- Open access
- Published: 18 October 2019

A detailed overview of xylanases: an emerging biomolecule for current and future prospective
- Nisha Bhardwaj 1 na1 ,
- Bikash Kumar 1 na1 &
- Pradeep Verma ORCID: orcid.org/0000-0003-2266-9437 1
Bioresources and Bioprocessing volume 6 , Article number: 40 ( 2019 ) Cite this article
46k Accesses
247 Citations
Metrics details
Xylan is the second most abundant naturally occurring renewable polysaccharide available on earth. It is a complex heteropolysaccharide consisting of different monosaccharides such as l -arabinose, d -galactose, d -mannoses and organic acids such as acetic acid, ferulic acid, glucuronic acid interwoven together with help of glycosidic and ester bonds. The breakdown of xylan is restricted due to its heterogeneous nature and it can be overcome by xylanases which are capable of cleaving the heterogeneous β-1,4-glycoside linkage. Xylanases are abundantly present in nature (e.g., molluscs, insects and microorganisms) and several microorganisms such as bacteria, fungi, yeast, and algae are used extensively for its production. Microbial xylanases show varying substrate specificities and biochemical properties which makes it suitable for various applications in industrial and biotechnological sectors. The suitability of xylanases for its application in food and feed, paper and pulp, textile, pharmaceuticals, and lignocellulosic biorefinery has led to an increase in demand of xylanases globally. The present review gives an insight of using microbial xylanases as an “Emerging Green Tool” along with its current status and future prospective.
Introduction
The major constituent of the plant cell wall is “lignocelluloses”, as the name suggests it consists of lignin (15–20%), hemicellulose (25–30%) and cellulose (40–50%) (Gray et al. 2006 ; Singla et al. 2012 ). These components together form a three-dimensional complex network with the help of covalent and non-covalent interactions (Sánchez 2009 ). Hemicelluloses consist of xylan, a heteropolysaccharide substituted with monosaccharides such as l -arabinose, d -galactose, d -mannoses and organic acids such as acetic acid, ferulic acid, glucuronic acid interwoven together with help of glycosidic and ester bonds (Collins et al. 2005 ; Ahmed et al. 2007 ; Motta et al. 2013 ; Sharma 2017 ). Xylan is readily available in nature, followed by cellulose the second most abundant polysaccharide which covers 33% of total lignocellulosic biomass found on the globe (Collins et al. 2005 ; Polizeli et al. 2005 ; Chávez et al. 2006 ; Walia et al. 2017 ). It accounts for 15–30% in hardwoods and 7–10% in softwood (Walia et al. 2017 ). There is a need for depolymerization of this complex polymer for its efficient utilization in different industrial application. Xylanase is a group of enzymes consisting of endo -1,4-β- d -xylanases (EC 3.2.1.8), β- d -xylosidases (E.C. 3.2.1.37), α-glucuronidase (EC 3.2.1.139) acetylxylan esterase (EC 3.1.1.72), α- l -arabinofuranosidases (E.C. 3.2.1.55), p -coumaric esterase (3.1.1.B10) and ferulic acid esterase (EC 3.1.1.73) involved in the depolymerization of xylan into simple monosaccharide and xylooligosaccharides (Gomez et al. 2008 ; Juturu and Wu 2014 ; Walia et al. 2017 ; Romero-Fernández et al. 2018 ).
Xylanases are produced by different living organisms such as microorganisms, protozoans, and molluscs, and also found in the rumen of higher animals (Beg et al. 2001 ). The xylanases are mainly produced by microorganisms, e.g., bacteria, fungi, and actinomycetes at industrial scale (Motta et al. 2013 ). The utilization of lignocellulosic biomass (LCB) for production of different biochemicals such as bioethanol, enzymes, and value-added compounds has tremendously improved in recent years. It results in providing opportunities for scientists to explore the hydrolytic potential of xylanase for efficient saccharification of LCB for ethanol and xylooligosaccharides generation. Xylanase also finds application in several industries like pulp and paper bleaching, food, feed, and pharmaceuticals.
