Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

Hepatitis B Surveillance Guidance
Cases and clusters of potential public health importance, interpretation of laboratory test results, recommended reportable laboratory markers, case reporting and national notification, surveillance of acute and chronic hepatitis b, surveillance of hepatitis b during pregnancy and perinatal hepatitis b.
Hepatitis B is a disease caused by the hepatitis B virus (HBV) that can be self-limited for some and lifelong for others. HBV is transmitted through the blood or bodily fluids of an infected person. In the United States, injection drug use (IDU) and having multiple sexual partners are the first and second most common risk behaviors or exposures reported for acute hepatitis B, respectively ( 3 ). Approximately 50–70% of people with acute hepatitis B are not symptomatic ( 45 ), resulting in many undiagnosed and unreported infections. HBV is highly transmissible and infectious on environmental surfaces for at least 7 days ( 46 ).
The epidemiology of hepatitis B in the United States has evolved since the hepatitis B vaccine first became available in 1982 ( 47 ). Declines in acute hepatitis B incidence following the expansion of vaccination recommendations ceased during 2010–2019. Decreases occurred in 2020 and 2021 during which disruptions caused by the COVID-19 pandemic made it difficult to determine the impact of prevention efforts ( 3 ). The incidence of acute hepatitis B is highest among non-Hispanic White people and non-Hispanic Black people ( 3 ). National chronic hepatitis B prevalence and death rates have remained relatively stable ( 1 , 3 ).
During January 2017–March 2020, approximately 0.2% of the non-institutionalized US population, representing approximately 660,000 people, were estimated to be living with chronic hepatitis B ( 1 ). The prevalence of chronic hepatitis B was highest among non-US-born people and those of Asian/Pacific Islander descent ( 1 ). Approximately 50% of people living with chronic hepatitis B were unaware of their infection status ( 1 ). Such people could unknowingly transmit their infection to others and are at risk for developing chronic liver disease.
There are clinical guidelines by the American Association for the Study of Liver Diseases (AASLD) for the prevention, diagnosis, and treatment of chronic hepatitis B ( 48 ). Several antiviral medications are available to effectively lower HBV DNA levels and slow the progression of liver disease; however, hepatitis B is not yet curable.
Recommendations for universal adult hepatitis B screening were released by the CDC in ( 49 ). They recommend that all adults > 18 years of age be screened at least once. All pregnant people are recommended for universal hepatitis B screening, preferably during the first trimester and regardless of vaccination status or testing history, because of the risk for perinatal transmission. People being tested for the first time should be tested using the “triple panel” – which includes hepatitis B surface antigen (HBsAg), total antibody to hepatitis B core antigen (anti-HBc), and hepatitis B surface antibody (anti-HBs) – to identify people who have current HBV infection, have resolved acute HBV infection and may be at risk for reactivation, are susceptible and need vaccination, or are vaccinated with immune protection. Infants who are perinatally exposed to HBV should receive the first dose of hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth, complete the vaccine series, and receive post-vaccination serologic testing (PVST) for HBsAg and anti-HBs during 9–12 months of age and 1–2 months after the final dose of the vaccine. Without preventive interventions, chronic infection develops in approximately 90% of infected infants compared with 25%–30% of children who acquire HBV infection during 1–5 years of age and about 10% of people infected at >5 years of age ( 50 , 51 ).
All unvaccinated people with ongoing risk behaviors or exposures should be tested for hepatitis B periodically while risk behaviors or exposures persist. People for whom HBV exposure is suspected should receive timely post-exposure prophylaxis and immediate testing, which can prevent HBV infection and interrupt transmission. In 2022, the ACIP recommended hepatitis B vaccination for all adults 19–59 years removing the need for risk factor screening among this age group ( 52 ). Infants and people <19 years of age regardless of risk factors for hepatitis B and adults > 60 years of age with risk factors for hepatitis B are also recommended to receive vaccination. Adults aged ≥60 years without known risk factors for hepatitis B may receive vaccination.
The purpose of this section is to provide guidance to jurisdictions as they implement and improve hepatitis B surveillance. It contains information regarding reporting requirements, collection of relevant laboratory data, and case investigation. Given that current systems for the surveillance and follow-up of hepatitis B cases differ by jurisdiction, the standards outlined in this document are designed to provide models for best practices, recognizing that not every jurisdiction can meet those standards with available resources.
Top of Page
Jurisdictions should review and analyze hepatitis B data regularly to identify cases and clusters of hepatitis B that merit further investigation. When resources are limited, these should be prioritized for investigation based on the degree of public health importance. The following are examples of high priority cases and clusters:
- People of childbearing age who are or have the potential to become pregnant, indicating the potential for perinatal transmission
- Children ≤ 24 months of age to detect perinatal transmission
- ≥70 years of age (indicating possible health care-associated transmission)
- People who were previously vaccinated to characterize possible vaccine failures (see Section 1.10 Case Investigation )
- People born after 1990 to distinguish between failure of vaccine and failure to vaccinate
- People receiving hemodialysis with evidence of acute hepatitis B (including those with test conversions)
- People lacking typical behavioral risk behaviors or exposures for hepatitis B (e.g., IDU) who have evidence of acute infection (including test conversions) to identify other potential causes of HBV transmission (e.g., health care-associated exposures) (information on investigation of health care-associated outbreaks can be found on the CDC DVH Viral Hepatitis Outbreaks website)
- with elevated ALT or jaundice;
- with positive immunoglobulin class M antibody to hepatitis B core antigen (anti-HBc IgM);
- with recent or current IDU history;
- who were tested at locations frequented by people at high-risk for acute infection (e.g., STI and HIV clinics, SSPs, correctional facilities, and medication-assisted treatment for opioid use disorder [MAT] centers); or
- who were in a residential facility or custodial care, including long-term care or correctional facilities, for ≥6 months prior to the onset of symptoms.
A description of hepatitis B laboratory markers can be found in Appendix B .
Understanding Changes in Biomarkers During Disease Progression
Understanding the changes in HBV biomarkers over the course of a person’s infection and recovery is key to interpreting the test results. Figure 3-1 and Figure 3-2 depict the typical biomarker changes over the course of hepatitis B disease.
Figure 3-1. Typical serologic course of acute hepatitis B to recovery

Weeks after Exposure
Figure 3-2. Typical serologic course of the progression to chronic hepatitis B

Acute, resolved, and chronic hepatitis B Approximately 90% of people >5 years of age with acute hepatitis B will spontaneously clear their infection ( 50 , 51 ). People with resolved hepatitis B will remain positive for total anti-HBc and develop anti-HBs that protect against future HBV infection ( Figure 3-1 ). Chronic hepatitis B is defined as an HBV infection lasting > 6 months. During the typical course of chronic infection, the total anti-HBc and HBsAg markers will always be present, whereas anti-HBc IgM will disappear ( Figure 3-2 ). Hepatitis B e antigen (HBeAg) and hepatitis B e antibody (anti-HBe) are variably present. HBV DNA levels vary during the course of chronic infection. Any detectable HBV DNA level is considered positive for surveillance purposes.
Isolated total anti-HBc positive A person with a positive total anti-HBc with corresponding negative HBsAg and anti-HBs results is considered to be isolated total anti-HBc positive. A small fraction of these people could have low level chronic viremia, also known as occult hepatitis B, in which HBsAg is absent in the serum but HBV DNA is detectable ( 53 ) ( Table 3-1 ). Cases of occult hepatitis B may be missed through surveillance in the absence of a provider report indicating occult infection or in the absence of total anti-HBc and HBV DNA results. To determine if occult hepatitis B is present, those who are isolated total anti-HBc positive should be tested for the presence of HBV DNA.
People with mutations in HBsAg that cannot be detected by current serologic assays may present with a negative HBsAg result despite high blood levels of HBV DNA. Some laboratories have the capacity to detect HBsAg mutants. Any HD interested in determining which laboratories can detect HBsAg mutants should follow-up with the major laboratories that perform HBsAg testing in their jurisdiction.
Hepatitis B reactivation People with a history of hepatitis B – either inactive chronic hepatitis B or resolved acute hepatitis B – can experience hepatitis B reactivation. Hepatitis B reactivation is the loss of hepatitis B immune control, which can be followed by a hepatitis flare characterized by ALT elevation (increase to greater than 3 times the baseline level and >100 IU/L with or without symptoms; in some cases, illness can be severe and result in death ( 48 ). In general, people with inactive chronic hepatitis B (i.e., those positive for HBsAg) are at greater risk for reactivation than are those with resolved hepatitis B (i.e., those negative for HBsAg and positive for total anti-HBc and anti-HBs). People at greatest risk of hepatitis B reactivation include those
- undergoing cancer chemotherapy,
- receiving immunosuppressive therapy (particularly anti-B cell therapy),
- with HIV infection who have discontinued antiretroviral drugs with activity against HBV (e.g., tenofovir),
- undergoing solid organ or bone marrow transplantation, and
- co-infected with hepatitis C virus (HCV) who are undergoing treatment with direct-acting antivirals (DAAs).
Among people with previously inactive chronic hepatitis B (i.e., those positive for HBsAg and total anti-HBc), laboratory evidence of reactivation includes meeting any one of the following criteria:
- a ≥100-fold increase in HBV DNA compared to the baseline level,
- HBV DNA ≥1,000 IU/mL in a patient with previously undetectable level, or
- HBV DNA ≥10,000 IU/mL if the baseline level is not available ( 48 ).
Among people with resolved acute hepatitis B (i.e., negative for HBsAg and positive for total anti-HBc and anti-HBs), laboratory evidence of reactivation includes meeting either of the following criteria:
- HBV DNA is now detectable or
- HBsAg test conversion occurs (negative HBsAg to positive HBsAg) ( 48 ).
People with hepatitis B reactivation are frequently positive for anti-HBc IgM. People with previously resolved infection who reactivate can have clinical signs and symptoms while also being transiently positive for anti-HBc IgM, therefore, mimicking acute infection. If it is determined that the case under investigation has test results known to be due to reactivation (e.g., a prior history of acute or chronic hepatitis B), the positive anti-HBc IgM test result should not be used for case classification purposes.
Obtaining a clinical history from the patient’s provider and/or checking the surveillance system or registry might provide clarification. A history of acute or chronic hepatitis B can help distinguish between a hepatitis B reactivation case (history of hepatitis B) and a newly diagnosed acute or chronic hepatitis B case (no history of hepatitis B). History of acute or chronic hepatitis B includes those that were previously reported to and documented in a jurisdiction’s surveillance system, were reported by a separate jurisdiction as having such a history, or a provider report of prior infection.
If reactivation is identified in a previously reported probable or confirmed acute hepatitis B case in a prior reporting year, and the person meets chronic case classification criteria with the latest report, this case should be submitted as a new chronic hepatitis B case. If reactivation is identified in a previously reported probable or confirmed chronic hepatitis B case, this case should not be counted again; however, reactivation may be noted in the jurisdiction’s hepatitis B surveillance database if the jurisdiction chooses to do so.
Interpreting Hepatitis B Laboratory Results
Many jurisdictions have regulations requiring laboratories to report all positive HBsAg, HBeAg, HBV DNA, and anti-HBc IgM laboratory results to the HD while a subset might also routinely receive positive total anti-HBc and anti-HBs results.
Additionally, some HDs might receive negative hepatitis B laboratory results, which are useful for determining false-positive results and monitoring patients through their infection and recovery. In jurisdictions that receive laboratory results performed as part of a reflex test panel, implementation of the initial screening triple panel (HBsAg, total anti-HBc, and anti-HBs) may result in more complete reporting of hepatitis B laboratory results among people with HBV infection. Table 3-1 shows how to interpret the combinations of laboratory results frequently available in hepatitis B test panels, following the biomarker changes over the course of disease as shown in Figure 3-1 .
Table 3-1. Interpretation of hepatitis B laboratory results
| HBsAg | Total anti-HBc | Anti-HBc IgM | Anti-HBs | HBV DNA | Possible Interpretation* |
|---|---|---|---|---|---|
| – | – | – | – | – | Never infected; susceptible if never vaccinated or vaccine failure |
| + | – | – | – | + or – | Early acute infection (if HBV DNA is positive); transiently positive for HBsAg after vaccination (if HBV DNA is negative)† |
| + | + | + | – | + | Acute infection |
| – | + | + | + or – | + or – | Acute resolving infection; “window period” if anti-HBs is negative |
| – | + | – | + | – | Recovered from past infection and immune |
| + | + | – | – | + | Chronic HBV infection |
| – | – | – | + | – | Immune from vaccination; passive anti-HBs transfer after hepatitis B immune globulin administration |
| – | + | – | – | + or – | Isolated total anti-HBc positive‡ |
| – | + or – | – | + or – | + | Occult HBV infection§ |
| + or – § | + | + or – | + or – | + | Possible HBsAg mutant infection |
Table modified from https://www.cdc.gov/mmwr/volumes/67/rr/pdfs/rr6701-H.PDF .
Abbreviations: – = negative; + = positive; anti-HBc = antibody to hepatitis B core antigen; anti-HBs = antibody to hepatitis B surface antigen; HBsAg = hepatitis B surface antigen; HBV DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
*Ingestion of high levels of biotin can significantly interfere with certain commonly used biotinylated immunoassays and cause false-positive or false-negative laboratory test results. The US Food and Drug Administration (FDA) is investigating thresholds associated with false-positive and false-negative tests. This section will be updated as more information becomes available. Reference: https://www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication .
†People who receive hepatitis B vaccine might be transiently positive for HBsAg, with reports of transient positivity 18 days post-vaccination ( 56 ). Retesting of patients who are positive for HBsAg shortly after hepatitis B vaccination at a later time is needed to determine the true HBV infection status.
‡Could result from:
- Loss of anti-HBs after past resolved infection. HBV DNA is negative.
- False-positive total anti-HBc, i.e., susceptible. HBV DNA is negative. To resolve the ambiguity of a false-positive total anti-HBc result, test a follow-up sample 4–8 weeks later. If found positive, interpret as a resolved infection. If negative, interpret as false-positive.
- Passive maternal transfer of total anti-HBc to infant born to a HBsAg-positive gestational parent for up to 24 months. HBV DNA is negative.
- Occult HBV infection. HBV DNA is positive, typically at low levels. Anti-HBs might or might not be positive.
- HBsAg mutant infection. HBV DNA is positive, typically at high levels. Anti-HBs might or might not be positive.
§ HBsAg mutants will not be detectable if testing was performed using an older assay that cannot detect HBsAg mutants. HBsAg mutant strains can be detected by some HBsAg assays that first became available in the United States in 2015, including Abbot ARCHITECT instrument, ETI-MAK-2 PLUS, and Siemens Advia Centaur XP or XPT instrument. Though specimens should be tested using an assay that can detect HBsAg mutants, older HBsAg assays that cannot detect HBsAg mutants remain available. Reference: Apata I W, Nguyen D B, Khudyakov Y, et al. Hepatitis B virus mutant infections in hemodialysis patients: A case series. Kidney Medicine 2019; 1( 6 ): 347-353. DOI: https://doi.org/10.1016/j.xkme.2019.07.011 . Downloads of this figure: PDF | PPT
The following laboratory markers are recommended for reporting to public health, as they can aid in case ascertainment, case classification, and monitoring care continua for hepatitis B:
- Positive HBsAg
- Positive/detectable HBV DNA (including quantitative, qualitative, and genotype testing)
- Positive HBeAg
- Positive anti-HBc IgM
- Pregnancy status
- Concurrent ALT and total bilirubin result
- Other hepatitis serological results (e.g., hepatitis A, hepatitis C, hepatitis D, and/or hepatitis E)
- Total anti-HBc * results
- Negative HBsAg
- Negative/undetectable HBV DNA † results
* Total anti-HBc is detectable, on average, approximately 5 weeks post-HBV exposure, remains detectable indefinitely following exposure, and indicates past or current infection. In the presence of total anti-HBc, a positive HBsAg, HBeAg, or anti-HBc IgM result is a more reliable indication of current or recent infection while negative total anti-HBc results can be used to determine that a person is not a case. Jurisdictions that receive total anti-HBc laboratory results can use these results to clarify a person’s HBV infection status and confirm chronic cases in conjunction with evidence of positive tests for HBsAg or HBeAg.
† Jurisdictions are encouraged to require reporting of negative/undetectable HBV DNA results as these results can be a proxy indicator that people living with hepatitis B have been linked to care. This information can support accurate longitudinal surveillance and measurement of hepatitis B care continua.
Cases of acute, chronic, and perinatal hepatitis B, and hepatitis B during pregnancy should be reported to HDs as specified by state, territorial, or local regulations. Acute, chronic, and perinatal hepatitis B are nationally notifiable conditions ( 5 ). Hepatitis B cases are identified using an event code corresponding to the hepatitis B condition ( Table 1-2 ). Data are sent weekly or more frequently, depending on the infrastructure of the jurisdiction sending the data. Cases might be re-classified or removed as needed after the initial transmission to CDC, as long as the changes occur before surveillance data are finalized each year.
The national incidence of acute hepatitis B dramatically declined after incremental recommendations for vaccinating people at-risk for infection and severe outcomes were released beginning in 1982 ( 47 ) and for infants and children in 1991 ( 55 ). The number of acute hepatitis B cases reported each year in the United States has remained relatively stable during 2014–2019 but decreased abruptly in 2020 and 2021 ( 3 ). The decrease in reported cases could be due to prevention efforts as well as disruptions of the COVID-19 pandemic. IDU and having multiple sexual partners are major risk behaviors associated with acute hepatitis B in the United States, and incidence is highest among non-Hispanic White people, non-Hispanic Black people, and those 30–59 years of age ( 3 ).
In the United States, chronic hepatitis B occurs primarily among people born in countries with intermediate or high hepatitis B prevalence, where the primary mode of transmission is perinatal transmission. Chronic hepatitis B occurs in about 1.0% of non-US-born adults ( 1 ). During January 2017–March 2020, approximately 660,000 people were estimated to be living with chronic hepatitis B in the United States ( 1 ).
