can bolster home support systems. Likewise, spiritual beliefs of patients and their care partners may differ and may be dynamic throughout the disease course. Early referral to spiritual care professionals can reconcile these differences and can provide much-needed QOL benefit before the EOL. 68 , 69 Providing quality palliative care in COPD requires sensitivity and understanding to a patient’s and their family’s culture and considers how culture relates to decision-making, illness understanding, and coping with symptoms, grief, and dying. Members of the COPD palliative care team also must be aware of their own biases and must seek opportunities to learn about the provision of culturally sensitive care. 69
Domains 7 and 8: Care of the Patient Nearing the EOL and Ethical and Legal Aspects of Care
Domains 7 and 8 focus on helping patients with COPD and their families approach the EOL. An essential element of palliative care is advance care planning, which includes engaging patients and their care partners in early values-based discussions, completing advance directives or similar documents, and addressing ethical concerns, such as designating a health-care power of attorney, that may arise near the EOL. 11 Advance care planning is complex and can help to achieve concordance between a patient’s preferences for the EOL and the care they receive and is associated with a reduction in anxiety and depression experienced by their care partners. 70 Specific considerations regarding COPD and advance care planning include discussions about lung transplantation and the period after transplantation, as well as noninvasive ventilation and invasive mechanical ventilation, with parameters for their success and failure. Discussing time-limited trials of noninvasive ventilation and invasive mechanical ventilation may be an option for select patients with COPD who require treatment in the ICU. 71
Many pulmonary clinicians are adept in having conversations with patients and their families through ICU experiences. However, these conversations occur during a time of crisis and often at or near the very the EOL. Barriers to upstream advance care planning in the clinic in COPD include insufficient time in ambulatory settings, prognostic uncertainty, and a lack of coordination with primary care clinicians. 72 , 73 , 74 Deciding when to begin these critical conversations with patients with COPD is further challenging because patients may live an extended time with little decline in lung function or QOL, whereas others can progress rapidly and can experience frequent exacerbations with poor QOL. 75 These factors present multiple barriers to build prognostic awareness and to plan for the future in this population. Nonetheless, advance care planning in COPD can improve communication around the EOL and is currently reimbursed through the Centers for Medicare and Medicaid Services. 76 , 77
Components of a Comprehensive Palliative Care COPD Assessment
Integrating primary palliative care principles into routine COPD practice begins with a comprehensive COPD-focused palliative care assessment that incorporates the NCP domains to identify symptoms and care needs that impact QOL and functional status and are not addressed adequately ( Table 2 , Fig 2 ). Doing so can enhance COPD-focused assessments and can facilitate more comprehensive palliative care for this population. Guideline-directed COPD symptom assessments include the modified Medical Research Council dyspnea scale and the COPD Assessment Test, which can be implemented easily in routine practice and have clinically significant thresholds that help to identify those who could benefit from early palliative care. For example, using these instruments along with an exacerbation history can help clinicians to categorize patients into GOLD letter grades from A through D. In one study, patients with GOLD grades B and D (ie, more severe symptoms and frequent exacerbations) compared with those with GOLD grade A showed a threefold higher frequency of anxiety and depression symptoms and were more likely not to receive medications to treat those symptoms. 60 Another example of a comprehensive palliative care assessment includes the Support Needs Approach for Patients Tool, 78 a 15-item questionnaire that identifies support needs of patients across broad domains and guides further interventions. Regardless of the instruments chosen, we recommend regularly reassessing symptoms and needs at critical points along the COPD trajectory and using these data to identify when patients may benefit from palliative care ( Fig 1 ).
Table 2
How a Comprehensive COPD Palliative Care Assessment Can Enhance Traditional COPD-Focused Assessments
| COPD-Focused Assessment | COPD Palliative Care Assessment |
|---|---|
| Comprehensive COPD assessment: | Comprehensive COPD-palliative care assessment (patient and family): |
| Focused comorbidities: coronary artery disease, heart failure, pulmonary hypertension, OSA, gastroesophageal reflux disease | Broader evaluation of multimorbidity, frailty, and psychiatric conditions |
| COPD-directed therapies: | |
| Prognosis-based discussions |
ADL = activities of daily living; CAT = COPD Assessment Test; HADS = Hospital Anxiety and Depression Scale; IADL = instrumental activities of daily living; NIV = noninvasive ventilation; PHQ-9 = Patient Health Questionnaire 9; SNAP = Support Needs Approach for Patients.
When to Manage and When to Refer?
A frequent struggle for clinicians who care for those living with COPD is the decision of when to initiate primary palliative care and when to seek expert guidance and refer to specialist palliative care. As shown in Table 3 , previously proposed hospice referral criteria for COPD often are present only in very advanced COPD stages. 79 At that point, initiating early palliative care may not have as much impact as if it had been initiated more proactively. An evolving concept that we have been developing and is illustrated in Figure 3 conceptualizes potential triggers for early palliative care in COPD that include the following “levers”: (1) worsening lung function, (2) severe symptoms or high burden of care needs, (3) poor prognosis, and (4) frequent severe exacerbations. 80 This “levers model” triggers palliative care (primary, specialist, or both) when one lever or more cross a clinically significant threshold. The overall point here is that integrating palliative care into COPD practice is not an on-off switch; rather, it should be based on multiple factors and can evolve over time. Furthermore, the levers model accounts for the fact that the dose of palliative care may be different and should be individualized. This allows a person with clinically stable and very severe COPD and someone with less severe COPD yet high symptom burden both to receive individualized early palliative care with varying doses of advance care planning or symptom management. Although a defined threshold for lung function referral has not been established, we previously demonstrated that patients with COPD accept palliative care as early as moderate COPD (FEV 1 < 80%), 4 so patients may be ready sooner than clinicians think. The threshold for severe symptoms is a broader category and could be defined using patient-reported outcomes as we described previously (eg, COPD Assessment Test score of > 20). The level lever for poor prognosis could pass the threshold when a patient falls into in the third or fourth quartiles, as an example, of the Body Mass Index, Obstruction, Dyspnea, and Exercise Tolerance Index. 81 Certainly, if prognosis is such a concern that a clinician is considering referral for lung transplant evaluation, then concurrent referral to specialist palliative care should be routine practice. Finally, frequent severe exacerbations, that is, those that require hospitalization or an ED visit, carry a high risk of mortality after hospitalization and are ideal inflection points in the illness trajectory of COPD. 82
Table 3
Potential Hospice Care Referral Criteria in COPD
| Indication Type | Criteria |
|---|---|
| Cardiopulmonary | < 30%) or significant disease progression ≤ 55 mm Hg on room air or oxygen saturation ≤ 88% on room air > 50 mm Hg) |
| Nutritional | |
| Diminished functional status |
ADL = activities of daily living; GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease; IADL = instrumental activities of daily living.