Xylanase is required in huge amount for industrial level application with characteristic properties to survive the harsh industrial level processing’s (Qiu et al. 2010 ). Therefore, there is a need to select potent microorganisms for xylanase production, followed by optimization of media components for enhanced production. The understanding of the genetic constituents of the microbe will help in deducing the mode of action of the enzyme. This will help in regulating the enzyme action for employment in desired industrial application. The microorganisms also produce other protein and metabolites with desired xylanase enzyme. Therefore, purification of the crude enzyme is a prerequisite to obtain purified enzymes. The characterization of purified xylanase will help in elucidating its stability and specificity toward different substrates. This will help in selecting the suitable industrial process in which it can be utilized. With the advent of advanced biotechnological techniques such as recombinant DNA technology, several attempts have been made to identify, isolate and clone the gene encoding for xylanase in a suitable system. This approach helps in the engineering of efficient microorganisms for enhanced xylanase production with desired properties. This review gives a comprehensive insight into xylanase classification, its mode of action, different xylanase sources with available production methods and its optimization strategies for enhanced production. The review also gives a brief idea about different strategies employed for xylanase purification and characterization, biotechnological approach for enhanced xylanase production with desired properties which are further used for different industrial applications.
Structure of xylan and role of xylanolytic enzymes in its breakdown
Xylan consists of d -xylose backbone linked with β-1,4-glycosidic bonds and l -arabinose traces forming into a complex heteropolymeric structure. Xylan is present in various biomasses that have several forms such as in hardwoods as O -acetyl-4- O -methylglucuronoxylan, in softwoods as arabino-4- O -methylglucuronoxylan and in grasses and annual plants as arabinoxylans. These residues can be substituted with acetyl, feruloyl, glucopyranosyl, 4- O -methyl- d -glucuronopyranosyl, p -coumaroyl or α- l -arabinofuranosyl side-chain groups with varying degrees. Xylanolytic enzymes play a key role in the breakdown of the complex structure of xylan. Hence, for complete and efficient hydrolysis of xylan into its constituent sugars requires synergistic action of various enzymes with specifically targeting appropriate bonds of xylan.
The multifunction xylanolytic system exists in bacteria (Zhang et al. 2016a , 2016b ), fungi (Driss et al. 2011 ; Bhardwaj et al. 2018 ) and actinomycetes (Hunt et al. 2016 ) where xylan backbone is randomly cleaved by the action of endo -1,4-β- d -xylanases; xylose polymer is broken down to its monomeric form by action of β- d -xylosidases. Acetyl and phenolic side branches were removed by the action of α-glucuronidase and acetylxylan esterase. α- l -Arabinofuranosidases catalyze the removal of the side groups. The ester bonds present on the xylan are cleaved by the action of p -coumaric esterase and ferulic acid esterase (Beg et al. 2001 ; Collins et al. 2005 ; Chakdar et al. 2016 ; Walia et al. 2017 ). The schematic structure of xylan showing bonds which are attacked by a specific xylanolytic enzyme for complete hydrolysis of xylan to its constituent monomeric units is represented in Fig. 1 .
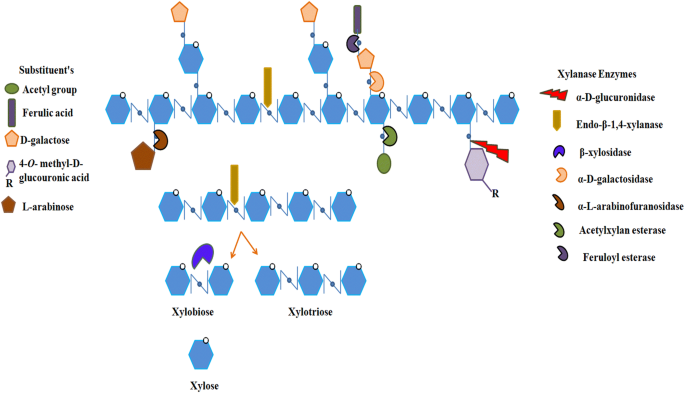
(Adapted from Beg et al. 2001 , Lange 2017 )
Structure of xylan showing bonds which are attacked by specific xylanolytic enzyme for complete hydrolysis of xylan to its constituents
Classification of xylanase
Xylanase can be broadly classified into three types on the basis of (a) molecular mass and isoelectric point, (b) crystal structure and (c) catalytic/kinetic property (Wong et al. 1988 ; Jeffries 1996 ; Biely et al. 1997 ; Liu and Kokare 2017 ). On basis of molecular mass and isoelectric point, the xylanase was classified into two groups, i.e., (a) high-molecular weight with low isoelectric (acidic) point (HMWLI) and (b) low-molecular weight with high isoelectric (basic) point (LMWHI). However, several exceptions to this classification have been observed where not all xylanases fall in the category of HMWLI (above 30 kDa) or LMWHI (below 30 kDa) (Collins et al. 2002 , 2005 ). Therefore, a more appropriate system including primary structure (crystal), comparison of catalytic domain with mechanistic features such as kinetic, catalytic property, substrate specificity, and product description was introduced (Henrissat and Coutinho 2001 ; Collins et al. 2005 ). The genomic, structural (3D crystal structure) and functional information of xylanase is available under glycoside hydrolase (GH) families available on carbohydrate-active enzyme (CAZy) database.