CDC has provided guidelines for hepatitis B screening among all adults > 18 years of age at least once during a lifetime and pregnant people during each pregnancy ( 49 ). Periodic testing among susceptible people with ongoing risks for exposures is recommended while risks for exposures persist. Undiagnosed hepatitis B cannot be detected using traditional surveillance methods. Improving hepatitis B surveillance by improving screening is an important component of national and jurisdictional strategies for the prevention and control of hepatitis B.
Uses of Surveillance Data
Acute and chronic hepatitis B surveillance data can be used to inform and improve public health interventions in the following ways:
- Monitoring trends in disease incidence and determining risk behaviors or exposures . Acute hepatitis B surveillance data should be analyzed at regular intervals by person, place, and time to monitor disease incidence. Risk factor information should be analyzed to monitor disease transmission patterns and identify groups at higher risk for infection.
- Identifying outbreaks . An outbreak is defined as the occurrence of more cases of disease than expected in a given area or among a specific group of people over a particular time period. Detailed guidance on viral hepatitis outbreaks, including examples of hepatitis B outbreaks, can be found on the CDC DVH Viral Hepatitis Outbreaks website .
- Patients whose infection source was reported as being a household or sexual contact should be investigated to determine whether they should have been vaccinated when the source case was identified. Potential barriers to administering post-exposure prophylaxis should be explored to mitigate future missed opportunities for prevention.
- Surveillance data can be used to provide information on cases occurring among adults at high risk for infection, creating opportunities to provide education and awareness to the health care community and the public about the importance of vaccinating high-risk populations as recommended by ACIP.
- Missed opportunities for vaccination should also be assessed for cases occurring among people born after 1990. Understanding the frequency and characteristics of these cases enables monitoring of the effectiveness of routine childhood vaccination programs and identification of barriers to childhood vaccination.
- Assessing the frequency and causes of vaccine failure . When available, vaccination history should be obtained. Though vaccine failure is rare, any case in a person who was previously vaccinated requires additional investigation to identify potential instances of vaccine failure. Where available, jurisdiction immunization registries can provide valuable information for such investigations Section 1.10 . Health care professionals or public health authorities who have questions on these cases should contact CDC’s DVH at [email protected] . Reporting and investigating these cases through surveillance is important for informing vaccination policies and education.
- Tracking cases of chronic hepatitis B . Surveillance systems and databases that track chronic hepatitis B cases can aid in monitoring trends in the prevalence of chronic infection.
- determine whether infections have resolved or reactivated;
- identify probable cases that need additional testing for diagnosis;
- identify health-related disparities;
- facilitate identification of HBV-infected pregnant people for enrollment in the Perinatal Hepatitis B Prevention Program (PHBPP);
- facilitate monitoring of perinatal hepatitis B;
- monitor the movement of cases in or out of the jurisdiction; and
- track the occurrence of related adverse health outcomes.
- Public health management of people with chronic hepatitis B and their contacts . Surveillance data can be used to identify and follow-up on chronic hepatitis B cases (especially among those who were recently diagnosed), link them to appropriate medical care and harm reduction services, and ensure contacts are protected and/or referred to care or testing, as appropriate.
Surveillance Case Definitions
Table 3-2 depicts the surveillance case definition for acute hepatitis B, adopted by CSTE and CDC in 2024 ( 56 , 57 ). See Appendix C for classification scenarios of cases of acute hepatitis B.
Table 3-2. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for acute hepatitis B, 2024
| Criteria Type | Criteria |
|---|---|
| Age | 24 months of age and the mode of exposure was not perinatal |
| Clinical | ) |
| Confirmatory Laboratory* | detection of at least one of the following: ) , HBeAg, or NAT for HBV DNA within 12 months (365 days) of a negative HBsAg test result (i.e., HBsAg seroconversion) detection of at least one of the following: |
| Presumptive Laboratory | negative or not done for HBsAg , HBeAg, or NAT for HBV DNA |
| Confirmed Acute* | 24 months of age and the mode of exposure was not perinatal, meets the clinical criteria and Has Tier 2 confirmatory laboratory evidence |
| Probable Acute | 24 months of age and the mode of exposure was not perinatal, |
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate. †If information on HBsAg test method is available and HBsAg confirmatory neutralization was performed as recommended, HBsAg positive by confirmatory neutralization.
Download of this table: PDF
Table 3-3 specifies the surveillance case definition for chronic hepatitis B, adopted by CSTE and CDC in 2024 ( 57 , 58 ). See Appendix C for classification scenarios of cases of chronic hepatitis B.
Table 3-3. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for chronic hepatitis B, 2024
| Criteria Type | Criteria |
|---|---|
| Age | 24 months of age and the mode of exposure was not perinatal |
| Clinical | |
| Confirmatory Laboratory | ) in two clinical specimens taken 6 months apart, 6 months apart, OR HBeAg] AND detection of total antibody to hepatitis B core antigen (anti-HBc), AND detection of HBeAg, |
| Presumptive Laboratory | OR HBeAg |
| Case Status | Classification |
| Confirmed Chronic | 24 months of age and the mode of exposure was not perinatal, |
| Probable Chronic | 24 months of age and the mode of exposure was not perinatal, |
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate. †If information on HBsAg test method is available and HBsAg confirmatory neutralization was performed as recommended, HBsAg positive by confirmatory neutralization. ‡A confirmed or probable acute hepatitis B case may be additionally enumerated as a new confirmed chronic hepatitis B case if a positive HBV viral detection test is reported 6 months or longer after acute case onset or, if asymptomatic, after the initial positive test result.
All Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories should be performing HBsAg confirmatory neutralization testing in accordance with CLIA regulations and the signal-to-cutoff values criteria listed in the instructions for use insert of the laboratory test assay. See Appendix D for more information on the HBsAg testing sequence. Therefore, HDs should not need to investigate every positive HBsAg result to determine whether confirmatory neutralization was performed. However, if a HD receives a negative or indeterminate HBsAg confirmatory neutralization result, the initial screened positive HBsAg result should not be used to count a person as a case.
False-positive HBsAg and HBeAg test results are possible. This can be due to multiple factors, including hepatitis B vaccination in the prior 30 days (e.g., can be longer if the person is a hemodialysis patient) and certain health conditions. If a false-positive result is suspected, jurisdictions should consider other available test results, such as the total anti-HBc result, to aid with interpretation. If results are determined to be false-positive, they should not be used to classify cases as confirmed or probable.
Multiple hepatitis B laboratory tests may be performed simultaneously on the same patient specimen as part of a “hepatitis panel.” Testing performed in this manner may lead to seemingly discordant results, e.g., negative HBsAg and positive HBV DNA. For the purposes of these case definitions, any positive result among the three viral detection tests (HBsAg, HBeAg, and HBV DNA) is acceptable for case classification, regardless of other testing results. Negative HBeAg results and HBV DNA levels below the positive cutoff level do not rule out HBV infection.
HBV DNA results might be reported as below the lower limit of quantification (e.g., < 15 IU/mL, etc.) yet simultaneously indicate that HBV DNA was detected. HBV DNA results that are above the limit of detection but below the lower limit of quantification should be considered “detectable” or “positive” for surveillance classification purposes.
The serologic course of acute hepatitis B lasts for <6 months. Therefore, the clinical and laboratory evidence used to classify a case as acute hepatitis B must have occurred within a time that is no longer than 6 months to meet classification requirements.
The “absence of a more likely diagnosis” clause of the acute clinical criteria can be applied when there is documentation of an alternative diagnosis that would more likely explain the presence of clinical evidence (i.e., a provider report of jaundice, total bilirubin > 3.0 mg/dL, or ALT >200 IU/L). If there is documentation from the patient’s health care provider explaining that the clinical evidence is due to another reason other than acute hepatitis B, the patient should not be evaluated under the acute hepatitis B case definition.
A case meeting the requirements for being classified as an acute hepatitis B case might also meet requirements for being classified as a chronic hepatitis B case. In this circumstance, unless there is evidence that the case is not acutely infected (e.g., hepatitis B reactivation), the case should only be classified as an acute case. If after 6 months from the initial event date, the case continues to have test results reported that indicate current HBV infection, they can then be additionally classified as a chronic hepatitis B case. People with hepatitis B reactivation should not be counted as new acute hepatitis B cases. People with hepatitis B reactivation may be counted as new confirmed chronic hepatitis B cases if they were not previously classified and reported as such.
Since hepatitis D only occurs in the presence of HBV infection, reports of hepatitis D without known HBV infection should be investigated as suspected cases of hepatitis B.
Case Ascertainment
The primary method to ascertain suspected cases is through investigation of reports from clinical laboratories, health care facilities, and health care providers suggestive of hepatitis B. Rules or regulations requiring that facilities and providers report hepatitis B to public health agencies vary by jurisdiction. See Section 1.6 and Section 3.4 for information on the recommended reporting requirements for hepatitis B.
Additional sources of information include medical records, hospital discharge databases, death certificates, and birth certificates. Section 5.4 provides more information on these data sources. Figure 3-3 illustrates a potential approach for acute and chronic hepatitis B case ascertainment and classification.
Specific procedures can vary by jurisdiction, but should generally follow the scheme below, in accordance with the CDC/CSTE Position Statement for the 2024 acute and chronic case definitions ( 56-58 ). Children < 24 months of age and born in the United States to a gestational parent with documented evidence of HBV infection should be classified and reported using the 2017 perinatal hepatitis B case definition ( 59 , 60 ), unless there is evidence that exposure occurred via a non-perinatal mechanism (e.g., health care-acquired). See Section 3.7.4 for case ascertainment guidance of perinatal hepatitis B cases.
Laboratory Reporting Laboratory reporting of HBV infection is required in all states for which acute and/or chronic hepatitis B are reportable. While case-defining infection markers (e.g., positive HBsAg or anti-HBc IgM) are reportable in most jurisdictions, regulations vary regarding which markers should be reported (e.g., any or all positive indicators within the panel, only positive results of selected biomarkers, or selected combinations of markers).
Some jurisdictions require reporting of negative hepatitis B laboratory results for some or all the infection markers or when accompanied with positive hepatitis B laboratory results. Receiving negative hepatitis B laboratory results or complete reporting of all tests in the hepatitis panel allows public health officials to interpret results more accurately. However, this also requires more sophistication in information systems to efficiently send, process, and utilize the information received.
Health Care Facility and Provider Reporting Many states require health care facilities and providers to report hepatitis B diagnoses.
Figure 3-3. Process for classifying cases of hepatitis B as acute and chronic
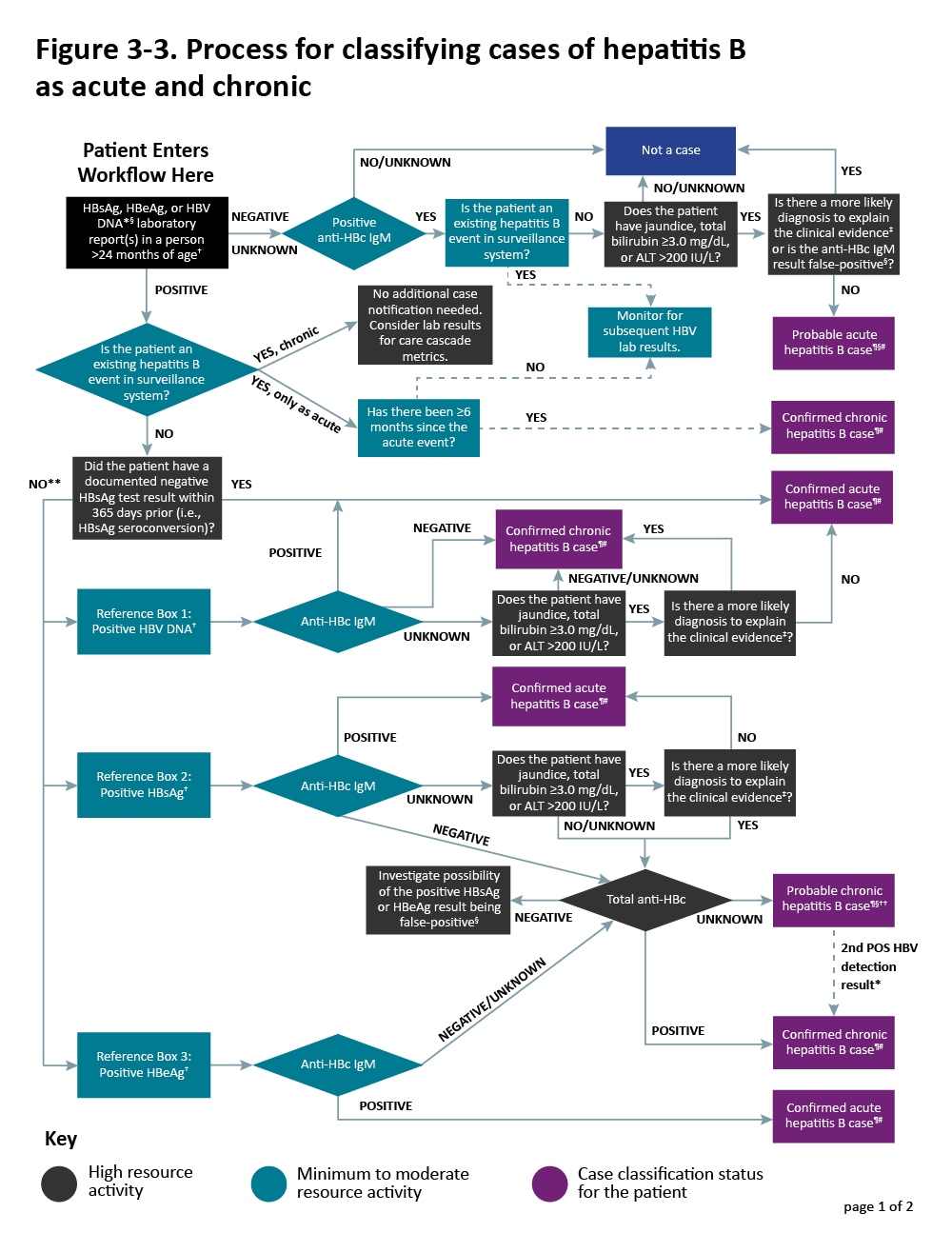
Case Investigation
The original report may be sufficient to classify a case as an acute or chronic infection. Resource limitations may not allow all chronic cases to be investigated in the same way as acute cases. Additional investigation may be necessary depending on the priority level of the case. The level of investigation will depend on the situation, the objectives, and the available resources. The following is a description of the type of information that should be collected during case investigations:
Information from the Laboratory Newly reported positive anti-HBc IgM, HBsAg, HBeAg, and HBV DNA laboratory results should be reported to the HD. Concurrent ALT and total bilirubin results reported with positive hepatitis B laboratory results can be helpful in identifying cases that might be acute. Total anti-HBc indicates past or current infection, and in its presence, a positive HBsAg, HBeAg, or anti-HBc IgM result is more likely to be a true positive.
Information from the Provider or Medical Records Medical records can provide the following types of information:
- Demographic information. Includes name, date of birth, sex at birth, current gender, race, ethnicity, country of birth, and residential address (including zip code).
- Clinical features. Includes reason for testing, illness onset date, clinical signs (jaundice) and symptoms (if available*), hospitalization status and date of death (if applicable), and whether an alternate diagnosis is suspected. HDs should inquire about the potential of past infection to confirm whether current clinical features are due to a newly acquired infection. The medical record may also provide evidence of chronic liver disease. *Includes fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, and abdominal pain.
- Pregnancy status. Pregnancy status should be checked for all people of childbearing age with childbearing potential. HBV-infected pregnant people should be referred to the PHBPP to ensure their infants receive appropriate post-exposure management according to ACIP recommendations. Children born to an HBV-infected gestational parent should be tested for infection, and if infected, classified according to the CDC/CSTE perinatal hepatitis B case definition .
- Diagnostic test results. If additional laboratory testing (e.g., ALT levels, total bilirubin levels, and results from a hepatitis panel) are needed to classify the case, HD staff may work with the provider to order/obtain these test results.
- Risk behaviors or exposures. Includes history of IDU, sexual and household exposures, experience of homelessness, recent medical procedures, hemodialysis, incarceration, and residence in a long-term care facility.
- Vaccination information . Vaccination history may be obtained from the patient’s medical provider or from the jurisdiction’s immunization registry. Note that recent hepatitis B vaccination can cause transient HBsAg positivity for up to 18 days post-vaccination ( 54 ). Retesting is needed to determine the true HBV infection status in patients who tested positive for HBsAg shortly after hepatitis B vaccination.
Information from the Patient All patients with acute hepatitis B should be contacted for an interview using the jurisdiction-specific case investigation form. If resources are limited, at a minimum, all patients who are classified as “confirmed” per the CDC/CSTE case definition and those flagged as having public health importance ( Section 3.2 ) should be interviewed. Decisions to contact the patient are often jurisdiction-specific and depend on available resources. In many situations, patient contact is reserved for those cases deemed highest priority for preventing further transmission or for referral for additional care and treatment, as needed.
The patient interview should ideally include the following components:
- Risk behaviors or exposures. This allows identification of a potential source or presence of risk behaviors or exposures for infection during the 60–150 days prior to symptom onset. For chronic cases, if it is determined that the person has current risk behaviors or exposures for ongoing transmission or was identified as part of a cluster of cases, additional information relevant to risk might be prioritized.
- Education and referral for follow-up. Newly diagnosed acute and chronic hepatitis B patients should be advised on how to prevent transmission to others. Patients should also be referred for hepatitis B-directed medical care and recommended to receive vaccination against hepatitis A, if indicated.
- Identification of contacts requiring post-exposure prophylaxis and testing. If resources allow, contacts should be identified and testing, post-exposure prophylaxis, counseling, and linkage to care coordinated, as appropriate. Information regarding hepatitis B vaccination and prophylaxis can be found on the Hepatitis B ACIP Vaccine Recommendations website.