Diagram showing the levers model for palliative care integration in COPD. This figure illustrates four potential triggers, or “levers,” for palliative care (primary, specialist, or both) in COPD. Each lever can be tuned up or down, and any one or more can pass a critical threshold to integrate palliative care. In this example, the patient meets referral criteria by high symptoms and severe exacerbations. Sample thresholds for each category could be as follows: (1) lung function: moderate stage COPD (GOLD grade II; FEV 1 , 50%-80%); (2) symptoms and care needs: refractory breathlessness, unintentional weight loss, declining functional status, high burden of social determinants of health, or caregiver needs; (3) poor prognosis: high BODE Index; and (4) ≥ 1 severe exacerbation. BODE = Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Tolerance; CAT = COPD Assessment Test; GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease; mMRC = modified Medical Research Council dyspnea scale. (Adapted by permission from Springer Nature, Palliative care in Lung Disease, Lindell KO and Danoff SY, Copyright 2021. 80 )
Table 4 illustrates four hypothetical cases informed by patients with COPD. The people in these cases warrant palliative care in different ways and with varying degrees of primary vs specialist integration. We challenge readers to reflect on how they would integrate palliative care in each case and if they would have considered each patient appropriate.
Table 4
Case-Based Implementation of Primary and Specialist Palliative Care in COPD
| Case | Palliative Care | |
|---|---|---|
| Primary | Specialist | |
| Patient 1 (a classic case): 74-y-old White man with COPD and very severe airflow obstruction (FEV 40%, GOLD grade III) who has been living with COPD for 20 y. He is a heavy smoker and quit 10 y ago. He is severely limited because of refractory breathlessness (mMRC dyspnea scale, 4). Changing the dog food bowl has now become cumbersome. He has been admitted to the ICU twice in the past year and once required invasive mechanical ventilation. He is adherent to triple inhaler therapy, a nebulizer, and supplemental oxygen. He completed pulmonary rehabilitation 1 y ago. Comorbidities are notable for hypertension. He is married and lives with his wife at home. He depends on her for transportation to his appointments and for medication support. On examination, he is a thin man receiving continuous supplemental oxygen. He pauses often while speaking because of breathlessness. He demonstrates diffuse centrilobular emphysema on CT scan. | ||
| Patient 2 (a missed opportunity): 67-y-old Black woman with COPD and very severe airflow obstruction (FEV 25%, GOLD grade IV). She requires continuous oxygen and has upper lobe predominant emphysema on CT scan with air trapping. She has breathlessness (mMRC dyspnea scale, 3). She is widowed and lives alone. She has not been hospitalized for an exacerbation. | ||
| Patient 3 (an early start): 52-y-old White woman with COPD and moderate airflow obstruction (FEV 60%, GOLD grade II). Her biggest issue has been frequent severe exacerbations: three in the past year, with one requiring IMV. She continues to smoke and has refractory breathlessness (mMRC dyspnea scale, 4) and a productive cough with sputum (CAT score, 25), placing her in GOLD category D. She does not adhere to her prescribed inhaler regimen. Her husband is ill with multiple comorbidities, and she is his primary caregiver. | ||
| Patient 4 (older adult): 82-y-old Black man with COPD and moderate airflow obstruction (FEV 70%, GOLD grade II). His primary symptom is a productive cough. Dyspnea is mild (mMRC dyspnea scale, 1). He uses supplemental oxygen and a cane to ambulate around his home. He is married, and his wife is healthy. He brings a grocery bag with 15 medications to the clinical visit. | ||
ADL = activities of daily living; CAT = COPD Assessment Test; EOL = end of life; GOLD = Global Initiative for Chronic Obstructive Lung Disease; IADLs = instrumental activities of daily living; IMV = invasive mechanical ventilation; mMRC = modified Medical Research Council; NIV = noninvasive ventilation.
Conclusions
In conclusion, palliative care should be integrated long before end-stage COPD to provide comprehensive supportive care for patients and their care partners and to prepare them better for the EOL. Palliative care should be integrated early and concurrently with COPD-directed therapies, and its intensity should increase over time as symptoms, needs, and exacerbations worsen approaching the EOL. When integrated proactively, primary and specialist palliative care can help patients with COPD and their care partners to manage difficult physical, emotional, social, and spiritual care needs and can help them to transition seamlessly to a focus on comfort near the EOL. NCP domains can guide a comprehensive palliative care approach triggered by multiple factors and initiated at various inflection points along the long and winding COPD trajectory.
Acknowledgments
Author contributions: A. S. I., D. R. S., K. O. L., and L. F. R. contributed to the design and content of this manuscript.
Financial/nonfinancial disclosures: A. S. I. reports funding from the National Institute on Aging of the National Institutes of Health. None declared (D. R. S., K. O. L., L. F. R.).
FUNDING/SUPPORT: A. S. I. reports funding from the National Institute on Aging [Grant K76 AG064327] of the National Institutes of Health.
DISCLAIMER: The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
- Research article
- Open access
- Published: 24 August 2021
Benefits, for patients with late stage chronic obstructive pulmonary disease, of being cared for in specialized palliative care compared to hospital. A nationwide register study
- Ingela Henoch ORCID: orcid.org/0000-0002-1987-5419 1 , 2 ,
- Ann Ekberg-Jansson 3 ,
- Claes-Göran Löfdahl 4 , 5 &
- Peter Strang 6 , 7
BMC Palliative Care volume 20 , Article number: 130 ( 2021 ) Cite this article
5898 Accesses
8 Citations
19 Altmetric
Metrics details
In early stage chronic obstructive pulmonary disease (COPD), dyspnea has been reported as the main symptom; but at the end of life, patients dying from COPD have a heavy symptom burden. Still, specialist palliative care is seldom offered to patients with COPD; they more often receive end of life care in hospitals. Furthermore, symptoms, symptom relief and care activities in the last week of life for COPD patients are rarely studied. The aim of this study was to compare patient and care characteristics in late stage COPD patients treated in specialized palliative care (SPC) versus hospital.
Two nationwide registers were merged, the Swedish National Airway Register (SNAR) and the Swedish Register of Palliative Care (SRPC). Patients with COPD and < 50% of predicted forced expiratory volume in 1 s (FEV 1 ), who had died in inpatient or outpatient SPC ( n = 159) or in hospital ( n = 439), were identified. Clinical COPD characteristics were extracted from the SNAR, and end of life (EOL) care characteristics from the SRPC. Descriptive statistics were used to describe the sample and the registered care and treatments. Independent samples t -test, Mantel–Haenszel chi-square test and Fisher’s exact test was used to compare variables. To examine predictors of place of death, bivariate and multivariate logistic regression analyses were performed with a dependent variable with demographic and clinical variables used as independent variables.
The patients in hospitals were older and more likely to have heart failure or hypertension. Pain was more frequently reported and relieved in SPC than in hospitals ( p = 0.001). Rattle, anxiety, delirium and nausea were reported at similar frequencies between the settings; but rattle, anxiety, delirium, and dyspnea were more frequently relieved in SPC (all p < 0.001). Compared to hospital, SPC was more often the preferred place of care ( p < 0.001). In SPC, EOL discussions with patients and families were more frequently held than in hospital ( p < 0.001). Heart failure increased the probability of dying in hospital while lung cancer increased the probability of dying in SPC.