The CAZy is knowledge-based, highly curated database on enzymes that play a key role in breakdown, modification, and assembly of glycosidic bonds in carbohydrates and glycoconjugates. It consists of genomic, sequence annotation, family classifications, structural (3D crystal) and functional (biochemical) information on carbohydrate-active enzyme from publicly available resources such as National Center for Biotechnology Information, NCBI (Lombard et al. 2014 ).
The major GH families associated with xylanase are 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51, and 62. The GH families 5, 7, 8, 10, 11, and 43 have a single distinct catalytic domain, whereas enzymes grouped under GH families 16, 51, and 62 have two catalytic domains with bi-functional property (Collins et al. 2005 ). The enzyme grouped under GH families 9, 12, 26, 30, and 44 has secondary xylanase activity. Based on the hydrophobic cluster analysis of the catalytic domains along with similarities studies of amino acid sequences, xylanases have been primarily classified as GH 10 and GH 11 (Verma and Satyanarayana 2012a ). The catalytic properties of GH 10 and GH 11 have been studied extensively, whereas the information on GH families 5, 7, 8 and 43 is very limited (Taibi et al. 2012 ). Different structural and functional properties of different GH families are tabulated in Table 1 .
Mode of action of xylanases grouped under various GH families
There is the difference in structure, physicochemical properties, substrate specificities and mode of action of members of GH families 5, 7, 8, 10, 11 and 43 (Collins et al. 2005 ). The hydrolysis of xylan by xylanase may occur by two different mechanisms, i.e., retention or inversion (Subramaniyan and Prema 2002 ; Lombard et al. 2014 ).
This process is represented by double displacement mechanism with α-glycosyl and oxo -carbonium intermediate formation followed by its subsequent hydrolysis. Glutamate residues play a vital role in the catalytic mechanism. First, two carboxylic acid residues present in the active site result in α-glycosyl enzyme intermediate formation. The intermediate formation occurs via protonation of the substrate by a carboxylic acid residue acting as an acid catalyst and departure of the leaving group due to nucleophilic attack caused by another carboxylic acid. This collectively results in β to α inversion due to the α-glycosyl enzyme intermediate formation. Second, the first carboxylate group abstracts a proton from a nucleophilic water molecule and attacks the anomeric carbon resulting in second substitution, where the anomeric carbon gives rise to product with the β configuration (α to β inversion) via a transition state of oxo-carbonium ions (Fig. 2 ) (Collins et al. 2005 ; Lombard et al. 2014 ). Enzymes of families 5, 7, 10, and 11 mostly work on the principle of retention.

Mode of action of xylanase: retention
The enzymes of families 8 and 43 act via inversion of the anomeric center with glutamate and aspartate as the major catalytic residue. This is a single displacement mechanism, in which only one carboxylate ion offers for overall acid catalyzed group departure (Fig. 3 ). This enzyme also acts as the base for activating a nucleophilic water molecule to attack the anomeric carbon (depending on the distance between two molecules) for breaking the glycosidic bonds and causing inversion of anomeric carbon configuration (Collins et al. 2005 ; Motta et al. 2013 ; Lombard et al. 2014 ).
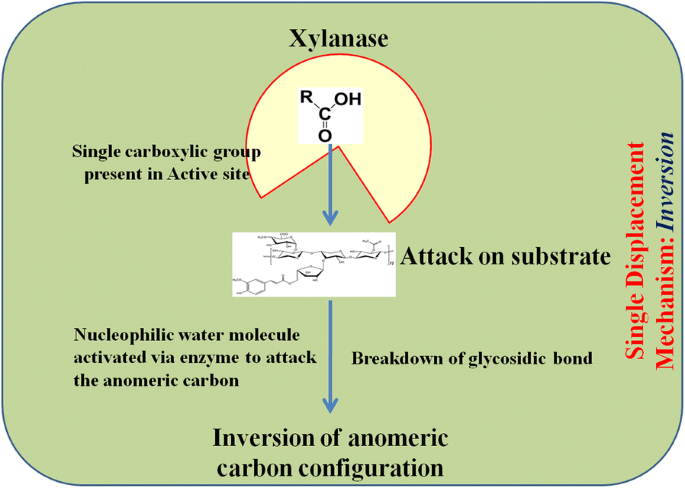
Mode of action of xylanase: inversion
Several attempts have been made to understand the mode of action of xylanase obtained for different organisms. An unusual mode of action of GH8 xylanase (β-xylosidase, an α-arabinofuranosidase, and an acetylesterase activity) was observed in Pseudoalteromonas atlantica , which showed the presence of a long tail of unsubstituted xylose residue on the reducing end of oligosaccharides produced (Ray et al. 2019 ). Thermophilic xylanase obtained from Bacillus licheniformis DMS has novel hydrolysis properties similar to GH30. It breaks the linear β-(1-4) linkage of beech wood and birchwood xylan along with glucuronoxylan and arabinoxylan. B. licheniformis DMS xylanase had both the properties of endoxylanase and appendage dependent xylanase activity. It showed equal production of both xylobiose and xylotriose by hydrolysis of the commercial substrate and agro-waste such as corn cob (Ghosh et al. 2019 ).