Special Considerations When Investigating Certain Populations or Settings at Risk for Rapid Disease Transmission Considerations when investigating hepatitis B cases among certain populations at risk for rapid transmission are provided in Section 1.10 .
Case Investigation Prioritization Providers are required to report acute infections directly to the HD, and laboratories should provide HDs with hepatitis B test results electronically. The automated collection of hepatitis B laboratory results will, in many jurisdictions, lead to a high volume of reporting. Many HDs might lack the resources needed to conduct investigations for all acute cases. If resources allow, automate the collection of ALT and total bilirubin results through electronic laboratory reporting (ELR) or electronic medical record (EMR) reporting, and prioritize data collection to confirm those cases with abnormal results. Jurisdictions can also consider the following when prioritizing case follow-up:
- Semi-automated/preliminary collection of risk data combined with more targeted follow-up on cases without identified risk history
- Pregnant people
- Elderly patients (e.g., >70 years of age)
- Cases that might represent emerging risks
- People infected with HIV, hepatitis C virus (HCV), or STIs
- Children and adult cases who were born after 1990
- Cases who are documented to have received hepatitis B vaccination
- SSPs or SUD treatment facilities
- Correctional facilities
- Retirement/nursing facilities
- Providers of people experiencing homelessness
- Areas where known risk behaviors are occurring, or rates of newly reported infections are increasing
- People tested at public health clinics
- SAMHSA/state drug use, overdose, and EMS data
- HIV, HCV, and STI incidence data to identify coinfections
- Ongoing outbreak and cluster investigations, if applicable
- Hospital discharge data
- Syndromic surveillance data on IDU-related emergency department care
Considerations for Conducting a Chronic Hepatitis B Case Investigation Conducting an investigation on a chronic hepatitis B case can involve the following considerations:
- Check the jurisdiction’s hepatitis B registry/surveillance system to ensure the case is newly reported and not previously documented.
- Review the information in the initial report to determine if the case potentially falls within a group prioritized for investigation, such as those outlined in Section 3.2. At a minimum, pregnancy status should be checked for all people with chronic hepatitis B who are of childbearing age with childbearing potential; reports of HBV-infected pregnant people should be shared with the PHBPP.
- When possible, contact the health care provider and/or review medical records to obtain additional information to help prioritize which cases should receive a patient interview.
- If the case has one of the risks for hepatitis B reactivation outlined in Section 3.3 under the subsection “Understanding Changes in Biomarkers during Disease Progression” (e.g., the patient is hepatitis C co-infected and is receiving DAA treatment), consider follow-up with the health care provider to ensure the patient receives medical management according to clinical guidelines ( 61 ).
- For patients who are interviewed, collect relevant demographic and risk history information using the jurisdiction-specific case report form.
- Investigate likely health care exposures according to the jurisdiction’s procedures, ideally in collaboration with the health care-associated infection team.
- Provide patient education about ways to avoid the spread of infection to others and ways to avoid further harm to the liver.
- Educate long-term sexual contacts and people who have had direct exposure to the patient’s blood about HBV transmission and the need to be tested for hepatitis B if they are not known to be immune or infected. If a contact is susceptible, they should complete the hepatitis B vaccine series; contacts deemed unlikely to return for test results should be vaccinated when testing is initiated.
- If the patient is a child, screen the parents and household members for evidence of infection.
- If resources allow, contact the provider and/or refer the patient to a patient navigator to ensure the patient is receiving care services.
Cases of acute and chronic hepatitis B are nationally notifiable to CDC using a condition-specific event code ( Table 1-2 ). Cases can be re-classified or removed after the initial transmission to CDC if changes are made before surveillance data are finalized each year.
A confirmed acute hepatitis B case may be additionally enumerated as a new confirmed chronic hepatitis B case if a positive HBV detection test (HBsAg, HBeAg, or HBV DNA) is reported 6 months or longer after acute case onset, or if asymptomatic, after the initial positive test result. A case initially classified as confirmed or probable chronic hepatitis B that is determined to be an acute hepatitis B case during the same reporting year should be corrected and reclassified as a confirmed or probable acute hepatitis B case.
A probable acute hepatitis B case that confirms within the same reporting year (before the NNDSS close-out date) can be sent as an update to the same case, but if the case confirms following the initial reporting year, it should not be reported to NNDSS again. A probable chronic hepatitis B case that confirms within the same reporting year (before the NNDSS close-out date) can be sent as an update to the same case, but if the case confirms following the initial reporting year, it should not be reported to NNDSS again. If a case is determined to meet the acute or chronic probable case classification in one reporting year, if additional data are received in a following reporting year that would confirm the case, it should not be reported again to NNDSS.
All confirmed and probable cases are recommended to be transmitted to NNDSS for inclusion in CDC case counts in print criteria. Event conditions for a person should be linked in NNDSS using the same patient ID if submitting via HL7 messages or NBS. See Section 5.2 for additional guidance on transmitting multiple viral hepatitis events for the same person. See Section 3.5 for more information on hepatitis B case reporting and national notification.
Surveillance Activities for Chronic Hepatitis B
The following section provides best practice models for core and enhanced surveillance activities that jurisdictions should consider. Enhanced surveillance activities should be defined based on local priorities.
Best Practice Models For Core and Enhanced Chronic Hepatitis B Surveillance
Core Surveillance
Ascertainment and Reporting
- Create or maintain an electronic system for systematically collecting and storing hepatitis B laboratory results and other case data (e.g., demographic, risk, and clinical information) longitudinally for unique (deduplicated) persons.
- Establish or maintain a method to receive hepatitis B laboratory data and enter it into the hepatitis B surveillance system or registry, preferably through an automated ELR system. ELR is the most efficient way to receive these data, especially if the ELR system can automatically enter the hepatitis B records into the surveillance system.
- If an ELR system for other conditions is used in the jurisdiction, include hepatitis B.
- If ELR is not possible, work with high volume testers to receive data in another way (e.g., periodic flat files).
- Implement a process to review and classify cases within the surveillance system or registry.
- Extract data from the hepatitis B surveillance system or registry and transmit cases to CDC according to NNDSS procedures.
- Include extended data elements in addition to core data elements, when feasible.
Investigations
- Document local procedures for investigations, including defining priority populations, or identifying priority reports for investigation. See Section 3.2 for types of priority cases.
- Conduct investigations for priority reports or populations. See Section 3.6.5 for chronic hepatitis B case investigation guidance.
- Establish a protocol for identifying and investigating health care-associated infections or coordinate with the department’s health care-associated infections program. Use CDC’s health care-associated infection toolkit as a resource.
- Establish a protocol for identifying and investigating other unique exposures.
Quality Assurance
- Identify and review potential duplicate reports so that only the initial report of each chronic hepatitis B case is counted, and subsequent reports can be used for confirming cases or longer-term monitoring.
- Establish a process for cleaning, reviewing, and standardizing case data and test results.
- Assess case reports and test results for completeness and accuracy.
- Create an annual report, situational analysis, or other data product that can be widely shared with providers, advocates, and other public health professionals.
- Research existing health code/policy related to hepatitis B reporting and the process for changing such policies (if necessary).
- Identify who should report hepatitis B cases – health care providers, health care facilities, and/or laboratories.
- Determine what should be reportable. At a minimum, positive HBsAg, HBeAg, anti-HBc IgM, and HBV DNA results (including genotype) should be reportable. If possible, pregnancy status and concurrent ALT and total bilirubin results should be reported with positive hepatitis B laboratory results, and negative HBsAg and undetectable HBV DNA results should also be reportable. Jurisdictions that receive total anti-HBc laboratory results can use these results to clarify a person’s HBV infection status and confirm chronic cases in conjunction with evidence of positive tests for HBsAg or HBeAg.
- Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to treatment and care, as appropriate.
Other Data Sources
- Explore how to obtain access to additional sources of data (e.g., vital statistics person-level data).
Enhanced Surveillance (where resources permit) Ascertainment and Reporting
- Use additional data sources to identify cases not reported to the hepatitis B surveillance system or registry (e.g., vital records and medical records review). See Section 5.4 for a description of optional data sources.
- Conduct vital statistics death registry matches to update vital status and death date
- Conduct vital statistics birth registry matches to update pregnancy information, and to link gestational parent-infant pairs within the surveillance system in coordination with perinatal hepatitis B prevention program
- Conduct data linkage matches to other disease registries (e.g., HIV, cancer) to find missing information (e.g., race, ethnicity, co-infection, co-morbidities)
- Use a medical record extraction system to identify additional cases and pregnancy status that might not otherwise be reported or to improve efficiency of those reports
- Implement a process for updating cases in the surveillance system or registry with potential health care systems data to track patients along the care continuum (e.g., insurance and pharmacy claims data, hospital discharge data).
- Conduct chronic hepatitis B case investigations for additional priority populations. See Section 3.6.5 for chronic hepatitis B case investigation guidance.
- Establish a protocol for identifying and investigating other unique exposures, including clusters or outbreaks.
- Establish methods for identifying reactivations (i.e., determine whether the patient has a history of acute or chronic hepatitis B) to distinguish a new case of disease from reports or notifications that should not be enumerated as a new case.
- If personnel and other resources allow, consider in-depth investigation of a random sample of chronic cases to evaluate demographic variables, reason for testing, access and barriers to prevention and treatment services, and other questions of importance for viral hepatitis elimination activities in the jurisdiction. Personnel with expertise in study design, data collection, and analytic skills should develop and oversee these types of in-depth investigations.
- Assure linkage to care, treatment, and harm reduction services for priority populations where resources allow.
- Establish additional quality assurance processes for case reports and test results.
- Implement quality assurance improvement measures to ensure completeness and accuracy of case investigations and interpretation of laboratory results.
- Establish systems to identify and address decreases in laboratory reporting by test type volume and laboratory that might represent coding or transmission issues.
- Establish systems to identify and address deficiencies in provider reporting (e.g., incomplete or missing reports).
- Use data linkage matches to other disease databases/registries (e.g., HIV, HCV, and cancer) for analysis of co-infections and identification of receipt of care.
- Use vital statistics birth registry matches for analysis of infants born to HBV-infected gestational parents.
- Use death registry matches to describe hepatitis B-associated mortality.
- Create provider-level indicators (such as complete reporting, complete diagnostic testing, linkage to care, and treatment initiation) to work with providers to improve these outcomes.
- Identify methods for establishing surveillance-based chronic hepatitis B prevalence estimates.
- Identify and describe trends and disparities in liver cancer incidence and mortality.
- Create hepatitis B care continua, including determining and validating surveillance-based definitions for hepatitis B treatment and outcome indicators.
- Identify and describe trends and disparities along the care continuum (e.g., disparities in screening, viremia, linkage to care, and treatment initiation).
- Expand the data reports available to external partners.
- Use surveillance data and best practices from other jurisdictions to recommend health code changes related to reporting (e.g., obtaining non-positive test results), as allowable within the jurisdiction.
- Use surveillance data to support evidence-based for health code changes related to expanding access to syringe services programs and other harm reduction services for populations affected by hepatitis B, as allowable within the jurisdiction.
- Use analysis of surveillance data on trends and disparities to guide resource allocation and inform public health action, prioritizing those communities most disproportionately affected.
- Obtain access to supplemental data sources wherever possible and incorporate their usage into routine practices. See Section 5.4 for a description of optional data sources.
Considerations for Hepatitis B Cases who were Transplant Recipients
Organ and tissue donor-derived HBV infection is rare and commonly associated with IDU in a deceased donor ( 66 ). In the 2020 Public Health Service (PHS) guidelines ( 67 ), it is recommended that all organ recipients in the United States receive hepatitis B vaccination, pre-transplant testing for total anti-HBc, HBsAg and anti-HBs, and post-transplant testing for HBV DNA at 4–6 weeks.
However, clinical manifestations of post-transplant HBV infection can be delayed by many months after liver transplantation ( 68 , 69 ). As such, the 2020 PHS guidelines also recommend that health care providers caring for liver recipients consider conducting additional testing by HBV NAT or assessing signs or symptoms of liver injury (e.g., jaundice or elevated liver function tests) at one-year post-transplant. All donors are screened for total anti-HBc, HBsAg, and HBV DNA prior to organ procurement. In situations where the donor is known to be positive for any of these tests, recipient HBV infection is expected and does not require investigation by the HD beyond notifying CDC that the recipient case is donor derived. As these patients are already linked to testing and treatment, these infections are notifiable to CDC as new acute cases, but the jurisdiction need not investigate beyond indicating that the infection was donor-derived.
Organ transplantation from deceased donors dying of overdose and IDU has increased recently ( 70 ). To facilitate identification of suspected donor-derived cases of viral hepatitis, jurisdictional viral hepatitis surveillance programs should consider reaching out to transplant centers proactively. In most jurisdictions, there are a relatively small number of medical centers that perform transplantation ( 71 ). A listing of transplant facilities in the United States, including facility location and phone number, can be found on the Organ Procurement and Transplantation Network (OPTN) website ( 71 ).
Knowing whether the transplant center is using organs from deceased donors who injected drugs or who were positive for anti-HCV or HCV RNA is important, as this might increase the very small risk of donor-derived HBV infection ( 69 ). When donor-derived viral hepatitis is suspected, the transplant center is required to report the infection to the Disease Transmission Advisory Committee (DTAC) of OPTN, which often consults with CDC about a possible investigation. If CDC accepts the investigation, it is coordinated by the CDC Office of Blood, Organ, and Other Tissue Safety with consultation from CDC DVH. CDC only investigates selected reports of “unexpected” viral hepatitis transmission, meaning that both the donor and recipient tested negative for hepatitis B (including anti-HBc, HBsAg and HBV DNA, if available) prior to the transplant.
Investigation includes review of all laboratory and clinical data for donor and recipients and testing archived donor samples (e.g., serum, lymphocytes, and liver biopsy), if available, for HBV DNA. When the initial investigation is complete, CDC DVH contacts the public health jurisdiction to complete the rest of the investigation. Typically, there are two outstanding questions that only the public health jurisdiction can answer: 1) Did the recipient have any behavioral or other risks for hepatitis B (e.g., IDU) and 2) Does the jurisdiction have any ongoing investigations of health care-associated hepatitis B that might be related to this investigation?
Case classification in patients with a documented transplant should consider reports of laboratory test results prior to and post-transplant and potential health care exposures, if suspected. Table 3-4 outlines considerations for hepatitis B cases who were transplant recipients of a solid organ.
Table 3-4. Considerations for hepatitis B cases who received a solid organ from a donor*
| Organ Recipient Pre-transplant Laboratory Result† | Organ Recipient Post-transplant Laboratory Result† | Case Classification |
|---|---|---|
| Positive hepatitis B surface antigen (HBsAg), hepatitis B ‘e’ antigen (HBeAg), or HBV DNA | Positive HBsAg, HBeAg, or HBV DNA | Should not be considered a new case due to organ transplant, but rather an infection documented prior to transplant. To determine whether this case should be considered newly reported, follow . |
| Evidence of resolved prior infection: | Evidence of reactivation: | Should not be considered a new case, but reactivation of prior infection. Reactivation information should be appended to the case record of the existing case in the jurisdiction’s surveillance system. |
| Negative HBsAg Negative total anti-HBc | Positive HBsAg, HBeAg, or HBV DNA | Three major potential possibilities should be considered: Centers for Disease Control and Prevention (CDC)’s Division of Viral Hepatitis (DVH) might already have been notified and is available for consultation and coordination of investigation. |
| No prior HBV laboratory results‡ |
*All donors should be tested for total anti-HBc, HBsAg and HBV DNA prior to organ procurement ( 63 ). This table applies to recipients of organs from donors who tested negative for all these markers. †Because of the large number of tests performed on transplant recipients, irreproducible positive results are rarely reported. Investigators should evaluate all available results in context. CDC DVH is available for consultation. ‡Pre-transplant hepatitis B screening (total anti-HBc, HBsAg and anti-HBs) is recommended for all transplant recipient candidates in accordance with guidelines published by the US Public Health Service ( 63 ). If a transplant recipient does not have hepatitis B laboratory results prior to transplantation of an organ, consider following-up with the transplant facility to discuss appropriate pre-transplant hepatitis B screening protocols. Download of this table: PDF
Cases of viral hepatitis identified among living organ transplant donors and recipients should be submitted to NNDSS in a standardized way, when possible. The CDC case report forms used for NBS and HL7 transmission both include a reason for testing variable in the core section of the form. For HDs transmitting data via NBS or HL7, under the “reason for testing” field, “blood/organ donor screening” should be selected for organ donor cases; “other” should be selected for organ transplant recipient cases with a specification of “transplant recipient” in the free text for the “other reason for testing” field. For state and territorial HDs transmitting case data via NETSS, there is no field on the case report form to indicate that the case was an organ transplant donor or recipient.
Monitoring Infection Trends and Disease Outcomes Using a Person-Level Database and Supplemental Data Sources
A person-level surveillance database can support hepatitis B elimination efforts by allowing a jurisdiction to document hepatitis B laboratory results and testing history. By doing so, jurisdictions are able to:
- track the number of unique persons living with hepatitis B longitudinally, which can inform more accurate estimates of incidence and prevalence;
- identify pregnant people and infants for the PHBPP;
- identify and link people living with hepatitis B to medical care;
- evaluate the impact of public health and clinical services;
- match with secondary data sources (e.g., Vital Statistics, Medicaid, cancer registry, HIV registries); and
- provide information on the number of people at each phase of the hepatitis B care continuum to identify areas for improvement, for example, by supplementing surveillance data with clinical and pharmacy data.