This study provides evidence for referring more COPD patients to SPC, which is more focused on symptom management and psychosocial and existential support.
Peer Review reports
Chronic obstructive pulmonary disease (COPD) is predicted to become the third leading cause of death globally by the year 2030 [ 1 ]. In its early stages, COPD is a lung disease with airway symptoms, such as shortness of breath as a main problem, especially with physical activity [ 2 , 3 ]. Later in the disease trajectory, comorbidities are common, e.g. weight loss, sometimes associated with cachexia, and heart failure, resulting in increased dyspnea [ 4 ]. The risk of thromboses as well as pulmonary embolism increases, resulting in further symptoms [ 5 , 6 ]. Also, depression is commonplace and is associated with poorer survival prospects [ 7 ].
At the very end of life, patients dying from COPD have similar and comparable symptoms to those dying from lung cancer [ 8 ], and are therefore in need of qualified care. For this reason, several studies comparing lung cancer and COPD have been performed [ 9 , 10 , 11 , 12 ]. These studies found that patients with lung cancer were more likely to receive home palliative care [ 9 , 10 ] and die at home [ 11 , 12 , 13 ]. Specialized palliative care (SPC) was offered only in the last few weeks of life to COPD-only patients [ 12 , 14 ], while COPD patients with comorbid lung cancer were far more likely to receive palliative care earlier in the disease trajectory [ 9 ]. Patients who did not receive palliative care during the last 3 months of life were more likely to die in an acute care setting [ 10 ]. Higginson et al. [ 15 ] who followed patients with COPD and interstitial pulmonary disease from 2001 to 2014, found a high prevalence of hospital deaths in both diseases, but presence of comorbidities increased the probability to die in hospital. Research in patients with advanced COPD disease has shown beneficial effects of home palliative care services, compared to usual care, on reducing symptom burden for patients [ 13 ].
Specialized palliative care focuses on symptom control, as well as on psychosocial and existential support, which includes end of life (EOL) discussions about future planning, goals of care, optimal (but not maximal) care and aims to support family well-being. In other words, the focus is on the individual patient’s wellbeing. In Sweden, most palliative care patients are enrolled in advanced palliative home care that operates on a 24/7 basis and is provided by multi-professional teams, typically including physicians, nurses, and allied health professionals. In Sweden, palliative incare services, with similar staffing, constitute an alternative for dying patients who, for certain reasons, do not want to receive care in their own homes, but prefer incare services.
Dying COPD patients have severe symptoms that need to be relieved, and SPC is a viable option. However, in contrast to lung cancer patients, COPD patients are not as likely to die in SPC: [ 10 , 16 ] a considerable percentage receive their EOL care in acute hospitals, instead.
Although much is known about palliative care for COPD and lung cancer [ 8 , 10 , 16 ], the last week of life is rarely characterized in respect of symptoms, symptom relief, and care activities.
The Swedish Register of Palliative Care (SRPC) is a validated, nationwide quality register for EOL care with focus on symptoms and symptom relief during the last week of life [ 17 , 18 ]. It is retrospectively completed and provides important data that can be compared across settings.
The aim of this study was to compare patients with late stage COPD who were being treated in SPC versus COPD patients receiving treatment in hospital. The following research questions were asked:
What are the demographic and clinical characteristics of patients with COPD and < 50% of predicted FEV 1 receiving SPC, compared to hospital care?
What characterizes the care, including symptom relief, EOL discussions, anyone present at death, and bereavement support provided to families, provided in SPC versus that provided in hospitals to patients with COPD and < 50% of predicted FEV 1 ?
This is a register study where two nationwide registers were merged, the Swedish National Airway Register (SNAR) [ 19 ] and the Swedish Register of Palliative Care (SRPC). The SNAR contains data on patients diagnosed with either COPD or asthma. Health care professionals (HCPs) in outpatient units made registrations of each patient visit. Most of the registrations were made in primary health care and only 14% of registrations were made in specialized pulmonary clinics. Registrations from the SNAR included demographic, clinical, and patient-reported data. In the present study, the last registrations for COPD patients were identified.
The SRPC, a nationwide quality register of EOL care, encourages all county councils and municipalities in Sweden to retrospectively complete a questionnaire about EOL care with focus on the last week of life. Health care professionals, registered nurses in the absolute majority of cases, at the unit where the patients had died report demographic and clinical characteristics of the patients, as well as place of death, some characteristics of the EOL care, and symptoms in the last week of life. The SRPC has been validated and has previously been described in detail [ 17 , 18 ]. It has a coverage of about 60% of all deaths in the country; and some of the questions from the SRPC have been adopted by the National Board of Health and Welfare as national quality indicators for a good death in Sweden [ 20 ].
Data from the two registers were merged based on patients’ personal security number. The patients included in the present study had died between 2009 and 2016.
Of the registered patients in the SNAR, 3,114 who had died between 2009 and 2016 were identified by the Swedish Tax authorities, which registers all deaths of Swedish citizens. Of these, patients with COPD and < 50% of predicted FEV 1 [ 21 ], corresponding to GOLD C and D, were identified, altogether 1,382. From this population, those who had died either in SPC or in hospital were extracted from the SRPC.
Data collection
The data collection is similar to another study made by our group where we compared patients with COPD dying in nursing home with patients dying in hospitals [ 14 ]. The demographic variables retrieved from the SNAR were age, sex, and living situation, i.e., living alone or cohabiting. As in our previous study, clinical characteristics included values for FEV 1 (forced expiratory volume during 1 s), presented as per cent of predicted, number of exacerbations and hospitalization in the last 12 months, comorbidities, and exercise capacity, measured by the number of days per week that the patient had been physically active. Patient-reported variables included smoking habits, divided into non-smokers, ex-smokers, and still smokers. Dyspnea was measured using the modified Medical Research Council (mMRC) dyspnea scale [ 22 ], ranging from 0 to 4, where 4 indicate more severe dyspnea. Health-related quality of life (HRQoL) was measured by the Clinical COPD Questionnaire (CCQ) [ 23 ], a patient-rated questionnaire with ten items where each items are scored on a 7-point scale, from zero (0) to 6, where higher score indicate more severe impact on HRQoL. In later registrations, health status was measured by the patient-rated COPD Assessment Test (CAT) [ 24 ]. The CAT consists of eight items ranging from zero (0) to 5, where 5 indicate more severe problems. The scores are summated to obtain a single total score ranging from 0 to 40.
Variables from the SRPC concerned whether death was expected; whether the patient would have preferred the place of death; presence of anyone at time death; and whether any EOL discussion about the impending death with either the patient or the family was performed; whether the family was invited to a post-death discussion; and length of stay in the setting. Concerning length of stay, seven patients with more than 1,000 days in the setting were excluded. Descriptive data and data about items such as clinical routines, symptom prevalence, and symptom management during the last week of life were also retrieved, including presence of pressure ulcers, symptom assessments, symptom prevalence, prescribed medications, and whether the symptom was alleviated. The following breakthrough symptoms during the last week of life were registered: pain, rattle, nausea, anxiety, dyspnea, and delirium (Yes/No format). The assessment of symptom relief was made on a three-grade scale: Complete – Partial – No relief. A summary of the variables is presented in Table 1 .