Thermothelomyces thermophila ( Tt Xyn30A) that hydrolyzes xylan into xylose and two acidic xylooligosaccharides, namely xylotriose (MeGlcA 2 Xyl3) and xylobiose, i.e., MeGlcA 2 Xyl 2 , was studied. TtXyn30A catalyzed the release of the disaccharide xylobiose from the non-reducing end of xylooligosaccharides, thus exhibiting an exo-acting catalytic behavior. TtXyn30A also showed the capability to cleave linear parts of xylan and uronic xylooligosaccharides as well as resulting in the formation of aldotriuronic and aldotetrauronic acid (Katsimpouras et al. 2019 ). Puchart et al. ( 2018 ) have reported the mode of action of hydrolysis of eucalyptus plant using endoxylanase belonging to GH10, GH11, and GH30 family. All the endoxylanse resulted in the formation of acetylated XOS. The GH10 endoxylanase results in short xylooligosaccharides, whereas GH30 endoxylanase results in longer xylooligosaccharides. An acetyl esterase (AcXEs) played a key role in understanding the plant decay or depolymerization mechanism and also showed efficiency in plant biomass bioconversion (Rytioja et al. 2014 ).
A novel modular endoxylanase with transglycosylation activity was reported from Cellulosimicrobium sp. HY-13 belonging to GH6 family (Ham et al. 2012 ). A GH30 family xylanase XynA was reported from Erwinia chrysanthemi belonging to subfamily 8 with the special property of hydrolyzing 4- O -methyl-glucuronoxylan (Urbániková et al. 2011 ). Xyn11B from thermophilic fungus Humicola insolens Y1 encoding multi-cellular xylanase belonged to GH11 reported by Shi et al. ( 2015 ). Bacteroides intestinalis DSM17393, a xylan degrading human gut bacterium, reported the presence of two putative GH8 xylanases which hydrolyze both xylopentose and xylohexose (Hong et al. 2014 ). Endo xylanase XynB from marine bacterium Glaciecola mesophila KMM241 with xylan binding ability and GH8 catalytic domain was reported by Guo et al. ( 2013 ).
Mechanism for glycosidic hydrolase family 10 (GH10)
Among all the above-mentioned GH families, GH 10 consists of endoxylanase, e.g., endo -1,4-β-xylanases, endo -1,3-β-xylanases and cellobiohydrolases (Collins et al. 2005 ). Endo -1,4-β-xylanases or xylanase mainly comes under this GH10 family. It usually consists of high-molecular weight xylanase with low isoelectric points and displays an (α/β) 8 -barrel fold. This structure mimics the shape of a ‘Salad Bowl’, because of an enlarged loop architecture, one face of the molecule is having ~ 45 Å large radius and the other face is having ~ 30 Å radius because of simple (α/β) turns (Zhang et al. 2016a , 2016b ). However, these two categories are relatively the same because along with sharing similar fold also shares some common residues and has similar catalytic mechanisms. The xylanase belonging to GH 10 family has low substrate specificity and, however, exhibits high catalytic versatility than that of GH 11 family. Xylanase belonging to GH10 family exhibits greater catalytic versatility and lower substrate specificity as compared to those belonging to GH11 (Biely et al. 1997 ; Faulds et al. 2006 ; Motta et al. 2013 ). GH10 xylanase attacks the xylose linkages which are closer to the side-chain residues (Dodd and Cann 2009 ). This could be explained by fact that the xylose residues bind at subsites (Fig. 4 ) on xylanase that causes cleavage of the bond between the monomeric residues at the non-reducing (− 1) and the reducing end (+ 1) of the polysaccharide substrate (Davies et al. 1997 ).