Some of these monitoring capacities may only be possible in jurisdictions capable of capturing negative hepatitis B laboratory results. Jurisdictions are encouraged to require reporting of negative/undetectable HBV DNA results as these results can be a proxy indicator that people living with hepatitis B have been linked to care. This information can support accurate longitudinal surveillance and measurement of hepatitis B care continua. Linking a person-level surveillance database to other data sources not only allows for longitudinal monitoring of disease outcomes, but can also improve completeness of information in the surveillance system ( 68 ). Supplemental data sources are helpful for understanding the burden of co-morbidities (e.g., infection with HCV or HIV) by providing cross-sectional data over time and can inform interpretation of prevalence estimates. Section 5.4. describes supplemental data sources for HDs to consider.
Knowledge of a pregnant person’s HBV infection status is essential for preventing perinatal hepatitis B. The American College of Obstetrics and Gynecologists (ACOG) supports CDC’s recommendation that prenatal care providers should screen every pregnant person for HBV infection during an early prenatal visit, even if the person has already been vaccinated or tested for hepatitis B ( 73 ). HBV particles have also been detected in ova ( 74 , 75 ); though uncommon, the potential to vertically transmit HBV exists when an HBV-infected genetic parent donating ova elects to use a gestational carrier (i.e., surrogate). Transmission of HBV infection at birth leads to chronic infection in approximately 90% of infants who are not given immunoprophylaxis ( 76 ).
To improve the prevention and identification of perinatal hepatitis B and to facilitate the clinical care of pregnant and postpartum people, universal HBsAg screening during an early prenatal visit and treatment of infants born to HBsAg-positive gestational parents with hepatitis B immunoglobulin and hepatitis B vaccine at birth were recommended in 1988 by ACIP ( 73 ). To reduce perinatal transmission risks, it is recommended that pregnant people with an HBV DNA level >200,000 IU/mL receive antiviral therapy at 28–32 weeks of gestation and infants born to HBV-infected gestational parents receive HBIG at birth ( 50 , 73 ). However, it is estimated that approximately 1,000 newborns are infected annually despite these longstanding recommendations ( 77 ).
Surveillance should include monitoring HBV-infected pregnant people and monitoring infants born to them for receipt of immunoprophylaxis at birth, completion of the infant hepatitis B vaccination series, and PVST for evidence of infection (HBsAg-positivity or HBV DNA-positivity) and immunity (anti-HBs > 10 mIU/mL). PVST identifies infants who failed to respond to the hepatitis B vaccine and require re-vaccination.
The overall surveillance goals of hepatitis B during pregnancy include: 1) identifying pregnant people currently infected with HBV (as indicated by the presence of HBsAg or HBV DNA), and 2) among HBV-infected people of childbearing age with childbearing potential, identifying those who are currently pregnant or who have recently delivered a live birth to identify exposed infants for referral to the PHBPP.
Perinatal hepatitis B surveillance relies on screening for HBsAg during each pregnancy and conducting the appropriate follow-up tests on infants born to HBV-infected gestational parents. The overall goals of perinatal hepatitis B surveillance are to identify exposed infants and evaluate the effectiveness of the PHBPP to prevent perinatal transmission, and also the following:
- identify HBV-infected people of childbearing age with childbearing potential to link them to care to prevent infant HBV exposure during future pregnancies;
- provide data to improve assessment of the burden of perinatal hepatitis B;
- evaluate health outcomes of HBV-infected infants;
- educate clinicians and guardians on HBV transmission, clinical progression, and treatment; and
- measure the rate of progression to chronic hepatitis B, as determined by HBsAg-positivity or by the detection of HBV DNA after 24 months of age.
Surveillance data are used in the following ways to accomplish the above goals:
- Identifying HBV-infected pregnant people to prevent perinatal HBV transmission.
- Number of HBV-infected pregnant people identified
- Number of births to HBV-infected pregnant people identified
The following key indicators are used for exposed infants:
- Number and percentage of exposed infants who receive first dose of hepatitis B vaccine and HBIG within 12 hours of birth.
- Number and percentage of exposed infants who receive HBIG and complete the hepatitis B vaccine series by 6 months of age.
- Number who tested positive for HBsAg
- Number with anti-HBs titer level of <10 mIU/mL
- Number who test positive for HBsAg (reported as chronic hepatitis B)
- Number of erroneous tests performed (e.g., testing performed outside of the recommended age windows or wrong test ordered)
- Number of exposed infants who require additional doses of vaccine (non-responders)
- Number of exposed infants lost to follow-up
- Assessing the frequency and evaluating the causes of missed opportunities . This includes evaluating missed opportunities for testing during pregnancy and for antiviral therapy, when indicated. For HBV-exposed infants, this includes failure to provide timely immunoprophylaxis and vaccination failure. It is recommended that all exposed infants receive the first dose of the hepatitis B vaccine and HBIG within 12 hours of birth, even those with low birth weight (i.e., birth weight <2,000 g). Investigation of perinatal hepatitis B cases should evaluate causes of possible breakthrough infections and should include obtaining sera from the infant and gestational parent to test for the presence of HBV variants.
- Monitoring adherence to screening recommendations among pregnant people. Surveillance programs should ideally collect negative HBV laboratory results. Alternatively, surveillance can help track changes in hepatitis B incidence and be used to implement quality measures to monitor adherence to screening recommendations.
- Monitoring trends in disease incidence and prevalence among people of childbearing age with childbearing potential. Knowing the incidence and prevalence of hepatitis B in the population who are or can become pregnant is critical to the prevention and control of hepatitis B, and this population should be assessed independently from surveillance in the general population.
*Note that the outcome indicators for PHBPP are slightly different from the ACIP recommendations ( 56 ).
Surveillance Case Definition
No CDC/CSTE surveillance case definition exists for HBV infection during pregnancy. Instead, these cases should be classified in accordance with the CDC/CSTE acute and chronic hepatitis B case definitions (see Section 3.6.3 ).
Table 3-5 depicts the surveillance case definition for perinatal hepatitis B, adopted by CSTE and CDC in 2017 ( 63 , 64 ). See Appendix C for classification scenarios of cases of perinatal hepatitis B.
Table 3-5. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for perinatal hepatitis B, 2017
| Criteria Type | Ctriteria |
|---|---|
| Demographic | |
| Clinical | |
| Laboratory* | Child 24 months of age with evidence of hepatitis B as shown by the following laboratory results: from 1–24 months of age only if at least 4 weeks after last dose of Hep B vaccine |
| Epidemiologic Linkage | |
| Case Status | Classification |
| Confirmed Perinatal* | |
| Probable Perinatal* | |
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to care, as appropriate. †Positive HBsAg results obtained from infants ≤9 months of age who received hepatitis B vaccine should not be interpreted as positive due to the potential for transient HBsAg positivity. Downloads of this table: PDF | PPT
HBsAg test results obtained from infants ≤1 month of age, and HBeAg and HBV DNA results from those ≤9 months of age should not be used for classification. Cases in the specified age range that are known to have been exposed to HBV through health care and not perinatally should also be classified under the 2012 acute and chronic hepatitis B case definition. The event date of the perinatal hepatitis B case should be based on the earliest relevant laboratory test collection date within the test-specific age window.
To facilitate identification of HBV infection during pregnancy, the following measures are recommended:
- Screen for HBsAg during each pregnancy as part of prenatal care at an early prenatal visit (i.e., during the first trimester)
- For pregnant people who are isolated total anti-HBc positive, check HBV DNA status to determine if occult HBV infection or HBsAg mutant infection is present
- Document maternal HBV infection status on newborn metabolic screening card or birth certificate
- Report laboratory results indicating HBV infection for all pregnant people to the appropriate health jurisdiction
- Those whose HBsAg status is unknown
- Those with clinical hepatitis
- Those with high risk behaviors (e.g., history of recent or current IDU, multiple sexual partners within the past 6 months or an HBsAg-positive sexual partner, or evaluation or treatment for a sexually transmitted infection)
- Report admission of HBV-infected pregnant people to delivery facility
- Test for HBV infection status during each pregnancy at an early prenatal visit (i.e., during the first trimester)
- Receive and integrate electronic laboratory reports, electronic health records, and facsimiles into a disease surveillance system
- Perform enhanced surveillance methods to identify previously unreported HBV-infected people who have recently given birth by comparing birth certificate data to known HBV-infected cases in the disease surveillance system
- Determine pregnancy status of all HBsAg-positive people of childbearing age with childbearing potential
- Determine pregnancy status for all existing cases of hepatitis B among people of childbearing age with childbearing potential
The following steps are recommended to facilitate identification of perinatally HBV-exposed infants:
- Ensure delivery facilities have standing orders to report all births to HBsAg-positive or HBV DNA-positive gestational parents to local PHBPP jurisdiction
- Test all exposed infants for HBsAg and anti-HBs (PVST) at 9–12 months of age
- Routinely report the hepatitis B PVST results of exposed infants to the local health jurisdiction
Figure 3-4 illustrates a potential approach for perinatal hepatitis B case ascertainment and classification. Specific procedures vary by jurisdiction, but should generally follow the scheme below, in accordance with the CDC/CSTE Position Statement for the 2017 perinatal hepatitis B case definition ( 63 , 64 ).
Figure 3-4. Process for perinatal hepatitis B case ascertainment and classification
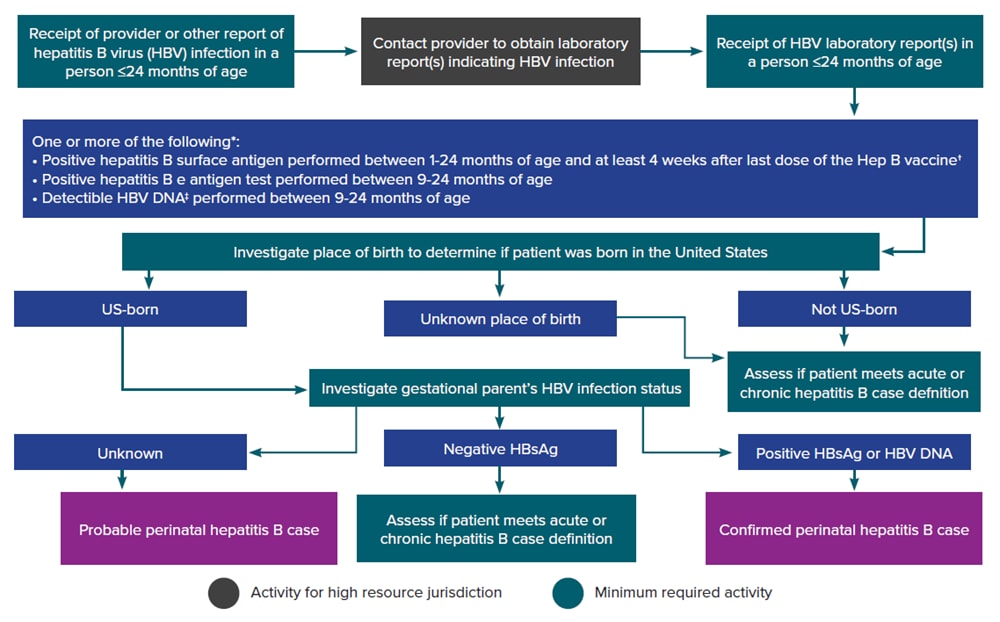
*Surveillance programs should provide prevention programs with information on people who have positive test outcomes for post-test counseling and referral to care, as appropriate. HBsAg test results obtained from infants ≤1 month of age and hepatitis B e antigen and HBV DNA results obtained from those ≤9 months of age should not be used for classification. Cases among children < 24 months of age who are known to have been exposed to HBV through health care (not perinatally) should be reported according to the 2012 acute and chronic hepatitis B case definitions. †Positive HBsAg results obtained from infants ≤9 months of age who received hepatitis B vaccine should not be interpreted as positive due to the potential for transient HBsAg positivity. ‡Nucleic acid testing for HBV DNA, including qualitative, quantitative, and genotype testing. Downloads of this figure: PDF | PPT
The following elements can inform investigation and management of HBV-infected pregnant people and infants:
- Demographic information . For the pregnant person, obtain date of birth, current gender, race, ethnicity, residential address (including zip code), insurance status, country of birth, and primary language spoken. For the infant, obtain the date, time, and place of birth, birth weight, sex, race, ethnicity, and insurance status. The contact information of the legal guardian(s) should also be collected.
- Patient and health care provider information . Includes prenatal care provider’s name and phone number to coordinate follow-up HBV DNA testing and treatment, if indicated. The contact information of the infant’s health care provider (to obtain PVST results) and legal guardian(s) as well as adoption or foster care status should also be collected.
- Delivery information . Includes the expected and actual due dates and the expected and actual delivery facilities.
- Diagnostic test results . Obtain documentation of positive HBV test results for both gestational parent and infant; obtain anti-HBs test result(s) for the infant.
- Clinical features . For the pregnant person, document the presence of symptoms and jaundice. Most infants with hepatitis B are asymptomatic. Obtain documentation of the gestational parent’s HBV DNA level and whether antiviral medication was administered during 28–32 weeks of gestation.
- Immunization and prophylaxis history . Ascertain the date and time for all administered doses of hepatitis B vaccine and HBIG.
- Epidemiologic link . For the infant, confirm birth to an HBV-infected gestational parent.
- Reporting information . Date reported to health jurisdiction, date of diagnosis, date investigation initiated, date of first contact with patient and/or health care provider, and date referred for medical evaluation.
- Education and referral for follow-up. Provide education to the patient/provider regarding the role of PHBPP, importance of immunoprophylaxis for the infant within 12 hours of birth, and timely completion of the hepatitis B vaccine series and PVST. Determine medical care provider for HBV-infected pregnant cases and educate on the importance of regular care and monitoring of hepatitis B, even after delivery.
Case Investigation Prioritization The following factors should be considered of high priority for investigation and follow-up:
- Investigation and follow-up should occur during pregnancy or as soon as possible thereafter. Successful follow-up might be more likely if contact information is utilized sooner. Early identification and timely follow-up will facilitate the prevention of perinatal HBV transmission.
- Investigation and follow-up should occur for those who can become pregnant and those whose pregnancy status is unknown who are co-infected with HIV/HCV/STIs or who have high HBV DNA levels (> 200,000 IU/mL).
Case Management
HBV-infected gestational parents and their infant(s) should be tracked in a surveillance database/system that can track case management processes and allow for sharing and/or linking parent and child events. The system should allow for each pregnancy to be considered unique and the pregnancy-specific data to be captured and maintained. It should also contain a mechanism to track hepatitis B vaccine doses given to the infant to ensure the proper number and spacing of doses.
HBV-infected pregnant people should be enrolled in the PHBPP. Upon enrollment, the hepatitis B coordinator should undertake the following case-management actions:
- Obtain a copy of the original laboratory results to provide to the anticipated delivery facility and infant’s anticipated health care provider. Original laboratory reports are strongly recommended to prevent misinterpretation or transcription errors.
- Notify of maternal HBV infection status (i.e., positive HBsAg or HBV DNA) and communicate the need for timely vaccination and PVST.
- For low birth weight babies (i.e., those <2,000 g), the initial dose of hepatitis B vaccine should still be administered as early as possible, but should not be counted as part of the vaccine series. The infant should receive 3 additional doses of the vaccine, typically starting at 1 month of age.
- PVST should include HBsAg testing to ensure the infant has not become infected and anti-HBs testing to ensure that immunity has been conferred (i.e., anti-HBs > 10 mIU/mL). Table 3-7 lists the most common laboratory codes for PVST.
- If the child’s anti-HBs titer is still <10 mIU/mL, complete the second revaccination series and retest for HBsAg and anti-HBs.
- If the anti-HBs titer is still <10 mIU/mL after full vaccination series, the child is considered a vaccine non-responder. Provide guidance to the parent(s) or legal guardian(s) of the child that remains susceptible. For more detailed guidance, see the Epidemiology and Prevention of Vaccine-Preventable Diseases from The Pink Book.
- It is recommended that pregnant people with an HBV DNA level >200,000 IU/mL start antiviral therapy at 28–32 weeks of gestation to reduce perinatal transmission risks ( 50 ).
- Document the gestational parent’s HBV DNA level at the time of delivery and whether antiviral medication was received during pregnancy.
- Ensure anticipated birth facility is aware of the pregnant person’s HBV infection status and anticipated due date and will report to local jurisdiction’s PHBPP upon delivery of infant.
- Parent or provider to confirm anticipated delivery facility
- provide timely post-exposure prophylaxis for infant,
- report birth and prophylaxis information to public health, and
- administer the first dose of the hepatitis B vaccine and HBIG in separate anatomical sites.
- Document the first dose of hepatitis B vaccine given within 12 hours of birth.
- Document completion of hepatitis B vaccine series in accordance with ACIP recommendations.
- confirmation of chronic hepatitis B (tests positive for HBsAg after 24 months), as spontaneous clearance is possible;
- assessment of evidence of chronic liver disease; and
- assessment of severity of disease and possible treatment according to current practice guidelines in consultation with, or by referral to, a specialist knowledgeable in this area.
- All parents should be provided with education regarding HBV vertical transmission and testing recommendations. Providers should also be provided with information on HBV transmission risk to the infant, hepatitis B testing recommendations (PVST), and HBV infection clinical care. Education should be provided according to evidence-based guidelines and include perinatal transmission risk, testing for the infant, and current treatment recommendations.
The following case management actions are recommended for delivery facilities:
- Report gestational parent’s HBV infection status (i.e., positive HBsAg or HBV DNA) on infant’s birth certificate and metabolic screening card (as applicable)
- Report birth to an HBV-infected gestational parent to appropriate health jurisdiction. This can be achieved by completing the jurisdiction-specific reporting form with all requested information
- Facilities that need information regarding immunoprophylaxis for infants born to HBV-infected gestational parents can contact the Perinatal Hepatitis B Prevention Program in their state or territory.
Case management is considered completed when the
- child has evidence of immunity,
- child has moved to another jurisdiction and notification to that jurisdiction and PHBPP has been completed, or
- gestational parent-infant pair is lost to follow-up.