Data analysis
To describe the sample and the registered care and treatments, descriptive statistics were used with mean values and standard deviations (SDs) for continuous variables, and numbers and percentages of the total sample for categorical variables. Independent samples t -test was used to compare continuous variables and the Mantel–Haenszel chi-square test and Fisher’s exact test were used to explore relationships between dichotomous categorical variables.
To examine predictors of place of death, bivariate logistic regression analyses were performed with a dependent variable, with SPC as place of death scored as 0 and hospital as place of death scored as 1. The following independent variables were used: age, sex, living situation, FEV 1 % predicted, number of exacerbations in the last 12 months, number of hospital admissions due to COPD in the last 12 months, exercise capacity, and smoking; as well as HRQoL measured using the CCQ or CAT; dyspnea measured by the mMRC dyspnea scale; and comorbidities. Independent variables that significantly predicted the dependent variable with p < 0.20 in the bivariate analyses were entered into the multivariate stepwise logistic regression analysis with the same dependent variable. A similar data analysis were made in our previous study, comparing patients with COPD dying in hospitals and in nursing homes [ 14 ].
In total, 159 patients who had died in SPC and 439 patients who had died in hospital were identified. The patients in SPC had died either in inpatient units ( n = 115) or in outpatient units, i.e. patients who died in their homes with support from advanced palliative home care teams ( n = 44). A flowchart of the sample is presented in Fig. 1 .
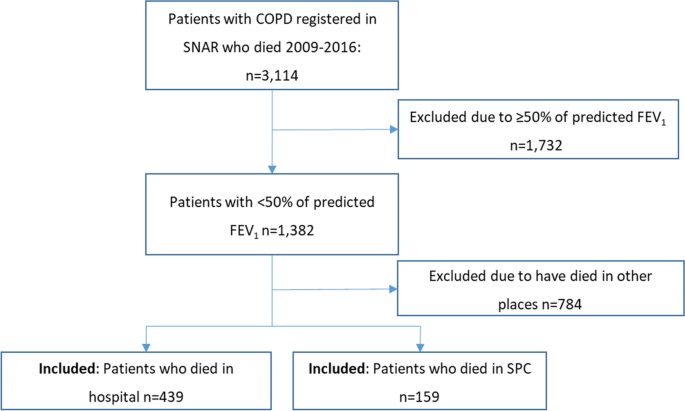
Flowchart of the patients included in the study
The patients who died in SPC were significantly younger than those who died in hospital. Significantly more men than women had died in SPC, while the opposite was true for those who had died in hospitals (Table 2 ). Length of stay in the setting was significantly longer for patients in SPC. The long time of care, 44 days was a mean value and as seen, the median value was 12 days. The much higher mean value (than median value) depended on certain patients who were enrolled in advanced palliative home care for long periods, as seen from the min–max values of 1 to 493 days. Patients who died in SPC inpatient units had shorter number of days in the setting compared to those dying at home with support from advanced palliative home care teams (mean 33 vs 81 days, p = 0.004, data not shown).
Concerning comorbidities, a greater percentage of patients dying in hospital had heart failure and hypertension, while a larger percentage of patients dying in SPC had lung cancer.
Comparisons of clinical characteristics between settings
For findings on symptom prevalence, assessment, and management in SPC versus hospitals, see Table 3 . Assessments of pain, other symptoms, and mouth health were more frequently reported in SPC than in hospitals. Although pain was more frequently reported in SPC, medication for pain was more frequently prescribed in SPC and consequently, pain was also more frequently relieved in SPC compared to hospitals. Rattle, nausea, anxiety, and delirium were reported at similar frequencies in both settings, but medication for rattle, nausea, and anxiety was more often prescribed in SPC than in hospitals. Dyspnea was more frequently reported in hospitals, but dyspnea, as well as delirium, was more frequently relieved in SPC. Pressure ulcers on admission were reported at similar levels in both settings, but pressure ulcers at death were more often reported in SPC. Parenteral infusions during the last 24 h of life were more often used in hospitals (Table 3 ). Patients who received an infusion had a significantly shorter length of stay in the setting, and less commonly had EOL discussions or relatives present at death, and more rarely received rescue medication for rattle and nausea (Table 4 ).
Comparisons of palliative care characteristics between settings
In patients being cared for in SPC, death was more often expected; and the place of care was more often the preferred place of care, compared to those cared for in hospitals. End of life discussions with both patients and their families were more frequently held in SPC than in hospitals, and bereavement support to families was also more common in SPC (Table 5 ).
In both settings, about 23% of patients died without anyone else present. In SPC, relatives only were more often present and in hospitals, HCPs only were more often present (Table 5 ).
Predictors of place of care
In the bivariate logistic regression, higher age, being a woman, living alone, having a lower number of exacerbations, having heart failure, having ischemic heart disease, and having hypertension, but not having lung cancer, predicted dying in hospital. In the multivariable stepwise logistic regression analysis, having heart failure and not having lung cancer predicted place of death, in that heart failure increased the probability of dying in hospital and lung cancer increased the probability of dying in SPC (Table 6 ).
Patient differences
There were some significant differences in symptom prevalence and symptom relief between COPD patients in hospital and those in SPC settings, in that dyspnea was more frequently seen in hospital care and pain was more prevalent in SPC. The differences could be due to comorbidities; heart failure, which can contribute to dyspnea, was more prevalent in hospitals, well in line with a cancer study where the presence of heart failure was related to hospitalisation [ 25 ]. Lung cancer, which regularly causes pain due to metastases, was more prevalent in SPC. That pain was more prevalent in SPC settings could indicate that patients with severe pain problems more often are referred to SPC, where it is assumed that pain problems are better managed [ 26 ].
Breathlessness is a prevalent and bothersome symptom in patients with COPD, which affects functional status, distress [ 27 ], and quality of life [ 28 , 29 ]. In the present study population, the prevalence of breathlessness in the last week of life was higher in hospitals. There are known differences in how patients experience breathlessness depending on which disease they have [ 30 ]. In previous research, patients with cancer described that breathlessness appeared suddenly and was frightening, while for patients with COPD, breathlessness developed gradually and was associated with episodes of distress, anxiety, panic, and fear of dying. Patients with heart failure have described the symptom in terms of limitations to daily functioning [ 30 ]. Palliative care has the potential to address breathlessness in a holistic way [ 30 ], but our study showed that patients who also suffered from diagnosed heart failure were less likely to receive palliative care, compared to patients with the comorbidity of lung cancer. This is in line with a recent review that showed that patients with lung cancer are more likely to receive palliative care compared to patients with COPD, despite a similar symptom burden [ 12 ]. However, the reason for a higher proportion of COPD patients with heart failure dying in hospitals and patients with concomitant lung cancer dying in SPC is partly explained by the nature of these comorbidities. As a rule, an acute heart failure leads to an acute hospital admission, whereas the course of a COPD patient with lung cancer is more foreseeable: a lung cancer diagnosis gives more opportunities to refer the patient to a palliative care service.