Sources of data on immunization and PVST include, but are not limited to
- jurisdiction’s immunization registry/information system,
- report from infant’s health care provider, and
- electronic health and laboratory records.
Table 3-6. Common laboratory codes for hepatitis B post-vaccination serologic testing
| Laboratory | Hepatitis B Surface Antigen | Antibody to Hepatitis B Surface Antigen |
|---|---|---|
| Affiliated Medical Services | 5196-1 | 10900-9 |
| LabCorp | 006510 | 006530 |
| Mayo Medical Labs | 9013 | 8254 |
| Quest Diagnostics | 498 | 8475 |
Downloads of this table: PDF | PPT
HBV infection during pregnancy should be a reportable event to the HD. Although no specific NNDSS event exists for hepatitis B during pregnancy, CDC recommends classifying these cases as acute or chronic using the appropriate event code ( Table 1-2 ) in accordance with the CDC/CSTE case definitions, and transmitting the pregnancy status to NNDSS for all cases among people of childbearing age with childbearing potential to allow for national tracking. At the jurisdiction-level, there may be classification category specifically for hepatitis B during pregnancy. Cases of perinatal hepatitis B are nationally notifiable to CDC and are submitted using a condition-specific event code ( Table 1-2 ). A case classified as perinatal hepatitis B can be additionally enumerated as a confirmed case of chronic hepatitis B if a positive HBV viral detection test (HBsAg, HBeAg, or HBV DNA) is obtained after the case is >24 months of age.
Back to Section Index
- Overview of Viral Hepatitis
- Statistics & Surveillance
- Populations & Settings
- State and Local Partners & Grantees
- Policy, Programs, and Science
- Resource Center
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Case studies of the hepatitis B patient: a panel discussion
Affiliation.
- 1 Cedars Sinai Medical Center, Los Angeles, California, USA.
- PMID: 10895442
In recent years, there have been significant advances in the treatment of patients with hepatitis B who are candidates for liver transplantation. This includes the prevention and management of hepatitis B posttransplantation. However, there is no established protocol for treating these patients. Ultimately, the goal would be to have patients HBV-DNA negative pretransplantation and then to prevent HBV recurrence posttransplantation to help ensure their quality of life. Several clinical case scenarios are presented and possible treatment solutions have been suggested. The timing of a transplant is critical due to the risk of viral mutation while the patient is on a nucleoside analogue antiviral agent and waiting for an organ. One successful option might be to start therapy pretransplant and continue it posttransplant. Combination therapy appears to provide the most effective course of treatment. This should include a nucleoside analogue and patients should be covered with hepatitis B immune globulin throughout the course of therapy. Several other variations of combination therapy are discussed, but many clinical issues remain to be resolved. Guidelines for future studies designed to answer these questions are proposed.
PubMed Disclaimer
Similar articles
- Management of hepatitis B in liver transplant patients. Mohanty SR, Cotler SJ. Mohanty SR, et al. J Clin Gastroenterol. 2005 Jan;39(1):58-63. J Clin Gastroenterol. 2005. PMID: 15599213 Review.
- Posttransplantation: future therapies. Peters MG, Shouval D, Bonham A, Vierling JM, Lok AS. Peters MG, et al. Semin Liver Dis. 2000;20 Suppl 1:19-24. Semin Liver Dis. 2000. PMID: 10895440 Review.
- [Prevention and treatment of hepatitis B virus reinfection after liver transplantation]. Liu J, Wu GC, Zhang ZT, Wu P, Zhang D, Sun MC, Gao DC, Wang Y, Jia JD, Wang BE. Liu J, et al. Zhonghua Wai Ke Za Zhi. 2005 Aug 1;43(15):976-9. Zhonghua Wai Ke Za Zhi. 2005. PMID: 16194352 Chinese.
- Liver transplantation in viral hepatitis: prevention of recurrence. Roche B, Samuel D. Roche B, et al. Best Pract Res Clin Gastroenterol. 2008;22(6):1153-69. doi: 10.1016/j.bpg.2008.12.004. Best Pract Res Clin Gastroenterol. 2008. PMID: 19187873 Review.
- Prophylaxis and treatment of recurrent hepatitis B after liver transplantation. Lo CM, Fan ST, Liu CL, Lai CL, Wong J. Lo CM, et al. Transplantation. 2003 Feb 15;75(3 Suppl):S41-4. doi: 10.1097/01.TP.0000047027.68167.07. Transplantation. 2003. PMID: 12589139 Review.
- Recent publications by ochsner authors. [No authors listed] [No authors listed] Ochsner J. 2001 Apr;3(2):117-9. Ochsner J. 2001. PMID: 21765731 Free PMC article. No abstract available.
Publication types
- Search in MeSH
LinkOut - more resources
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Public Health Response to Hepatitis B Exposure
A case study on gaps and opportunities to improve postexposure care.
Freeland, Catherine MPH * ; Cohen, Chari DrPH, MPH * ; Collier, Melissa G. MD, MPH †
From the * Hepatitis B Foundation, Doylestown, PA and
† Centers for Disease Control and Prevention, Atlanta, GA.
Correspondence to: Catherine Freeland, MPH, Hepatitis B Foundation, 3805 Old Easton Rd, Doylestown, PA 18902. E-mail: [email protected] .
The authors have no funding of conflicts of interest to disclose.
The anonymous individual described herein provided written consent for the publication of this case report.
In 2015, an estimated 257 million people were living with chronic hepatitis B virus (HBV) globally, with estimates in the United States as high as 2.2 million. 1–3 Only 25% of infected individuals in the United States are aware of their HBV infection, and less than 10% are able to access care and treatment. 2 If left untreated, chronic HBV infection can lead to serious liver complications, including liver cirrhosis and liver cancer, in up to 25% of infected individuals. 4,5 Hepatitis B can be transmitted through direct contact with infected blood, unprotected sex with an infected individual, use of illicit drugs, contaminated or unsterile injecting equipment, and most commonly worldwide from an infected mother to her newborn during childbirth (or during pregnancy in women with high viral load). 5 Likely attributed to the recent opioid epidemic, Centers for Disease Control and Prevention (CDC) has reported an increase in cases of acute HBV infections in 3 states among non-Hispanic whites aged 30 to 39 years who reported injection drug usage. 6 The opioid epidemic increases the risk of potential exposure to hepatitis B, and our public health system must be ready to provide timely postexposure prophylaxis (PEP).
Hepatitis B vaccination is highly effective at preventing HBV infection and is recommended in the United States for all infants at birth and adults considered to be high risk for hepatitis B (including persons who inject drugs, persons infected with human immunodeficiency virus, men who have sex with men, healthcare personnel, and persons with diabetes). 7,8 The hepatitis B vaccine is generally a 3-dose series administered on a 0, 1, and 6-month schedule. 8
Centers for Disease Control and Prevention has published PEP guidelines for both occupational and nonoccupational exposure to HBV. The guidelines, which are intended to increase immune response and prevent HBV infection after exposure, may consist of hepatitis B immune globulin (HBIG) and HBV vaccine, depending on the exposed person's vaccination status and source of exposure. 8,9 These guidelines state that after nonoccupational exposure to an HBV infected person, (1) persons who have written documentation of a complete hepatitis B vaccine series and who did not receive postvaccination testing should receive a single vaccine booster dose, (2) persons who are in the process of being vaccinated but who have not completed the vaccine series should receive the appropriate dose of HBIG and should complete the vaccine series, and (3) unvaccinated persons should receive both HBIG and hepatitis B vaccine as soon as possible after exposure (preferably within 24 hours), and the hepatitis B vaccine series should be completed in accordance with the age-appropriate vaccine dose and schedule. 8,9
CASE PRESENTATION
A 55-year-old Caucasian female was exposed to HBV through unprotected sexual contact on October 1, 2017 ( Fig. 1 ). The woman's partner had used intravenous drugs in the past and was chronically HBV infected. The woman had received 2 doses of the hepatitis B vaccine, with the first dose in June 2017 and the second dose in August 2017. Her third dose was scheduled for December 2017.

The woman went to her local health department within 24 hours of the exposure, but the recommended adult HBIG dosage (5 mL) was unavailable. She then called the Hepatitis B Foundation consultation phone line. The Hepatitis B Foundation assisted by contacting hospitals near the woman's residence to find the appropriate postexposure HBIG dose. The first hospital contacted was “uncomfortable” with the administration of HBIG and felt it was the responsibility of the local health department. The second hospital contacted carried the correct HBIG dose, but when the patient arrived, the hospital emergency department refused to administer it, asserting that HBIG administration was not done in the emergency setting and “it never has been done in this hospital.” The Hepatitis B Foundation contacted the state viral hepatitis program for assistance with providing the woman with the care she needed. Unfortunately, the state viral hepatitis program representative was uncomfortable with being asked to advocate for the patient with the hospital, primarily because “CDC's guidelines are a bit confusing and can seem to contradict themselves at times.” The woman ultimately did not receive HBIG.
Three days later, the woman received a PEP dose of HBV vaccine at her local health department because the hospital refused to administer it. The woman also plans to complete the series as scheduled in December 2017, receiving a total of 4 doses of hepatitis B vaccine. Although the woman did receive a dose of PEP hepatitis B vaccine, she was unable to receive the adequate HBIG.
The CDC PEP guidelines for nonoccupational exposure explicitly state: “persons who are in the process of being vaccinated but who have not completed the vaccine series should receive the appropriate dose of HBIG and should complete the vaccine series, preferably within 24 hours.” 8,9 However, the HBIG dose can still be effective in preventing infection if given within 14 days of exposure but is unlikely to have any effect after 14 days. This patient did not receive any HBIG and is at risk for developing HBV infection because of the confusion around administration. It is concerning that an incompletely vaccinated individual was unable to access HBIG after an exposure to HBV, even after seeking out HBIG and involving the Hepatitis B Foundation, 2 hospitals, and viral hepatitis program staff in her state. Although the CDC guidelines are straightforward, this event demonstrates a lack of knowledge of the CDC PEP guidelines and understanding of who is responsible for providing this very important service to patients. With the rise of the opioid epidemic and the increasing number of cases of acute HBV infections in the United States, clinical providers in emergency department settings should be prepared to administer HBV PEP and recognize this as their responsibility. In addition, providers should know how to report both exposures and hepatitis B infections to local and state health departments to assist in case management and prevention of additional cases. 10 All health departments and viral hepatitis program staff should have a clear understanding of the CDC HBV PEP guidelines, so that they can help individuals who need assistance finding HBIG at a site near them and advocate for patients who are unable to obtain PEP services in healthcare facilities.
- Cited Here |
- View Full Text | PubMed | CrossRef |
- Google Scholar
- PubMed | CrossRef |
- + Favorites
- View in Gallery
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- eClinicalMedicine
- v.76; 2024 Oct
- PMC11416547

Effectiveness and implementation of decentralized, community- and primary care-based strategies in promoting hepatitis B testing uptake: a systematic review and meta-analysis
Thanh van kim.
a Center of Excellence for Liver Disease in Viet Nam, Johns Hopkins School of Medicine, Baltimore, MD, USA
b Department of Epidemiology, Pham Ngoc Thach University of Medicine, Viet Nam
c Division of Gastroenterology and Hepatology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA
Trang Ngoc Doan Pham
d School of Public Health, University of Illinois at Chicago, Chicago, IL, USA
Minh Huu Nhat Le
e International Ph.D. Program in Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
f Research Center for Artificial Intelligence in Medicine, Taipei Medical University, Taipei, Taiwan
Phuong Thi Nguyen
g University of Health Sciences, Vietnam National University Ho Chi Minh City (VNUHCM-UHS), Binh Duong, Viet Nam
Ha Thi Nguyen
Dan xuan nguyen.
h Boston University School of Public Health, Boston, MA, USA
Chelsea Cao
Ahmet gurakar, christopher j. hoffmann.
i Division of Infectious Diseases, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA
j Department of Health, Behavior, and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Associated Data
Expanding chronic hepatitis B (CHB) testing through effective implementation strategies in primary- and community-care setting is crucial for elimination. Our study aimed to determine the effectiveness of all available strategies in the literature and evaluate their specifications and implementation outcomes, thereby informing future programming and policymaking.
We conducted a systematic review and meta-analysis (PROSPERO CRD42023455781), searching Scopus, Embase, PubMed, and CINAHL databases up to June 05, 2024, for randomized controlled trials investigating primary- and community-care-based implementation strategies to promote CHB testing. Studies were screened against a priori eligibility criteria, and their data were extracted using a standardized protocol if included. ROB-2 was used to assess the risk of bias. Implementation strategies' components were characterized using the Behavior Change Wheel (BCW) framework. Random-effect models were applied to pool the effectiveness estimate by strategy. Mixed-effect meta-regression was employed to investigate if effectiveness varied by the number of strategy's BCW components.
7146 unique records were identified. 25 studies were eligible for the review, contributing 130,598 participants. 19 studies were included in the meta-analysis. No studies were conducted in low-and-middle-income countries. Implementation outcomes were reported in only ten studies (40%). Community-based strategies included lay health workers-led education (Pooled Risk Difference = 27.9% [95% Confidence Interval = 3.4–52.4], I 2 = 99.3%) or crowdsourced education on social media (3.1% [−2.2 to 8.4], 0.0%). Primary care-based strategies consisted of electronic alert system (8.4% [3.7–13.1], 95.0%) and healthcare providers-led education (HCPs, 62.5% [53.1–71.9], 27.5%). The number of BCW-framework-driven strategy components showed a significant dose-response relationship with effectiveness.
Interpretation
HCPs-led education stands out, and more enriched multicomponent strategies had better effectiveness. Future implementation strategies should consider critical contextual factors and policies to achieve a sustainable impact towards hepatitis B elimination targets.
Tran Dolch Post-Doctoral Fellowship in Hepatology, Johns Hopkins University School of Medicine, Baltimore MD, USA.
Research in context
Evidence before this study.
Developing implementation strategies to expand chronic hepatitis B (CHB) testing is pivotal to addressing the impediment to the CHB treatment and elimination. We conducted a systematic review using PubMed, Embase, CINAHL, and Scopus for evidence on implementation strategies to expand CHB testing to community and primary care settings. Our search encompassed studies in English published until June 05, 2024, using key terms related to the hepatitis B virus, testing, primary health care, community care, clinical trials, and intervention models. We found six relevant systematic reviews, which focused on the effectiveness of optimizing different steps in the viral hepatitis care continuum of some implementation strategies (e.g., mHealth intervention, health worker intervention) on specific populations (e.g., high-risk or migrant populations in high-income countries). However, a notable knowledge gap persists concerning the effectiveness coupled with implementation features of strategies aimed explicitly at CHB testing in primary and community care settings worldwide.
Added value of this study
This study advances the CHB elimination efforts with actionable insights on the real-world effectiveness and implementation challenges of various strategies to promote CHB testing in primary and community care settings. It highlights the considerable evidence gap in low- and middle-income countries (LMICs). Among the assessed strategies, CHB education delivered by healthcare providers, coupled with decentralized testing, emerged as particularly effective. Notably, the specification of implementation strategies is considered essential. Strategies enriched with a greater number of framework-driven mechanisms, such as those exemplified by Lay Health Workers-driven CHB education, demonstrated larger effect sizes on CHB testing. However, it is concerning that less than half of the studies adequately evaluated the implementation outcomes of these strategies. This underreporting may hinder the generalizability of the findings and applicability of the strategies in various settings to achieve sustainable impact.

Implications of all the available evidence
Hepatitis B stands at a crossroads between a future of continued inequal access to care and a path towards rapid expansion of program scale-up for testing and subsequent linkage to care. To catalyze for the latter, our study identifies effective strategies, such as CHB education delivered by healthcare providers and decentralized testing in primary care settings. These strategies have demonstrated real world effectiveness in increasing testing rates for early detection and treatment initiation of CHB. Another important implication is the recognition of the evidence gap in LMICs, highlighting the need for targeted research and interventions in these settings. Addressing this gap is essential for ensuring equitable access to CHB testing and care services worldwide. Lastly, strategies can be synergistically bundled and integrated with policy and health system-level factors to maximize impact and sustainability. Taken together, the findings of the study provide valuable insights for policymakers, healthcare providers, and researchers involved in CHB elimination efforts.
Introduction
Despite global efforts and World Health Organization and United Nations calls for progress, chronic hepatitis B (CHB) remains a substantial global burden. In 2019, there were close to 300 million people living with CHB, accounting for a worldwide prevalence of 3.84%. 1 In the same year, 1,525,800 people developed acute hepatitis B, and 821,100 CHB-associated deaths occurred. 1 Deaths from CHB annually exceed the number of deaths from HIV, TB, or malaria. 2
In 2020, the global elimination targets of a 30% reduction in new infections and a 10% reduction in mortality were unmet, primarily due to gaps in the CHB care cascade. 1 Diagnosis and treatment coverage in 2019 were estimated at 10% and 2%, respectively. 1 For low and middle income countries (LMICs), the inequities in access to testing and treatment are even more amplified. 3 Rapid acceleration and expansion of diagnosis and treatment are demanded in the later stage of the elimination endeavor from 2020 to 2030.
Testing is the essential first step in the CHB care cascade. Despite the availability of accurate tests and global calls to expand CHB testing, delivery progress has been insufficient over the past years. 1 Effective strategies are needed and need to be used at scale for implementation to successfully bridge from policies to practice to reach the millions of people living with undiagnosed CHB. Decentralized testing in community- and primary-care settings have the potential to reduce the coverage gaps, as exemplified with robust evidence in HIV and HCV literature. 4 , 5
Various strategies have been tested and implemented to optimize the general CHB care cascade. Some systematic reviews attempted to evaluate the effectiveness of specific strategies, such as mHealth or education, in some particular populations, such as high-risk or migrant people. 6 , 7 , 8 , 9 , 10 , 11 However, a notable knowledge gap persists in understanding the effectiveness of implementation strategies aimed explicitly at CHB testing in the context of primary and community care settings. Importantly, further synthesis of strategy specifications (i.e., components or mechanisms) and their implementation outcomes appears necessary to strengthen the contextualization of meta-analytic effectiveness findings. 12
Taken together, we sought to fill this gap by conducting a systematic review and meta-analysis to 1) determine the effectiveness of community-based and primary care-based implementation strategies for promoting CHB testing in the general population, 2) evaluate the specifications of implementation strategies and its implementation outcomes, and 3) examine whether the enrichment of strategies’ components is related to an increase in their effectiveness. Through this study, we aim to provide actionable insights into real-world effectiveness and implementation challenges, thereby guiding future CHB testing programs toward achieving elimination goals.