Our findings show that pressure ulcers at death were more frequent in SPC. One explanation for this could be the longer length of stay in SPC, with a longer time of being confined to bed and therefore a higher risk for pressure ulcer development. When exploring the presence of pressure ulcers in the last week of life in relation to setting, one study found that specialist inpatient palliative care units had a higher prevalence (19%) compared to hospitals (ca. 14%), when all grades of pressure ulcers were included [ 31 ]. In some cases, pressure ulcers at the end of life are unavoidable and may develop rapidly. These are often named “Kennedy Terminal Ulcers (KTUs).” Patients at the end of life have risk factors for unavoidable pressure ulcers, as they are more immobile, more malnourished, and/or cachectic.
Patient care differences
The differences in care between SPC and hospitals were related to symptom relief, occurrence of EOL discussions, and prescription of parenteral infusions also during the last 24 h of life. Symptoms such as dyspnea, anxiety, delirium, and death rattle were more often relieved in SPC. The relieved symptoms coincide with symptom assessments, which were more frequently performed in SPC. Regular symptom assessment is associated with higher HRQoL in patients with cancer [ 32 ], and is also recommended in COPD care [ 33 , 34 ].
Rescue medication was more frequently prescribed in SPC. Rescue medication has previously been found to be helpful in patients with COPD suffering from disturbing symptoms [ 35 ]. Morphine is the primary rescue medication for breathlessness in cancer patients in palliative care, but there is also evidence that morphine is helpful for COPD patients with breathlessness [ 36 , 37 ].
In our population, only about one-third of patients in hospital had EOL discussions with an HCP, compared to 87% in SPC. The consequences of a lack of EOL discussions could be continued administration of unnecessary medical treatment, such as intravenous nutrition and hydration also during the last 24 h of life, which at this stage could contribute to nausea, dyspnea, and rattle. Moreover, lack of EOL discussions in the present study was also related to less prescription of rescue medication. This suggests that patients who have had EOL discussions may also receive higher quality care, possibly, as a result of higher awareness of the impending death. Previous studies report that patients with COPD were more satisfied with care after having had EOL discussions [ 38 , 39 , 40 ]. In the present study, higher rates of EOL discussions also coincide with higher ratings of the setting as the preferred place of death, which could be difficult for HCPs to know without bringing up the topic. Moreover, patients with lung disease and their relatives, as well as clinicians, have been reported to have a positive attitude to introducing advance care planning in a thoracic inpatients ward, especially when the focus of the discussions concerns symptom control [ 41 ].
Furthermore, in our study, patients who had parenteral infusion of fluids the last week of life had also lower rates of EOL discussions, which indicates that patients and relatives may not have been informed about the risks of nutrition and fluid in the acutely dying patient. In the present study, parenteral nutrition support was more common in hospitals than in SPC. To provide COPD patients with nutritional support is important in the early stages of the disease, but, at the end of life, total parenteral nutrition could cause nausea, due to an autonomic dysfunction in the dying, resulting in a gastric distension, but also in dyspnea and rattle, due to hyperhydration [ 42 ]. Moreover, in cases when HCPs do not initiate EOL discussions and nutritional support continues to be provided, this could signal to patients and their relatives that the patient is not immediately dying.
In the present study, there were similar levels, about 23%, of patients dying alone in both settings. A study comparing deaths of patients with cancer and patients with heart failure found that 20% of patients with heart failure, compared to 12% of patients with cancer, were alone at the moment of death [ 43 ]. Furthermore, another study that explored several aspects of palliative care in patients dying in nursing homes, found that about 16% died without anyone present [ 44 ]. This could indicate that patients with COPD are more often alone at the very moment of death, even in cases where death is expected within days. Dying alone is sometimes regarded as a failure of the HCP, but can happen when death occurs suddenly, unexpectedly, or during sleep. This is a topic that needs to be communicated with relatives and in health care teams, in order to reduce feelings of guilt for not providing optimal care.
Patients might be inclined to seek care in hospitals because of the high medical competence related to hospital care. In contrast to cancer, COPD is often regarded, by both the patient and HCPs, as a “chronic disease,” and is less often viewed as a palliative diagnosis, despite high mortality. This could be due to the unpredictability of the COPD disease trajectory, especially in combination with heart failure. When presenting with an exacerbation, neither the patient nor the HCPs know whether this exacerbation is the last one leading to death. Although it was significantly more common that death was expected in SPC, still 82.8% of deaths in hospitals were expected. This could indicate that there is reason to offer SPC earlier in the disease course, and more frequently.
Implications
In line with our results showing that breathlessness was relieved to a larger extent in SPC, early integration of palliative care with respiratory primary care and rehabilitation services has been associated with better management of dyspnea in patients with COPD [ 45 ]. Our study indicate that patients with COPD need support to manage severe symptoms including anxiety [ 46 ] and need both medical treatment and psychological support [ 26 ], which is provided in palliative care. Admissions to SPC should be considered more often, as recent Swedish data show that COPD patients admitted to SPC have a reduced need of emergency room visits and have more seldom hospital as their place of death [ 47 ]. A pre-emptive approach, instead of reacting when a high-intensity symptom already is present, is a main issue in palliative care, which is also applicable to hospital care of COPD patients. To be able to detect symptoms early, regular symptom assessment is an important prerequisite for the improvement of symptom management in all settings.
Strengths and limitations
Strengths of this study are that breakthrough of symptoms and the degree of relief were registered systematically with a validated questionnaire, where several of the questions are among those adopted by the National Board of Health and Welfare as national quality indicators for good care of the dying [ 20 ]. Using SRPC data, the prevalence of symptoms as well as symptom relief can be compared in different settings. We have no possibility to evaluate any differences between registered patients in the SNAR and not registered. However, with the great number of patients from almost all parts of the country, we feel confident that we get representative number of patients included in this study. In SRPC, 60% of the patients who die in hospitals and 90% of the patients in SPC were registered. If a clinic is committed to register, then most of the patients in that clinic will be registered, which increases the credibility of the study.
A weakness of our study is the observational design, without any random assignment to the care settings, e.g. COPD patients with acute heart failure are often admitted to hospitals. Some of them will recover whereas others will die. Future studies should address this type of different outcomes. Other limitations are that the data were collected by HCP retrospectively and that the specialty of the hospital wards was not registered. The comparison of two different registers could not fully exclude the risk that the symptom reporting habits differ between palliative care and hospital care. Although many initial factors to compare the two groups are similar, others vary considerably. Most importantly, the comorbidity spectrum is different between the groups, as, e.g., the hospital group more commonly had heart failure. The pace of disease progression can also be a factor that differs between the groups, influencing the selection of patients for the two care settings as emergency hospitals are equipped for emergency care, but not for planned palliative care. The mean days between the last visits registered in SNAR were 682 vs 612 (NS), indicating that the patients in the meantime probably have had health care contacts that were not registered in the SNAR.