Conceptual framework
Practically, we employed the Behavior Change Wheel (BCW) framework to characterize components within implementation strategies into strategy functions and sources of behavior to be included in qualitative and quantitative analyses. 13 This application facilitated the deeper understanding of mechanisms behind each implementation strategy, which were central to the second and third aims of our study. Strategy functions represent the tools employed in implementation strategies to influence healthcare providers (HCPs) or patients in ordering or obtaining CHB testing (the “what”). Targeted sources of behavior refer to why HCPs or participants decide to order or obtain CHB testing (the “why”). We chose the BCW because of its high validity, as evidenced by cross-validation in different settings, and its transparent coding guideline. 14 The definitions of BCW framework's components are provided in Table 1 .
Table 1
Definition of implementation strategy mechanisms delineated through Behavior Change Wheel framework.
| Mechanism | Definitions |
|---|---|
| Strategy functions: the tools employed in implementation strategies to influence HCPs or participants in ordering or obtaining CHB testing (the “what”) | |
| Increasing knowledge or understanding | |
| Using communication to induce positive or negative feelings or stimulate action | |
| Creating an expectation of reward and incentives. | |
| Creating an expectation of punishment or cost. | |
| Imparting knowledge and skills. | |
| Using rules or laws to reduce the opportunity to engage in the target behavior (or to increase the target behavior by reducing the opportunity to engage in competing behaviors) | |
| Changing the physical or social context | |
| Providing a role model or an example for people to aspire to or imitate. | |
| Increasing means/reducing barriers to increase capability (beyond education and training) or opportunity (beyond environmental restructuring. | |
| Sources of behavior: the reasons why HCPs or participants decide to order or obtain CHB testing (the “why”) | |
| Having the physical skills, strength, or stamina to order HBV tests (for health care workers) or obtain HBV tests. | |
| Having the knowledge, psychological skills, strength, or stamina to order/obtain HBV testing. | |
| The environment allows or facilitates ordering/obtaining HBV testing in terms of time, triggers, resources, locations, physical barriers, etc. | |
| Interpersonal influences, social cues, and cultural norms (from peers and networks) allow or facilitate ordering/obtaining HBV testing, etc. | |
| Self-conscious planning and evaluation (beliefs about what is good and bad) about HBV testing | |
| More intrinsic, such as emotional reactions, desires (wants & needs), impulses, inhibitions, drive states, and reflex responses regarding ordering and obtaining HBV testing. | |
Eligibility criteria
We (TVK, TNDP, CJH, DYD) developed preliminary criteria for study selection. Based on the team's experience, publications considered most relevant to the preliminary criteria were identified. TVK piloted the preliminary criteria on those publications and looked for more relevant publications through reference lists and citation tracing. During this pilot phase, the eligibility criteria were finalized during whole-team discussions. The eligibility criteria were as follows.
We retrieved studies conducted on populations who were recommended for CHB testing (e.g., people who were born or lived in high prevalence countries, institutionalized populations, vulnerable or marginalized populations, people who inject drugs, people who live with HIV, or males who have sex with males). These populations also included children and pregnant women. We excluded studies focused solely on blood donors, or cancer patients.
The primary focus of this study is on primary care and community care settings. Primary care settings encompass healthcare facilities that serve as the initial point of contact for individuals seeking care for general or specific health concerns within the health system. On the other hand, the community care setting extends beyond clinical environments and is primarily provided outside the traditional clinic setting.
Our study defines implementation strategies as a compilation, or “bundle,” of implementation interventions that aim to promote CHB testing. 15 We included studies using community-based and primary care-based implementation strategies. This included but was not restricted to health education (e.g., by lay health workers (LHWs) or healthcare providers (HCPs), different group sizes, and different types of delivery), active or passive navigation to testing services, financial incentivization to physicians or test takers, simplified testing (e.g., home-based or community-based testing, point-of-care (POC) testing, self-testing, rapid diagnostic testing, dried blood spot testing), application of eHealth or mHealth, electronic reminder or best practice alert systems, and health policies.
Studies compared different implementation strategies or periods with and without the strategy of interest.
The primary outcome includes HBsAg testing uptake in patients (self-reporting or confirmed diagnosis by chart validation or proof of testing). When studies reported more than one measure, confirmed HBsAg testing was selected for analysis due to less risk of misclassification biases that affect self-reporting, including recall bias, social desirability bias, and others.
The secondary outcome is the uptake of tests for diagnosing hepatitis B immunity (i.e., anti-HBs antibody and anti-HBc antibody) and linkage to care such as hepatitis B vaccination and treatment evaluation (e.g., viral load testing or hepatologist consultation). Implementation outcomes such as acceptability, adoption, appropriateness, costs, feasibility, fidelity, penetration and sustainability were also collected when available. 16
Time point/timing
Eligible studies published until the day of the final search were included. Also, no restrictions were made on timing of outcome assessment.
Study designs
Primary studies included experimental designs with a randomization component, specifically including randomized controlled trials and cluster randomized trials.
Information sources and search strategies
An information specialist translated the eligibility criteria into search terms relevant to each database (Scopus, Embase, Pubmed, CINAHL). Additionally, MeSH (Medical Subject Headings) terms were extracted from the preliminary publications using the Yale MeSH Analyzer and added to the search terms. 17 The research team then reviewed and edited the search strategies. The included databases and the search strategies are shown in Supplemental Material 1 . The final search was conducted on June 05, 2024.
Manual searches were conducted by tracing the reference lists of the included studies and relevant systematic reviews. We also traced and screened studies that cited the included studies and related studies using the “Cited by” and “Related articles” functions on PubMed and Google Scholar. As mentioned in detail in the next section, two independent reviewers conducted the study tracing and conformed to the similar voting and conflict resolution.
Selection process
We managed all search results on Covidence ( https://www.covidence.org/ ), including screening, extraction, and quality assessment. Duplicate studies were excluded by built-in algorithms deployed by Covidence or manually by reviewers during the study.
First, two independent reviewers (TVK, PP, BT, CC, QL, HN, PN, DN, MHNL) screened the titles and abstracts against the eligibility criteria. Studies voted “included” or “maybe included” by consensus were carried to the next step, while studies voted “not included” by both reviewers were excluded. Discrepancies in voting were resolved by two-way discussions, or in case any conflicts remained, by seeking a third opinion and casting a third vote (TNDP, CJH, DYD). Next, full texts were uploaded onto Covidence. Two independent reviewers screened the full text against the eligibility criteria using a similar voting and conflict resolution system in full-text screening. The number of studies screened was assigned to TVK as the first reviewer and other members (PP, BT, CC, QL, HN, PN, DN, MHNL) as second reviewers.
Data extraction and risk of bias assessment
A data extraction form, which had been reviewed, piloted, and edited, was used to extract relevant information from the included studies. The form comprised data fields for study identification, methods, populations, implementation strategies, and outcomes. Details of the form are presented in Supplemental Material 2 . Behavior Change Wheel and TIDieR checklist were used to guide the design of implementation strategy-related data fields. 14 , 18 Other data fields were developed based on suggestions in the Cochrane handbook. 19 When relevant information was unavailable in the texts, the fields were entered as “not reported.” The risk of bias assessment form was developed using the ROB-2 system for individual RCTs and its variant for CRTs. 20
The data extraction and risk of bias assessment were conducted simultaneously. Two independent reviewers extracted data using the form. Each data field was compared head-to-head between the two reviewers. The same voting and conflict resolution were performed to achieve consensus for each data field.
We used the BCW framework to characterize the mechanisms of the implementation strategies. The coding system for strategy functions and targeted sources of behavior follows the steps described by Michie et al.‘s guidebook. 14 Extracted implementation characteristics were thematically matched with the behavioral change techniques defined in Step 7 of the guidebook. After that, we linked those behavioral techniques to corresponding behavior targets and implementation strategy functions. All team members underwent group training and were familiar with this coding process ( Supplemental Material 3 ).
Synthesis methods and effect measure
The outcome measure was the proportion of CHB testing among those who were recommended for CHB testing. We have used the term CHB testing rather than HBsAg or HBV testing to indicate the role of HBsAg testing in identifying individuals with surface antigenemia for further assessment and possible CHB treatment. The effect measure of effectiveness was the risk difference (RD), which represents the absolute incremental increase in the proportion tested. When possible, effect estimates from individual- and cluster-randomized trials employing the same implementation strategies were pooled in the same meta-analysis. The grouping by implementation strategy was specified a priori based on prior literature and overall descriptions by the authors of the included studies. 6 , 7 , 8 , 9 , 10 , 11 As we anticipated considerable between-study heterogeneity, a random-effects model was used to pool effect sizes. The Higgins & Thompson's I 2 statistic was used to quantify the heterogeneity. 21 , 22 The synthesis results were presented in a forest plot.
We conducted a mixed-effects meta-regression analysis to investigate if the enrichment of strategies' mechanisms is related to an increase in strategies' effectiveness, or in other words, whether the strategy effectiveness increased by the number of BCW components (i.e., the number of targeted sources of behavior or the number of strategy functions involved) in multicomponent strategies. 23 Publication bias would be assessed using funnel plots and Egger's test, and the corresponding adjustment would be performed if the heterogeneity was small to moderate (<25%) to avoid adding further bias. 24
For individual RCTs, RDs and their 95% confidence interval were computed using extracted raw values. Adjusted effect estimates reported in the RCTs would be used if multiple adjustment analyses were mentioned in their a priori analysis plans. For cluster-randomized trials, if the authors calculated the effect estimates using cluster-effect-adjusted univariate or multivariate analyses (e.g., design-based model, mixed-effects models, or generalized estimating equations), their effect estimates were extracted directly. Effect estimates presented in relative risks (RR) or odds ratios were converted to RDs (conversion formulas shown in the Supplemental Material 4 ). In one study, a reported covariate- and cluster-adjusted incidence rate ratio was assumed to approximate RR and then converted to RD. 25
Some cluster-randomized trials did not report accounting for survey design in the outcome analysis, potentially leading to overestimated precisions. We applied an approximately correct analysis to these trials, by which their observed sample sizes were reduced to effective sample size 26 ( Supplemental Material 4 ). Intra-cluster correlation was assumed to be 0.03, which was based on one of the included CRT. 25 The meta-analysis did not include studies that only reported the changes between baseline and study.
The alpha significance level was 0.05. All data were processed and analyzed using R version 4.3.2 (2023-10-31). The protocol had been registered on PROSPERO (CRD42023455781).
Role of the funding source
The sponsor of TVK's postdoctoral fellowship had no involvement in data collection, analysis, interpretation, writing of the manuscript and the decision to submit.
Study selection
Our search strategy identified 8652 results, of which 7146 remained after removing duplicates ( Fig. 1 ). After screening titles and abstracts, 25 studies were included in the review, none of which involved children or pregnant women ( Table 2 ). 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 Each study's characteristics are presented in Supplemental Table S1 . The study by Fung et al. was excluded due to combining self-reported CHB screening uptake and intent to CHB screening. 51 Eight studies on pregnant women were excluded for non-randomized design, no CHB testing outcome, or already having positive HBsAg results as an entry criteria. 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 One pediatric study was excluded because of its non-randomized design and the setting of tertiary care oncology. 60 Studies excluded at full-text screening stage are shown in Supplemental Table S2 . Nineteen studies were included in five meta-analyses and two meta-regression because their implementation strategies and outcomes could be directly compared to at least one other included study. 25 , 27 , 28 , 30 , 31 , 32 , 34 , 35 , 37 , 38 , 40 , 41 , 42 , 44 , 46 , 47 , 50 , 61

The flow of study inclusion .
Table 2
Trials of implementation strategies meeting inclusion criteria.
| Study | Study design/setting | Study population | Implementation strategy arm vs. control arm | N of strategy arm vs. control arm | %Female | Mean age (SD) | Risk difference (95% CI) |
|---|---|---|---|---|---|---|---|
| Ahmadi 2019 | CRT/Community | People with substance-related disorders in Iran | CHB education by LHWs vs. Usual practice | 50 vs. 50 | 100% | 28.6 (6.5) | 34.0% (12.6–55.4) |
| Bastani 2015 | CRT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 543 vs. 580 | 65.3% | 45.5 (12.5) | 17.1% (7–31.7) |
| Bottero 2015 | RCT/Primary care | Migrant patients visiting PCCs in France | Point-of-care CHB testing vs. Usual practice | 162 vs. 162 | 38.0% | 38 (12.2) | 27.2% (19.9–34.5) |
| Chak 2018 | RCT/Primary care | HCPs (to order CHB tests for Asian patients in the US) | CHB testing electronic reminder vs. Usual practice | 1542 vs. 1568 | 53.3% | 42.8 (14.7) | 9.0% (6.6–11.3) |
| Chak 2020 | RCT/Primary care | HCPs (to order CHB tests for Asian patients in the US) | CHB testing electronic reminder vs. Usual practice | 2599 vs. 2590 | 53.3% | 51.5 (20.8) | 2.9% (2–3.9) |
| ChenMSJr 2013 | RCT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 130 vs. 130 | 59.6% | NA | 10.8% (2.5–19.1) |
| Fitzpatrick 2019 | RCT/Community | Males who have sex with males in China | Crowdsourced CHB education vs. Usual practice | 280 vs. 276 | 0.0% | 25.5 (7) | 2.3% (−2.9 to 10.9) |
| Flanagan 2019 | CRT/Primary care | HCPs/Migrant patients visiting PCCs in the UK | Financial incentivization vs. Attention control | 58,512 vs. 31,738 | 51.8% | NA | 4.6% (0.5–16.3) |
| Hsu 2013 | RCT/Primary care | HCPs/Asian patients visiting PCCs in the US | CHB testing electronic reminder vs. Usual practice | 88 vs. 87 | 62.9% | 40.8 (12.3) | 34.1% (24.1–44.1) |
| Juon 2014 | CRT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 220 vs. 226 | NA | 45.1 (13.5) | 23.6% (10.1–40.4) |
| Khalili 2022 | CRT/Primary care | HCPs/Asian patients visiting PCCs in the US | CHB education on digital platforms vs. Attention control | 270 vs. 182 | 63.9% | 56.8 (16.9) | 30.7% (15.5–48.6) |
| Ma 2017 | CRT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 1131 vs. 1206 | 56.2% | 55.4 (15.1) | 78.1% (73.7–82.5) |
| Ma 2018 | CRT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 972 vs. 862 | 58.0% | 51.6 (13.5) | 91.3% (80.4–93.3) |
| Richens 2010 | RCT/Primary care | Patients with sexual health problems in the UK | Computer-assisted self-interviewing vs. Attention control | 795 vs. 779 | NA | NA | 0.6% (−3.1 to 4.2) |
| Rosenberg 2010 | RCT/Primary care | Patients with mental health problems in the US | CHB education by HCPs with decentr. testing vs. Usual practice | 95 vs. 93 | 47.9% | 46.5 (8.9) | 57.6% (46–69.1) |
| Sahajian 2011 | CRT/Community | Shelter residents in France | CHB education by HCPs with decentr. testing vs. Usual practice | 211 vs. 793 | NA | NA | 67.2% (56.1–78.3) |
| Sequeira-Aymar 2022 | CRT/Primary care | HCPs (to order CHB tests for migrant patients in Spain) | CHB testing electronic reminder vs. Usual practice | 3445 vs. 2784 | NA | NA | 2.2% (0.6–4.1) |
| Shireman 2020 | CRT/Community | Community health workers in the US | Training modalities of LHWs vs. Usual practice | 481 vs. 523 | 53.5% | 46.2 (14.4) | 27.9% (18.9–36.8) |
| Taylor 2009 | RCT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 231 vs. 229 | 37.2% | NA | 2.6% (−0.3 to 5.5) |
| Taylor 2011 | CRT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 95 vs. 123 | 55.0% | NA | 4.6% (−2.6 to 11.8) |
| Taylor 2013 | RCT/Community | Asian community members in the US | CHB education by LHWs vs. Attention control | 125 vs. 125 | 50.0% | NA | 8.8% (3.7–13.9) |
| VanderVeen 2014 | RCT/Community | Turkish community members in the Netherlands | CHB education on digital platforms vs. Attention control | 472 vs. 496 | 50.2% | 33.6 (5.2) | −1.5% (−9.0 to 5.8) |
| Wong 2022 | RCT/Primary care | Patients visiting PCCs in China | Crowdsourced CHB education vs. Usual practice | 376 vs. 376 | 57.3% | 42.7 (11.2) | 4.3% (−4.7 to 11.9) |
| Xiao 2021 | RCT/Community | Asian community members in Australia | CHB education by LHWs vs. Attention control | 26 vs. 28 | NA | NA | 8.0% (−6.1 to 22) |
| Ye 2023 | CRT/Community | Males who have sex with males in China | Financial incentivization vs. Attention control | 160 vs. 162 | 0.0% | 29.5 (12.6) | 35.2% (24.1–46.3) |
Abbreviation: CRT, Clustered Randomized Trial; RCT, Randomized Controlled Trial; CHB, Chronic hepatitis B; LHWs, Lay health workers; HCPs, Healthcare providers; SD, Standard deviation; PCCs, Primary care clinics; decentr. testing, decentralized testing; NA, Not available.
Study characteristics
The characteristics of the studies are summarized in Table 3 . The 25 studies contributed a total of 130,598 participants, with 34,925 participants included in all the meta-analyses. Ten studies (40.0%) were conducted at primary care clinics, while 12 (48.0%) were clustered randomized trials. Four studies (16.0%) were conducted in upper-middle-income countries, and 21 (84%) were from high-income countries. 56.0% (14/25) and 16.0% (4/25) of studies worked solely with migrant Asian populations and those living in Asian territories, respectively. The most common implementation strategy to promote CHB testing in community care settings was health education by laypeople (10 studies, 67.0%). In contrast, in primary care settings, it was efforts to increase CHB testing by clinicians through electronic reminders (4 studies, 40.0%). Five out of 25 studies had strategies aimed at clinicians. Ten studies (40.0%) reported implementation outcomes such as fidelity, adoption, contamination, or cost-effectiveness. Nine studies (36.0%) were considered at high risk of bias ( Table 3 and Supplemental Figures S1 & S2 ).
Table 3
Summary estimates of studies’ characteristics by setting.
| Characteristic | Overall, n (col %) | Settings | |
|---|---|---|---|
| Community care, n (col %) | Primary care, n (col %) | ||
| Total | 25 (100%) | 15 (100%) | 10 (100%) |
| Study design | |||
| Clustered Randomized Trials | 12 (48.0%) | 9 (60.0%) | 3 (30.0%) |
| Randomized Controlled Trials | 13 (52.0%) | 6 (40.0%) | 7 (70.0%) |
| Country | |||
| China | 3 (12.0%) | 2 (13.3%) | 1 (10.0%) |
| Iran | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Australia | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Canada | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| France | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| Netherlands | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Spain | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| United Kingdom | 2 (8.0%) | 0 (0.0%) | 2 (20.0%) |
| United States and Canada | 13 (52.0%) | 8 (53.3%) | 5 (50.0%) |
| Race(s) in the study sample | |||
| Multi-racial | 7 (28.0%) | 2 (13.3%) | 5 (50.0%) |
| Only Asian (migrant) | 14 (56.0%) | 10 (66.7%) | 4 (40.0%) |
| Only Asian (native) | 4 (16.0%) | 3 (20.0%) | 1 (10.0%) |
| Implementation strategies | |||
| Computer-assisted interviewing of high-risk people | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| Crowdsourced CHB education on social media | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| CHB electronic reminder | 4 (16.0%) | 0 (0.0%) | 4 (40.0%) |
| Financial incentivization | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| CHB education by HCWs with decentralized testing | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| CHB education by LHWs | 10 (40.0%) | 10 (66.7%) | 0 (0.0%) |
| CHB education on digital platforms | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| Point-of-care CHB testing | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| Training modalities of LHWs | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Actors of implementation | |||
| Administrators | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| Community members | 11 (44.0%) | 10 (66.7%) | 1 (10.0%) |
| Digital applications | 3 (12.0%) | 1 (6.7%) | 2 (20.0%) |
| Electronic health record system | 3 (12.0%) | 0 (0.0%) | 3 (30.0%) |
| Health professionals | 4 (16.0%) | 1 (6.7%) | 3 (30.0%) |
| Peer members | 2 (8.0%) | 2 (13.3%) | 0 (0.0%) |
| Action targeting on healthcare providers | |||
| Healthcare Providers | 5 (20.0%) | 0 (0.0%) | 5 (50.0%) |
| No | 20 (80.0%) | 15 (100%) | 5 (50.0%) |
| Action targeting on patients or community members | |||
| Community health workers | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| General community members | 10 (40.0%) | 10 (66.7%) | 0 (0.0%) |
| Males who have sex with males | 2 (8.0%) | 2 (13.3%) | 0 (0.0%) |
| Patients visiting primary care clinics | 5 (20.0%) | 0 (0.0%) | 5 (50.0%) |
| Patients with mental health problems | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| Patients with sexual health problems | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| Patients with substance-related disorders | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Shelter residents | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| No | 3 (12.0%) | 0 (0.0%) | 3 (30.0%) |
| Mode of delivery | |||
| Distance | 3 (12.0%) | 2 (13.3%) | 1 (10.0%) |
| Electronic health record system prompts | 4 (16.0%) | 0 (0.0%) | 4 (40.0%) |
| Face-to-face, group | 10 (40.0%) | 10 (66.7%) | 0 (0.0%) |
| Face-to-face, individual | 8 (32.0%) | 3 (20.0%) | 5 (50.0%) |
| Location of implementation | |||
| Churches | 3 (12.0%) | 3 (20.0%) | 0 (0.0%) |
| Community-based organizations | 5 (20.0%) | 5 (33.3%) | 0 (0.0%) |
| Drop-in centers | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Homes | 3 (12.0%) | 3 (20.0%) | 0 (0.0%) |
| Mental health clinics | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| Primary care clinics | 7 (28.0%) | 0 (0.0%) | 7 (70.0%) |
| Sexual health clinics | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Shelters | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Social media | 2 (8.0%) | 1 (6.7%) | 1 (10.0%) |
| Websites | 1 (4.0%) | 1 (6.7%) | 0 (0.0%) |
| Reporting implementation outcomes | |||
| Yes | 10 (40.0%) | 6 (40.0%) | 4 (40.0%) |
| No | 15 (60.0%) | 9 (60.0%) | 6 (60.0%) |
| Time at outcome assessment | |||
| After 12 months | 3 (12.0%) | 1 (6.7%) | 2 (20.0%) |
| After 6 months | 10 (40.0%) | 9 (60.0%) | 1 (10.0%) |
| After 3 months | 3 (12.0%) | 1 (6.7%) | 2 (20.0%) |
| After 1 month | 4 (16.0%) | 2 (13.3%) | 2 (20.0%) |
| Within 1 week | 5 (20.0%) | 2 (13.3%) | 3 (30.0%) |
| Type of outcome report | |||
| Self-reporting testing | 6 (24.0%) | 5 (33.3%) | 1 (10.0%) |
| Confirmed testing | 19 (76.0%) | 10 (66.7%) | 9 (90.0%) |
| Overall risk of bias | |||
| High | 9 (36.0%) | 8 (53.3%) | 1 (10.0%) |
| Low | 6 (24.0%) | 1 (6.7%) | 5 (50.0%) |
| Some concerns | 10 (40.0%) | 6 (40.0%) | 4 (40.0%) |
Abbreviation: CHB, Chronic hepatitis B; HCWs, Health care workers; LHWs, Lay health workers.
Publication bias was performed for the implementation strategy of CHB education by lay health workers. The funnel plot shows a slight asymmetry ( Supplemental Figure S3 ); however, Egger's regression test failed to detect significant asymmetry, with a p-value of 0.430. Publication bias was not performed for other strategies due to the small number of studies and/or large heterogeneity (I 2 > 75.0%) in each meta-analysis. 24
Table 4 shows the summary statistics for strategies functions and sources of behavior characterized by the BCW framework. In the community care setting, most implementation strategies involved education and persuasion as strategy functions (84.0% and 76.0%, respectively) and psychological capability and reflective motivation as targeted sources of behavior (84.0% and 84.0%, respectively). In contrast, environmental restructuring to increase physical opportunity had a central role in the primary care setting (100% and 90.0%, respectively). However, no strategies involved the function of coercion or restriction. No policy-level factors were also involved in all strategies. BCW's components in each study are presented in Table 5 .
Table 4
Summary estimates for Behavior Change Wheel's components of implementation strategies by setting.
| Characteristic | Overall, n (% out of total) | Settings | |
|---|---|---|---|
| Community care, n (% out of total) | Primary care, n (% out of total) | ||
| Total | 25 (100%) | 15 (100%) | 10 (100%) |
| Sources of behavior | |||
| Physical Capability | 1 (4.0%) | 0 (0.0%) | 1 (10.0%) |
| Psychological Capability | 21 (84.0%) | 15 (100%) | 6 (60.0%) |
| Physical Opportunity | 14 (56.0%) | 5 (33.3%) | 9 (90.0%) |
| Social Opportunity | 11 (44.0%) | 10 (66.7%) | 1 (10.0%) |
| Reflective Motivation | 21 (84.0%) | 15 (100%) | 6 (60.0%) |
| Automatic Motivation | 13 (52.0%) | 7 (47.7%) | 6 (60.0%) |
| Strategy functions | |||
| Education | 21 (84.0%) | 15 (100%) | 6 (60.0%) |
| Persuasion | 19 (76.0%) | 15 (100%) | 4 (40.0%) |
| Incentivization | 3 (12.0%) | 1 (6.7%) | 2 (20.0%) |
| Coercion | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Training | 6 (24.0%) | 1 (6.7%) | 5 (50.0%) |
| Restriction | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Environmental Restructuring | 16 (64.0%) | 6 (40.0%) | 10 (100%) |
| Modelling | 4 (16.0%) | 3 (20.0%) | 1 (10.0%) |
| Enablement | 14 (56.0%) | 10 (66.7%) | 4 (40.0%) |
Note: One study may have more than one behavior targets or strategy functions, so the column numbers or proportion may not be mutually exclusive.
Table 5
Characterization of Behavior Change Wheel's components in each study.
| Study ID | Implementation strategy | Strategy functions | Sources of behavior targets | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Education | Persuasion | Training | Modelling | Enablement | Incentivization | Environment Restructure | Physical Capability | Psycho Capability | Physical Opportunity | Social Opportunity | Reflective Motivation | Automatic Motivation | ||
| Fitzpatrick 2019 | Crowdsourced education | |||||||||||||
| Wong 2022 | Crowdsourced education | |||||||||||||
| Ye 2023 | Financial incentivization | |||||||||||||
| Flanagan 2019 | Financial incentivization | |||||||||||||
| Sahajian 2011 | Education by HCPs | |||||||||||||
| Rosenberg 2010 | Education by HCPs | |||||||||||||
| Bastani 2015 | Education by LHWs | |||||||||||||
| Ahmadi 2019 | Education by LHWs | |||||||||||||
| Ma 2018 | Education by LHWs | |||||||||||||
| Ma 2017 | Education by LHWs | |||||||||||||
| Juon 2014 | Education by LHWs | |||||||||||||
| Taylor 2013 | Education by LHWs | |||||||||||||
| ChenMSJr 2013 | Education by LHWs | |||||||||||||
| Xiao 2021 | Education by LHWs | |||||||||||||
| Taylor 2011 | Education by LHWs | |||||||||||||
| Taylor 2009 | Education by LHWs | |||||||||||||
| VanderVeen 2014 | Digitalized education | |||||||||||||
| Khalili 2022 | Digitalized education | |||||||||||||
| Sequeira-Aymar 2022 | Electronic reminder | |||||||||||||
| Hsu 2013 | Electronic reminder | |||||||||||||
| Chak 2020 | Electronic reminder | |||||||||||||
| Chak 2018 | Electronic reminder | |||||||||||||
| Bottero 2015 | Point-of-care testing | |||||||||||||
| Shireman 2020 | Training modalities | |||||||||||||
| Richens 2010 | CAPI | |||||||||||||
Abbreviation: HCPs, Healthcare providers; LHWs, Lay health workers; CAPI, Computer-assisted Personal Interviewing.
CHB education by lay health workers (LHWs)
Ten studies, totaling 7451 participants, investigated the effectiveness of the strategy in which LHWs without clinical training provided CHB- or liver cancer-related education in the community. 27 , 28 , 32 , 35 , 37 , 38 , 44 , 46 , 47 , 61 Three studies had some concerns about bias, while the other seven showed high risks of bias. When pooled together in the meta-analysis, the LHWs-driven implementation strategy showed an increase of 27.9% (95% CI, 3.4–52.4%) in CHB testing uptake with large heterogeneity (I 2 = 99.3%) compared with control arms ( Fig. 2 ). Most studies were conducted on migrant Asian populations, except for the study by Ahmadi et al., which focused on a high-risk population (patients with substance-related disorders) living in Iran. 27

Forest plots of individual and pooled effect estimates in promoting CHB testing of implementation strategies. Moderate to large heterogeneity but with consistency of direction toward benefits can be observed for CHB education by lay health workers, CHB testing electronic reminder, CHB education by healthcare providers coupled with decentralized testing. Abbreviation: CHB, Chronic hepatitis B; RD, Risk Difference.
All ten studies included education and persuasion as functions in their strategy, hence increasing patients' psychological capability to change CHB testing behavior ( Table 5 ). Three studies restructured the environments to optimize the physical and social opportunities. 28 , 37 , 38 Ma et al., in 2017 and 2018 employed navigation services (e.g., transportation, language translation, scheduling appointments) and a community participatory approach (e.g., engaging community members in planning, development, and implementation) to alleviate any physical or social barriers for the target sample. 37 , 38
Implementation outcomes were reported in three studies, which showed suboptimal participants’ engagement in receiving the implementation or follow-up. The home-based education by Taylor et al., in 2009 only reached 63.0% of the participants in the strategy arm, and only as low as 34.0% of them were eventually exposed to one of the education materials. 47 A similar implementation by Taylor et al., in 2013 on Cambodian Americans delivered complete home-based education to 79.2% of the participants. 44 The other participants refused the education completely (9.6%) or partially (4.0%) or did not commit to follow-up (4.0%). 44 Group-based CHB education at Vietnamese American community-based organizations reached 100% of participants; however, only 52.0% received the testing navigation component. 37
One study further reported secondary outcomes. A multicomponent strategy by Ma et al., in 2018 effectively increased both CHB testing uptake and hepatitis B vaccination. 38 92.8% and 84.0% of people who tested negative for CHB initiated and completed vaccination series in the implementation strategy arm, while the figures in the control arm were 29.4% and 17.6%, respectively. 38
Meta-regression
Mixed-effect meta-regression analyses were conducted to investigate the large heterogeneity and to associate the number of implementation strategy mechanisms with effectiveness in promoting CHB testing uptake. This analysis was performed only for the LHWs-driven strategy thanks to the acceptable number of ten studies. 23 The number of targeted sources of behavior showed a dose–response relationship with CHB testing uptake, with a significant increase in the effectiveness of 23.8% (95% CI 14.6–33.0, R 2 = 84.6%) per additional source of behavior targeted. Likewise, for every additional function included in the implementation strategy, effectiveness significantly increased by 17.9% (95% CI 1.6–34.1%, R 2 = 59.2). For better visualization of the incremental change, the random-effect risk differences by sources of behavior or strategy functions were presented in Fig. 3 .

A whisker plot showing random-effect risk difference and its confidence interval sub-grouped by the number of strategy mechanisms (sources of behavior or strategy functions), with number of studies in each subgroup. Meta-regression shows that a significant increase in the effectiveness of 23.8% (95% CI 14.6–33.0, R 2 = 84.6%) was observed per additional source of behavior targeted. Likewise, for every additional function included in the implementation strategy, effectiveness significantly increased by 17.9% (95% CI 1.6–34.1%, R 2 = 59.2).
Sensitivity analyses
Subgroup analyses based on study characteristics were conducted where possible, as shown in Supplemental Table S3 . No significant differences were detected between subgroups. The I 2 value for heterogeneity remained over 50% in most subgroups, except for studies implementing only educational activities, where the I 2 value was 0.0%.
Training modalities for LHWs
One study with some concerns for bias investigated different training modalities for LHWs to conduct community-based health education. Shireman et al. found that in-person training significantly increased CHB testing uptake among church-goers (RD = 27.9% [95% CI 18.9–36.8%]) than online training with similar contents. 43 Also, the proportion of hepatitis B vaccination in the in-person training arm was higher than in the online training arm (17.0% vs. 5.9%). 43 Despite the difference in effectiveness in increasing CHB testing uptake and hepatitis B vaccination, the total training costs were comparable between the two arms. 43
CHB education by HCPs coupled with decentralized testing
A total of 1912 participants were included in two trials with some concerns of bias applying the model in which HCPs provided CHB education and decentralized testing. 40 , 41 The meta-analysis shows the pooled RD of 62.5% (95% CI 53.1–71.9%) with small heterogeneity of I 2 being 27.5% ( Fig. 2 ). An outreach strategy for underprivileged people living in shelters in France included individual consultation and group information regarding CHB and hepatitis C, followed by decentralized testing. 41 This model of patient group information helps to enable patients’ psychological capabilities and restructure the social norms regarding CHB testing ( Table 5 ). On the other hand, Rosenberg et al. conducted a bundle of care at primary mental health clinics, including individual education and pretest counseling about infectious diseases in general, including hepatitis B, followed by decentralized testing and further incentivized by immunization and risk reduction education. 40 The care bundle triggered patients' automatic motivation, making them desire to obtain CHB testing ( Table 5 ).
Digitalized CHB education
The effectiveness of health education delivered through digital applications was investigated in two studies with some concerns for bias, adding up to 1852 participants. 36 , 48 However, these two studies’ effect estimates were not pooled due to inconsistent settings and comparators. VanderVeen et al., 2014 deployed CHB education websites to educate people in the community and observed an RD of −1.5% (95% CI −9.0 to 5.8%). 48 Two arms received the web-based education; the contrast of interest was between the culture- and behavior-adapted CHB contents in one arm and generic CHB-related information in the other arm. 48
On the contrary, Khalli et al., in 2022 tested the implementation of an iPad-based mobile application to educate patients and facilitate the patient-provider discussion regarding hepatitis testing in primary care clinics. 36 Both patients and HCPs were beneficiaries. This implementation model engaged up to six strategy functions and four behavior targets, as shown in Table 5 . The CHB testing uptake was increased by 30.7% (95% CI 15.5–48.6%) compared to usual care. 36 Other implementation outcomes were also reported. 70.4% of patients started discussing with providers in the mobile app arm, while only 16.5% did in usual care. Additionally, 51.1% of providers recommended CHB testing, while only 13.2% recommended it in usual care. 36
Crowdsourced CHB education on social media
Two studies with 1308 participants and a high risk of bias tested an approach in which CHB educational content was crowdsourced and delivered on social media. 33 , 49 Meta-analysis in Fig. 2 shows a minimal increase in CHB testing proportion by 3.1% (95% CI −2.2 to 8.4%, I 2 = 0.0%). This innovative virtual community-based outreach enhanced the social opportunities for CHB knowledge and testing. Implementation-wise, challenges included the fidelity and contamination of crowdsourced education on social media. In Wong's study in 2022, 61.4% of participants received all educational materials, and 26.9% did not see any of them during the study period. 49 Fitzpatrick et al., in 2019 noted that 27.9% of participants in the crowdsourcing arm saw no educational materials, and 52.9% shared crowdsourced materials with others, while 9.0% of men in control were exposed to the crowdsourced materials.