The results from this study, examining the characteristics of end of life care for COPD patients in hospital versus specialized palliative care, indicate that: (1) symptoms are prevalent in both settings, but symptom relief is offered more often in specialized palliative care than in a hospital setting; and (2) end of life communication is more common in specialized palliative care. Based on the careful registration of items importantly related to the EOL treatment of patients with COPD, and in spite of the abovementioned limitations of this observational study, our findings indicate that referring COPD patients to specialized palliative care needs to be considered. An important option for that care is an outpatient setting, which can also be viewed as a transitional phase from hospital care.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request. As regards the primary databases used in this study, SNAR is found in the SNAR’s website [ 48 ] and SRPC is found in SRPC’s website [ 49 ]. SRPC is partly open, aggretated data are available from their website [ 49 ]. However, for individual data, an administrative permission is needed for both registers, which was received for SNAR 2015–08-01 and for SRPC 2017–03-28.
Abbreviations
COPD Assessment Test
Clinical COPD Questionnaire
- Chronic obstructive pulmonary disease
End of life
Per cent of predicted forced expiratory volume in 1 s
Health care professionals
Modified Medical Research Council
Swedish National Airway Register
- Specialized palliative care
Swedish Register of Palliative Care
Pocket Guide to COPD Diagnosis Management and Prevention. https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-POCKET-GUIDE-FINAL-pgsized-wms.pdf .
Rossi A, Butorac-Petanjek B, Chilosi M, Cosío BG, Flezar M, Koulouris N, Marin J, Miculinic N, Polese G, Samaržija M, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis. 2017;12:2593–610.
Article Google Scholar
Van Remoortel H, Hornikx M, Demeyer H, Langer D, Burtin C, Decramer M, Gosselink R, Janssens W, Troosters T. Daily physical activity in subjects with newly diagnosed COPD. Thorax. 2013;68(10):962–3.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195.5:557–82.
Cao YQ, Dong LX, Cao J. Pulmonary embolism in patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J. 2018;131(14):1732–7.
Aleva FE, Voets L, Simons SO, de Mast Q, van der Ven A, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2017;151(3):544–54.
Lecheler L, Richter M, Franzen DP, Rampini SK, Cheetham M, Jenewein J, Battegay E, Nowak A. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):170026. https://doi.org/10.1183/16000617.0026-2017 .
Wysham NG, Cox CE, Wolf SP, Kamal AH. Symptom burden of chronic lung disease compared with lung cancer at time of referral for palliative care consultation. Ann Am Thorac Soc. 2015;12(9):1294–301.
Bloom CI, Slaich B, Morales DR, et al. Low uptake of palliative care for COPD patients within primary care in the UK. Eur Respir J. 2018;51:1701879. https://doi.org/10.1183/13993003.01879-2017 .
Kendzerska T, Nickerson JW, Hsu AT, Gershon AS, Talarico R, Mulpuru S, Pakhale S, Tanuseputro P. End-of-life care in individuals with respiratory diseases: a population study comparing the dying experience between those with chronic obstructive pulmonary disease and lung cancer. Int J Chron Obstruct Pulmon Dis. 2019;14:1691–701.
Cohen J, Beernaert K, Van den Block L, Morin L, Hunt K, Miccinesi G, Cardenas-Turanzas M, Onwuteaka-Philipsen B, MacLeod R, Ruiz-Ramos M, et al. Differences in place of death between lung cancer and COPD patients: a 14-country study using death certificate data. NPJ primary care respiratory medicine. 2017;27(1):14–14.
Butler SJ, Ellerton L, Gershon AS, Goldstein RS, Brooks D. Comparison of end-of-life care in people with chronic obstructive pulmonary disease or lung cancer: a systematic review. Palliat Med. 2020;34.8:1030–43.
Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013(6):CD007760. https://doi.org/10.1002/14651858.CD007760.pub2 .
Henoch I, Strang P, Lofdahl CG, Ekberg-Jansson A. Equal palliative care for patients with COPD? A nationwide register study. Ups J Med Sci. 2019;124(2):140–7.
Higginson IJ, Reilly CC, Bajwah S, Maddocks M, Costantini M, Gao W, project GUC. Which patients with advanced respiratory disease die in hospital? A 14-year population-based study of trends and associated factors. BMC Med. 2017;15(1):19–19.
Kuo LC, Chen JH, Lee CH, Tsai CW, Lin CC. End-of-life health care utilization between chronic obstructive pulmonary disease and lung cancer patients. J Pain Symptom Manage. 2019;57(5):933–43.
Lundstrom S, Axelsson B, Heedman PA, Fransson G, Furst CJ. Developing a national quality register in end-of-life care: the Swedish experience. Palliat Med. 2012;26(4):313–21.
Martinsson L, Heedman PA, Lundstrom S, Axelsson B. Improved data validity in the Swedish Register of Palliative Care. PLoS One. 2017;12(10):e0186804.
Stridsman C, Konradsen JR, Vanfleteren L, Pedroletti C, Binnmyr J, Edfelt P, Fjällman Schärberg K, Sjöö Y, Nyberg F, Lindberg A, et al. The Swedish National Airway Register (SNAR): development, design and utility to date. European Clinical Respiratory Journal. 2020;7(1):1833412.
Article CAS Google Scholar
Socialstyrelsen. National guidelines - targets for quality indicators in palliative care by The Swedish National Board of Health and Welfare [Nationella riktlinjer - målnivåer. Palliativ vård i livets slutskede. Målnivåer för indikatorer]. In . Stockholm; 2017.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). [ http://goldcopd.org ].
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–6.
van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–54.
McDermott CL, Engelberg RA, Sibley J, Sorror ML, Curtis JR. The association between chronic conditions, end-of-life health care use, and documentation of advance care planning among patients with cancer. J Palliat Med. 2020;23.10:1335–41.
Nordstrom M, Strang P. High degree of satisfaction with the support given by multidisciplinary palliative home care teams in the county of Stockholm. J Palliat Care. 2018;33(2):109–14.
Weingaertner V, Scheve C, Gerdes V, Schwarz-Eywill M, Prenzel R, Bausewein C, Higginson IJ, Voltz R, Herich L, Simon ST. Breathlessness, functional status, distress, and palliative care needs over time in patients with advanced chronic obstructive pulmonary disease or lung cancer: a cohort study. J Pain Symptom Manage. 2014;48(4):569-581.e561.
Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115–23.
Ekström M, Williams M, Johnson MJ, Huang C, Currow DC. Agreement between breathlessness severity and unpleasantness in people with chronic breathlessness: a longitudinal clinical study. J Pain Symptom Manage. 2019;57(4):715-723.e715.
Gysels MH, Higginson IJ. The lived experience of breathlessness and its implications for care: a qualitative comparison in cancer, COPD, heart failure and MND. BMC Palliat Care. 2011;10:15.
Carlsson ME, Gunningberg L. Predictors for development of pressure ulcer in end-of-life care: a national quality register study. J Palliat Med. 2017;20(1):53–8.