CHB testing electronic reminder
Four studies were conducted on 24,254 patients to assess the effectiveness of electronic reminders in promoting CHB testing uptake in primary care clinic settings. 30 , 31 , 34 , 42 One study demonstrates some concerns for bias, while others at low risk. The pooled RD was 8.4% (95% CI 3.7–13.1%) with large heterogeneity (I 2 = 95.0%) in Fig. 2 . In these studies, electronic reminder pop-ups identified patients at higher risk of hepatitis B (i.e., Asian and Pacific Islanders, and people from countries with CHB prevalence >2%). The implementation by Chak et al., 2018 and 2020 only included electronic reminders, 30 , 31 while Hsu et al. and Sequeira-Aymar 2022 added an education persuasion component, which improved the psychological capabilities and reflective motivation of HCPs ( Table 5 ). 34 , 42 Of significant note, the tool used in the study by Sequeira-Aymar et al. was for multiple infections, one of which is hepatitis B. 42
Concerning implementation outcomes, only Hsu et al. reported on CHB test orders by healthcare providers. 34 In the electronic reminder arm, where all encounters were prompted with a CHB alert, 53.7% (36/67) of patients were ordered CHB tests, and 83.3% (30/36) completed the testing order. In the usual care arm, 1.6% (1/63) were ordered, and none completed the order.
Financial incentivization
Two studies with a low risk of bias implemented financial incentivization to increase CHB testing. Meta-analysis was not done due to the difference in the nature of the implementations. Flanagan et al. offered pay-for-performance financial incentives to primary care providers to encourage as many testing uptakes as possible in their clinics. 25 This strategy led to an increase of 4.6% (95% CI 0.5–16.3%) in CHB testing uptake and was deemed cost-effective at a willingness-to-pay thresholds above £8540 per QALY. In contrast, Zhang et al. targeted directly on the community with a one-off community-driven and pay-it-forward incentives program at community-based organizations. 50 Every person was offered a free test with community-generated messages and then asked if they would like to donate money to support others to receive free testing. The RD was 35.2% (95% CI 24.1–46.3%). The financial cost is $69 per case of viral hepatitis identified.
POC testing
One study with a low risk of bias compared the effectiveness of fingerstick POC HBsAg testing with a 30-min turnaround time to standard venipuncture-based testing with a one-week turnaround time. 29 This strategy, which was conducted in the setting of primary care clinics, increased the CHB testing uptake by 27.2% (95% CI 19.9–34.5%) compared with the standard care. Furthermore, linkage-to-care rates were also improved, with 90.0% of infected patients in the POC arm compared to 83.3% in the standard arm.
Computer-assisted interviewing of high-risk people
One study with some concerns for bias implemented computer-assisted self-interview (CASI) to promote CHB testing through mitigating social desirability in reporting activities at risk for CHB infection. 39 Patients who visited sexual health clinics would self-report their behaviors to a computer before moving on to consultation with care providers. Richens et al. found a minimal and insignificant increase in CHB testing (RD = 0.6% [95% −3.1 to 4.2%]) compared to the traditional approach in which patients reported their behaviors to providers face-to-face. 39
We have systematically synthesized evidence on the effectiveness and implementation of diverse strategies, uniformly characterized using BCW framework, to promote CHB testing in primary care and community settings. Among the evaluated strategies, CHB education delivered by HCPs, together with decentralized testing, demonstrated a prominent effect. Importantly, implementation strategies enriched with a larger number of BCW mechanisms, as exemplified in the case of LHWs-driven CHB education, showed larger effect on CHB testing. However, evidence from LMICs is absent. Additionally, less than half of the studies provided clear evaluations of the implementation outcomes of the strategies.
Interestingly, we found that multicomponent CHB education programs led by LHWs, if incorporating more BCW's targeted sources of behavior or strategy functions, demonstrated significantly higher effectiveness. This may suggest that combining multiple theory-driven components when developing an implementation strategy enhances its effectiveness in CHB testing promotion. 14 Due to the limited number of available studies in the literature, we could not identify independent components or optimal combinations of components or apply similar meta-regression models to other implementation strategies. Therefore, future studies are encouraged to validate our hypothesis and improve our analysis.
HCPs involvement in CHB education and testing recommendations was the most effective strategy in increasing CHB testing. 40 , 41 With the highest effect size and moderate heterogeneity, this strategy showed great potentials for application in different settings. However, as we consider expanding this strategy, we should appreciate that its success may rely on additional tools to drive behavior change among HCPs. For instance, reminders or best practice alerts for HCPs in electronic health records have been proven effectively in prompting testing orders. 30 , 31 , 34 , 42 Yet, these alerts may lead unintended side effects like alert fatigue. 62 Hsu et al. reported that only about half of the physicians complied with the alert in their study. 34 Another potential tool is financial incentivization for physicians. That said, this approach showed the minimal effectiveness and questionable cost-effectiveness of LMICs. 25
Implementation strategies utilizing LHWs for community-based CHB education were extensively studied. 27 , 28 , 32 , 35 , 37 , 38 , 43 , 44 , 46 , 47 , 61 LHWs understand the community, require no clinical training, are cost-effective, and so are considered suitable for resource-limited areas. 63 This approach has also proven effective in other health behaviors, such as the uptake of childhood immunization or the initiation of breastfeeding. 63 Additionally, the community can be extended to online platforms. The educational materials can be developed, distributed, and benefited by social media users per se in a crowdsourced approach. 33 , 49 However, challenges like low compliance and high contamination, especially on social media, may undermine the effectiveness of these community-based strategies. 33 , 37 , 44 , 47 , 49 Future implementations should proactively address these considerations.
On the other hand, none of the included studies explored the inherent interplay of system and societal factors with the implementation strategies. Understanding these critical contextual factors would help implementation practitioners decide if the findings are likely to generalize to given settings. 12 , 64 These contextual factors could be characterized by policy categories in the BCW framework, such as the availability of related fiscal measures or guideline. 14 For example, implementation strategies may interact with financial barriers in some countries, such as Viet Nam and the Philippines, where CHB tests are not covered for the general population. 65 Conversely, the release of CHB universal screening guideline by the Center of Disease Control and Prevention in the United States could accelerate testing progress. 66
Furthermore, the evidence gap persists, particularly concerning geography and race. None of the trials included in our review were conducted in LMICs, where the majority of the CHB burden exists. 2 The lack of studies from Africa and among African immigrants living in North America and Europe represents a substantial gap that future studies should address. Additionally, screening recommendations are evolving, and studies conducted in select populations (e.g., Asian immigrants) may have limited applicability in a setting where universal testing is now recommended, as is the case in the US. 66 Other critical contextual factors, such as culture, resources, and infrastructure, vary greatly by setting and profoundly impact the accessibility, acceptability and affordability of any implementation strategies. 12 The absence of evidence may impede the most affected countries from making informed policy decisions regarding CHB testing.
The limitations of this review are noted. Firstly, although we conducted comprehensive searches on four major medical and social literature databases, omitting LMIC-specific databases is inevitable due to the language barrier. To mitigate this, we traced references through reference lists and citations of included studies and relevant reviews and reviewed similar articles on large databases. Given the lack of published studies from LMICs, generalization of the findings to such settings should be done with consideration of variation in contextual factors that may mediate effectiveness. Secondly, caution is also warranted in generalizing the estimates to future programs or trials due to large heterogeneity in some strategies. Still, we stress that the consistency in the direction of effects suggests a degree of generalizability across varying population groups (at least those represented in the studies). The fact that strategies inevitably operate differently across contexts is a well-recognized reality in implementation. 12 , 64 This fact echoes the call for more studies focusing on both effectiveness and implementation on diverse races and in geographical and economic areas.
In conclusion, during our evaluation of implementation strategies, some have demonstrated high effectiveness in some settings. However, understanding critical contextual factors and relevance in LMICs contexts is paramount for optimizing real-world effectiveness across settings. Additionally, bundling strategies with policy and health system-level factors can amplify impact and sustainability, facilitating progress toward hepatitis B elimination targets.
Contributors
Conceptualization, TVK, TNDP, CJH, DYD; Methodology, TVK, TNDP, CJH, DYD; Validation, TVK, ML, PN, HN, TNDP, CJH, and DYD; Formal Analysis, TVK; Screening, Reviewing and Data Collection, TVK, PP, ML, QL, PN, HN, DN, BT, CC; Writing—Original Draft Preparation, TVK; Writing—Review & Editing, TVK TNDP, PP, ML, QL, PN, HN, DN, BT, CC; AG, CJH, DYD; Visualization, TVK; Supervision, TNDP, CJH, DYD; Project Administration, TVK; Access to and verification of data, TVK, CJH, and DYD; Responsibility for the decision to submit the manuscript: TVK, CJH, and DYD.
Data sharing statement
Datasets and R codes are available to readers upon request on GitHub.
Declaration of interests
DYD has received financial support from Mai Dolch for the Center of Excellence for Liver Disease in Vietnam at Johns Hopkins School of Medicine; research grants from the Ludwig Institute for Cancer Research, Gilead Sciences, Fujifilm Medical Systems, and Roche Diagnostics International Ltd.; honoraria and travel support from BMJ Best Practices, Fujifilm Medical Systems, Roche Diagnostics International Ltd., and Techno Orbits; and equipment and materials from Fujifilm Medical Systems and Roche Diagnostics International Ltd. DYD has also served on Data Safety Monitoring Boards (DSMBs) or advisory boards for IQVIA. HN has received payment from A. Menarini Singapore Pte. Ltd. for a presentation on the economic evaluation of tenofovir alafenamide in chronic hepatitis B in Vietnam; financial support from VinHealth for the economic evaluation of tenofovir alafenamide in Vietnam; and financial support from Mahidol University for evaluating strategies to prevent mother-to-child transmission of hepatitis B virus (HBV). PN has received financial support from VinHealth for the economic evaluation of tenofovir alafenamide in chronic hepatitis B in Vietnam. All other authors declare no competing interests.
Acknowledgements
Sincere thanks to Dr Jay Vaidya, MD PhD (BEADCore Team, Johns Hopkins Medicine) for his consultation on epidemiological and biostatistical methods and to Jacob White, MLS (Welch Medical Library, Johns Hopkins Medicine) for developing and conducting the search strategies.
TVK is the inaugural recipient of the Tran Dolch Post-Doctoral Fellowship in Hepatology at Johns Hopkins School of Medicine, Baltimore MD, USA.
Appendix A Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102818 .
Appendix A. Supplementary data

IMAGES
VIDEO
COMMENTS
The following topic will outline issues related to the management of hepatitis B through the use of cases studies that incorporate patient-specific clinical information and test results. Our approach to treatment is generally consistent with guidelines from the European Association for the Study of the Liver, Asian-Pacific Association for the ...
A Japanese nationwide study showed that genotypes A, B, C and D accounted for 4.1%, 17.5%, 77.6% and 0.6% of chronic HBV infections, respectively . ... We herein report a rare case of acute hepatitis B in an elderly woman caused by subgenotype D1 HBV. Case Report.
Patients may also skip the acute viral illness stage and present much later with a subclinical chronic infection. 2. Patients with chronic hepatitis B are at increased risk for developing hepatocellular carcinoma, even before reaching end-stage cirrhosis. 2. Acute hepatitis B is treated with supportive care.
The association of acute hepatitis—specifically hepatitis B—and non-immune haemolytic anaemia is rare. 5 It occurs usually in young patients without a significant medical history and presents with haemoglobin levels usually below 8 g/dL, as depicted in the case described above. Only a few cases of this severe association have been described ...
In 2015, an estimated 257 million people were living with chronic hepatitis B virus (HBV) globally, with estimates in the United States as high as 2.2 million. 1-3 Only 25% of infected individuals in the United States are aware of their HBV infection, and less than 10% are able to access care and treatment. 2 If left untreated, chronic HBV infection can lead to serious liver complications ...
The management of hepatitis B virus (HBV) infection is complex and depends upon multiple factors including clinical variables (eg, the presence or absence of liver inflammation and/or cirrhosis), the patient's immunologic response to infection (eg, hepatitis B e antigen status), virologic factors (eg, HBV DNA level and genotype), and risk ...
DOI: 10.1056/NEJMra2211764. VOL. 388 NO. 1. Chronic hepatitis B is caused by the hepatitis B virus (HBV), a hepatotropic DNA virus that can replicate at high levels and cause minimal disease or ...
INTRODUCTION. Hepatitis B virus (HBV) is a double-stranded deoxyribonucleic acid (DNA) virus belonging to the family of hepadnaviruses. HBV infection is a global public health problem. It is estimated that there are more than 250 million HBV carriers in the world, of whom approximately 800,000 die annually from HBV-related liver disease.
Here we describe an acute hepatitis B infection in a patient who received five hepatitis B vaccinations. Although his initial response to vaccination was moderate, he finally reached an excellent hepatitis B surface antibody level (anti-HBs) titres of more than 1000 IU/l in response to a booster vaccination with a recombinant DNA vaccine. . Nevertheless, he developed full-blown acute hepatitis ...
Acute, resolved, and chronic hepatitis B Approximately 90% of people >5 years of age with acute hepatitis B will spontaneously clear their infection (50, 51).People with resolved hepatitis B will remain positive for total anti-HBc and develop anti-HBs that protect against future HBV infection ().Chronic hepatitis B is defined as an HBV infection lasting >6 months.
Case 1 -Extensive Treatment Experience 44 yo man with longstanding HIV infection, stage 2 with nadir CD4 220 and chronic hepatitis B infection, e-Ag positive with high baseline HBV viral level and probable cirrhosis. • Persistent HBV viremia in 5 log 10 range on lamivudine/adefovir (& various ART) for many years until finally
Comparison of Hepatitis B virus (HBV) mutations described in the literature with those found in the HBV sequence of a patient presenting with acute biochemical hepatitis (patient 1745). Mutations are relating to chronic HBV with acute reactivation (CHB-AR), acute hepatitis B (AHB) and HBV associated acute on chronic liver failure.
INTRODUCTION. The management of hepatitis B virus (HBV) infection is complex and depends upon multiple factors including clinical variables (eg, the presence or absence of liver inflammation and/or cirrhosis), the patient's immunologic response to infection (eg, hepatitis B e antigen status), virologic factors (eg, HBV DNA level and genotype), and risk factors for disease progression (eg, age ...
This study sought to address the gap between current and optimal hepatitis B testing in a primary care clinic with a likely high population of undiagnosed hepatitis B. Between September 2015 and December 2016, four interventions aimed at enhancing general practitioner testing practices were implemented: staff education, quality improvement and ...
Hepatitis B is estimated to cause 500 000-900 000 deaths globally each year. WHO has targets for elimination by 2030; however, progress has stalled due to multiple barriers, notably a paucity of global funding and insufficient evidence on the economic burden of disease. Using a dynamic mathematical model of hepatitis B transmission, disease progression, and mortality in the six WHO regions ...
Hepatitis B virus (HBV) infection is a major public health problem, with an estimated 296 million people chronically infected and 820 000 deaths worldwide in 2019. Diagnosis of HBV infection requires serological testing for HBsAg and for acute infection additional testing for IgM hepatitis B core antibody (IgM anti-HBc, for the window period when neither HBsAg nor anti-HBs is detected).
Background: Hepatitis B is one of the major causes of mortality among viral diseases. To reduce morbidity rate and increase knowledge of people about potential risk factors, the aim of this study was to determine the prevalence of hepatitis B among the general population and the risk factors associated with hepatitis B virus (HBV) infection in Isfahan, Iran.
Antiviral therapy for chronic hepatitis B (CHB) has modified the clinical evolution of the disease with an improvement in terms of morbidity, mortality and quality of life. ... Contributors: BC and OS provided care of the patient in the case study. BC is the attending physician; he managed the patient's care from point of initial diagnosis, and ...
Combination therapy appears to provide the most effective course of treatment. This should include a nucleoside analogue and patients should be covered with hepatitis B immune globulin throughout the course of therapy. Several other variations of combination therapy are discussed, but many clinical issues remain to be resolved.
In the case of reactivation (HBsAg or HBeAg becomes positive, ALT levels increase, or HBV DNA reappears) ... She is Chair of the American Association for the Study of Liver Diseases (AASLD) Hepatitis B Special Interest Group and a member of the Governing Council of the International Association for the Study of Liver Diseases (IASL).
In 2015, an estimated 257 million people were living with chronic hepatitis B virus (HBV) globally, with estimates in the United States as high as 2.2 million. 1-3 Only 25% of infected individuals in the United States are aware of their HBV infection, and less than 10% are able to access care and treatment. 2 If left untreated, chronic HBV infection can lead to serious liver complications ...
Hepatitis B viral infection is a serious global healthcare problem. It is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). It is often transmitted via body fluids like blood, semen, and vaginal secretions. The majority (more than 95%) of immunocompetent adults infected with HBV can clear the infection spontaneously. Patients can present with acute ...
Hepatitis B stands at a crossroads between a future of continued inequal access to care and a path towards rapid expansion of program scale-up for testing and subsequent linkage to care. To catalyze for the latter, our study identifies effective strategies, such as CHB education delivered by healthcare providers and decentralized testing in ...