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–65.
van der Molen T, Miravitlles M, Kocks JW. COPD management: role of symptom assessment in routine clinical practice. Int J Chron Obstruct Pulmon Dis. 2013;8:461–71.
Warwick M, Gallagher R, Chenoweth L, Stein-Parbury J. Self-management and symptom monitoring among older adults with chronic obstructive pulmonary disease. J Adv Nurs. 2010;66(4):784–93.
Marx G, Nasse M, Stanze H, Boakye SO, Nauck F, Schneider N. Meaning of living with severe chronic obstructive lung disease: a qualitative study. BMJ Open. 2016;6(12):e011555.
Abdallah SJ, Wilkinson-Maitland C, Saad N, Li PZ, Smith BM, Bourbeau J, Jensen D. Effect of morphine on breathlessness and exercise endurance in advanced COPD: a randomised crossover trial. Eur Respir J. 2017;501701235. https://doi.org/10.1183/13993003.01235-2017 .
Ekstrom MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445.
Leung JM, Udris EM, Uman J, Au DH. The effect of end-of-life discussions on perceived quality of care and health status among patients with COPD. Chest. 2012;142(1):128–33.
Au DH, Udris EM, Engelberg RA, Diehr PH, Bryson CL, Reinke LF, Curtis JR. A randomized trial to improve communication about end-of-life care among patients with COPD. Chest. 2012;141(3):726–35.
Wentlandt K, Seccareccia D, Kevork N, Workentin K, Blacker S, Grossman D, Zimmermann C. Quality of care and satisfaction with care on palliative care units. J Pain Symptom Manage. 2016;51(2):184–92.
Hjorth NE, Schaufel MA, Sigurdardottir KR, Haugen DRF. Feasibility and acceptability of introducing advance care planning on a thoracic medicine inpatient ward: an exploratory mixed method study. BMJ Open Respir Res. 2020;7(1):e000485.
Bouleuc C, Anota A, Cornet C, Grodard G, Thiery-Vuillemin A, Dubroeucq O, Cretineau N, Frasie V, Gamblin V, Chvetzoff G, et al. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. 2020;25(5):e843–51.
Brannstrom M, Hagglund L, Furst CJ, Boman K. Unequal care for dying patients in Sweden: a comparative registry study of deaths from heart disease and cancer. Eur J Cardiovasc Nurs. 2012;11(4):454–9.
Smedback J, Ohlen J, Arestedt K, Alvariza A, Furst CJ, Hakanson C. Palliative care during the final week of life of older people in nursing homes: a register-based study. Palliat Support Care. 2017;15(4):417–24.
Ambrosino N, Fracchia C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. A narrative review. Pulmonology. 2019;25(5):289–98.
Gardener AC, Ewing G, Kuhn I, Farquhar M. Support needs of patients with COPD: a systematic literature search and narrative review. Int J Chron Obstruct Pulmon Dis. 2018;13:1021–35.
Strang P, Furst P, Hedman C, Bergqvist J, Adlitzer H, Schultz T. Chronic obstructive pulmonary disease and lung cancer: access to palliative care, emergency room visits and hospital deaths. BMC Pulm Med. 2021;21(1):170.
Swedish National Airway Register's official web-page [ https://lvr.registercentrum.se/ ].
Swedish Register of Palliative Care, data [ https://palliativregistret.se/utdata/ ].
Download references
Acknowledgements
We would like to thank Proper English AB for professional language editing.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Open access funding provided by University of Gothenburg.
Author information
Authors and affiliations.
Department of Research and Devlopment, Angered Hospital, Gothenburg, Sweden
Ingela Henoch
Institute of Health and Care Sciences, Sahlgrenska Academy, University of Gothenburg, Box 457, 405 30, Gothenburg, Sweden
Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Ann Ekberg-Jansson
University of Lund, Lund, Sweden
Claes-Göran Löfdahl
COPD Center, Institute of Medicine, Sahlgrenska University Hospital, University of Gothenburg, Gothenburg, Sweden
Department of Oncology–Pathology, Karolinska Institute, Stockholm, Sweden
Peter Strang
Research and Development Unit, Stockholms Sjukhem Foundation, Stockholm, Sweden
You can also search for this author in PubMed Google Scholar
Contributions
IH, AEJ and CGL designed the study; IH merged the registers and performed analyses of data; IH, PS, AEJ and CGL discussed results, finalized analyses and potential implications of the results; IH and PS drafted the manuscript and tables; all authors read and approved the final manuscript.
Corresponding author
Correspondence to Ingela Henoch .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from the Regional Ethics Committee in Gothenburg (Dnr. 317–15, date 2015–07-07). Patients gave verbal informed consent to have data registered in the SNAR. The Ethics Committee accepted verbal informed consent for registry studies. Data in the SRPC were collected after the death of the patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Henoch, I., Ekberg-Jansson, A., Löfdahl, CG. et al. Benefits, for patients with late stage chronic obstructive pulmonary disease, of being cared for in specialized palliative care compared to hospital. A nationwide register study. BMC Palliat Care 20 , 130 (2021). https://doi.org/10.1186/s12904-021-00826-y
Download citation
Received : 26 October 2020
Accepted : 06 August 2021
Published : 24 August 2021
DOI : https://doi.org/10.1186/s12904-021-00826-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Symptom management
- End of life care
- Register study
BMC Palliative Care
ISSN: 1472-684X
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Background: Patients with lung cancer, idiopathic pulmonary fibrosis (IPF), and chronic obstructive pulmonary disease (COPD) have high symptom burden, poor quality of life, and high healthcare utilization at the end of life. While proactive integration of palliative care in lung cancer can improve outcomes, it is unclear whether similar practices have been adopted in COPD and IPF care.
Results. Eleven eligible studies were included in this review with a total of 1535 patients. Compared to usual care, palliative care interventions demonstrated statistically significant effects on improving generic health-related quality of life (SMD, 0.30 [95 % CI, 0.12 to 0.48]) and heart failure-specific quality of life (SMD, 0.17 [95 % CI, 0.03 to 0.31]).
The following is a summary of "Differences in Healthcare and Palliative Care Utilization at the End of Life: a Comparison Study Between Lung Cancer, COPD, and IPF," published in the August 2024 issue of Pulmonology by Suen et al. Patients with lung cancer, idiopathic pulmonary fibrosis (IPF), and chronic obstructive pulmonary disease (COPD) often experience
Objectives The existing evidence for the impacts of continuity of care (COC) in patients with chronic obstructive pulmonary disease (COPD) is low to moderate. This study aimed to investigate the associations between relational COC within primary care and COPD-related hospitalisations using a robust methodology. Design Population-based cohort study. Setting National Health Insurance Service ...
Palliative care (derived from the Latin root palliare, or 'to cloak') is an interdisciplinary medical caregiving approach aimed at optimizing quality of life and mitigating suffering among people with serious, complex, and often terminal illnesses. [1] Within the published literature, many definitions of palliative care exist. The World Health Organization (WHO) describes palliative care as ...
The majority of palliative care patients express a preference for remaining at home for as long as possible. Despite progression of disease there is a strong desire to die at home. Nonetheless, there are transfers between care settings, demonstrating a discrepancy between desired and actual place of death. To map the prevalence of patients near death undergoing specialized palliative home care ...
Community Based Palliative Care is a special kind of medical care for seriously ill patients, focused on relieving physical symptoms and stress of their illness. Palliative care is patient-and family-centered care that helps you carry on your daily life, improve your ability to go through medical treatment, and enjoy the best possible quality ...
Heart failure (HF) is a chronic illness associated with a heavy burden of symptom and excessive cost of care. 1, 2 In the United States, the aging population and availability of improved treatments for HF over the past decade have resulted in an increased number of individuals living with the disease. 1, 3, 4 Currently, 6.7 million US adults aged ≥20 years have HF, and ≈1 in 4 people will ...
Palliative care focuses on providing relief from the symptoms and stress of a serious illness. It is appropriate at any stage of a serious illness and can be provided alongside curative treatments.
Learn more about Medicaid expansion and palliative care for advanced-stage liver cancer. 04/01/2024
Background: Delays and limitations of palliative care in patients with liver transplantation-ineligible end-stage hepatocellular carcinoma according to Barcelona Clinic Liver Cancer staging system may be explained by different perceptions between hepatologists and palliative care physicians in the absence of shared guidelines. Aim: To assess physicians' attitudes toward palliative care in end ...
Episode · The Sunflower- Palliative Care Podcasts · Earlier this year at the MetaECHO Conference in New Mexico, Dr Megan Doherty was able to catch up with Dr. Rajagopal. He shared his wisdom and insights about what it takes to provide compassionate care.
The purpose of this study was to assess the prevalence and prognosis of cachexia in patients with non-sarcopenic dysphagia. A retrospective cohort study was conducted using the Japanese sarcopenic dysphagia database. Cachexia was diagnosed using the Asian Working Group for Cachexia criteria, sarcopenia using the Asian Working Group for Sarcopenia 2019 criteria, and malnutrition using the ...
The Gombe State Government on Monday presented 380 bags of 25kg rice, 380 bags of 50kg sorghum, and 240 cartons of spaghetti to security agencies in the state, our correspondent has gathered.
Still, the effect of palliative care to reduce COPD readmissions has not been tested adequately. Moreover, the studies of palliative care for COPD have not shown any reduction in COPD-related hospital readmissions. However, ... Inhaler education, an action plan in case of clinical deterioration, home visits to provide education, and assessing ...
COPD is the fourth leading cause of death in the United States and is a serious respiratory illness characterized by years of progressively debilitating breathlessness, high prevalence of associated depression and anxiety, frequent hospitalizations, and diminished well-being. Despite the potential to confer significant quality-of-life benefits for patients and their care partners and to ...
Introduction. Patients with advanced Chronic Obstructive Pulmonary Disease (COPD) suffer from a high symptom burden and low quality of life, emphasizing the need for palliative care [].Palliative care is an approach that aims to optimize the quality of life of patients with a life-limiting illness through assessment and treatment of physical, psychological, social and spiritual problems [].
Objectives Little direction exists on how to effectively implement palliative care for patients with COPD. In the COMPASSION study, we developed, executed, and evaluated a multifaceted implementation strategy to improve the uptake of region-tailored palliative care intervention components into routine COPD care. We evaluated the implementation strategy and assessed the implementation process ...
Patients with COPD would benefit from an approach that integrates palliative care and standard care early in the disease course and that offers the flexibility of intensifying care as the patients' health status changes. In this issue of CHEST, Fu et al 4 conducted a qualitative study of patients with severe COPD (n = 20), their caregivers (n ...
Introduction. Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death worldwide. 1 Patients suffering from end-stage COPD experience severe breathlessness and other debilitating symptoms such as fatigue, pain, anxiety and depression, leading to poor quality of life and emphasizing the need for adequate palliative care. 2 Palliative care aims to improve the quality of ...
One reason why COPD patients receive poor quality palliative care is that patient-physician communication about this is unlikely to occur . 17 out of 37 papers highlighted that a variable percentage of COPD patients had discussed palliative care topics, this ranged from 0% to 56%, [5, 19, 21, 24, 25, 29, 30, 35-44].
Currently, there is a lack of palliative care support (PCS) for patients with end-stage COPD, despite evidence that it improves their quality of life [4, 5]. The natural course of physical decline for patients with COPD can be variable, but overall it is characterised by a long-term steady deterioration [6].
Chronic obstructive pulmonary disease (COPD) is a progressive condition characterized by airflow obstruction which ultimately kills many patients. It is common in both men and women and there is a 24-30% 5-year survival rate in the UK for those with severe disease. The annual death rate in the UK from COPD approaches that from lung cancer.
Introduction. COPD is the third leading cause of death worldwide and the seventh in the combination of years of life lost or lived with disability. 1 Several studies have shown that patients with severe COPD have a similar or greater number of symptoms than those of patients with inoperable lung cancer. 2 Nonetheless, COPD patients are less likely to receive palliative care (PC) compared with ...
POOR PALLIATIVE CARE IN COPD AND THE LINK TO POOR COMMUNICATION. The Study to Understand Prognosis and Preferences for Outcomes and Treatments (SUPPORT) enrolled seriously ill, hospitalised patients in one of five hospitals in the USA with one of nine life-limiting illnesses, including COPD 10.Compared with patients with lung cancer, patients with COPD were much more likely to die in the ...
Educational barriers include limited knowledge of palliative care on the part of patients, their care partners, and clinicians. 4, 12, 37 In one study, only 30% of patients with COPD and their care partners had heard of palliative care, and pulmonologists also frequently mix the terms palliative care and hospice, as discussed earlier. 4 ...
The Role of Palliative Care in COPD. Anand S. Iyer, MD, MSPH; Donald R. Sullivan, MD, MCR; Kathleen O. Lindell, PhD, RN; and Lynn F. Reinke, PhD, ARNP. COPD is the fourth leading cause of death in the United States and is a serious respiratory illness characterized by years of progressively debilitating breathlessness, high prevalence of asso ...
Background In early stage chronic obstructive pulmonary disease (COPD), dyspnea has been reported as the main symptom; but at the end of life, patients dying from COPD have a heavy symptom burden. Still, specialist palliative care is seldom offered to patients with COPD; they more often receive end of life care in hospitals. Furthermore, symptoms, symptom relief and care activities in the last ...
FOR EDITORIAL COMMENT, SEE PAGE 2133. Take-home Point. The goal of this study was to explore palliative care needs and integration of palliative care support in COPD services. The findings demonstrated that patients and their carers have unmet needs for managing uncertainty and fear, daily living, and finances.
3. Almagro P, Yun S, Sangil A, et al. Palliative care and prognosis in COPD: a systematic review with a validation cohort. Int J Chron Obstruct Pulmon Dis. 2017;12:1721-1729. 4. Fu Y, Mason A, Boland AC, et al. Palliative care needs and integration of palliative care support in COPD: a qualitative study. Chest. 2021;159(6):2222-2232. 5.