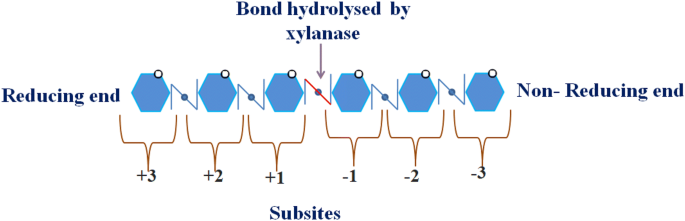
Schematic representation of site for attack of GH10 xylanase on xylan
Maslen et al. ( 2007 ) demonstrated that when arabinoxylan was hydrolyzed by GH10 and GH11 xylanase, the products generated have arabinose residues substituted on xylose at the + 1 subsite and + 2 subsites, respectively. Therefore, xylanases from family 11 and 10 preferentially cleave the unsubstituted regions of the arabinoxylan backbone and the unhampered substituted regions along the xylan backbone (Biely et al. 1997 ; Motta et al. 2013 ). The degree of side-chain decorations of xylan influences the specificity of the enzyme toward substrates and, thus, has an important implication on the hydrolytic product formation by xylan deconstruction (Dodd and Cann 2009 ). Yang and Han ( 2018 ) demonstrated the positional binding and substrate interaction of GH10 xylanase of Thermotoga maritime using molecular docking approach.
Researchers have reported in their previous studies that GH10 endoxylanase had better performance than GH11 in synergy with cellulase enzyme for pretreated lignocellulosic biomass hydrolysis. The reason behind this may be because GH11 endoxylanase has the lower accessibility toward acetylated xylan backbone (Faulds et al. 2006 ). Hu et al. ( 2011 ) proposed a model holocellulosic substrate, i.e., mixture of pure cellulose and 10% pre-deacetylated commercial birchwood xylan to understand the synergism between two family xylanase and cellulase (during the xylan extraction process). This study showed that substrate deacetylation has increased the hydrolytic performance of GH11 as the acetyl group restricted the accessibility of xylan more for GH11 than GH10. Thermostability is the second factor for better performance of GH10 endoxylanase over GH11 because lignocellulosic biomass hydrolysis occurs better at high temperature (50 °C) and 2–3 days long-time duration.
Source for xylanase production
The xylanase is ubiquitous in nature and its presence is observed diversely in a wide range of living organisms, such as marine, terrestrial and rumen bacteria (Chakdar et al. 2016 ), thermophilic and mesophilic fungi (Chadha et al. 2019 ; Singh et al. 2019 ), protozoa (Devillard et al. 1999 ; Béra-Maillet et al. 2005 ), crustaceans (Izumi et al. 1997 ), snails (Suzuki et al. 1991 ), insects (Brennan et al. 2004 ), algae (Jensen et al. 2018 ), plants and seeds (immature cucumber seeds and germinating barley) (Bae et al. 2008 ; Sizova et al. 2011 ). Bacteria and fungus are widely used for industrial production of xylanase. Several microbial sources of xylanase are classified in Table 2 .
Bacterial sources of xylanase
Among bacteria, Bacillus species have been reported widely as the most potent xylanolytic enzyme producers such as Bacillus sp., B. halodurans (Gupta et al. 2015 ) , B. pumilus (Thomas et al. 2014 ), B. subtilis (Banka et al. 2014 ), B. amyloliquefaciens , B. circulans , and B. stearothermophilus (Chakdar et al. 2016 ). Xylanase with high temperature stability, acid/alkali stability, and cold adaptability have been isolated and purified from a wide range of bacteria found in extreme environment. Thermotolerant xylanase active at a very high temperature of 60–70 °C has been reported from Bacillus spp. (Thomas et al. 2014 ), Bacillus Halodurans TSEV1 (Kumar and Satyanarayana 2014 ), Clostridium thermocellum (Fernandes et al. 2015 ), Rhodothermus marinus (Karlsson et al. 2004 ), Streptomyces sp. (Sukhumsirichart et al. 2014 ), Stenotrophomonas maltophila (Raj et al. 2013 ), Thermotoga thermarum (Shi et al. 2013 ). Psychrophilic xylanases are not very common but found to be isolated from several bacteria such as Clostridium sp. PXLY1 (Akila and Chandra 2003 ), Flavobacterium sp. MSY-2 and Flavobacterium frigidarium (Humphry et al. 2001 ; Dornez et al. 2011 ) Pseudoalteromonas haloplanktis TAH3A (Van Petegem et al. 2002 ).
Several alkali stable xylanases have been isolated from firmicutes such as B. pumilus (Thomas et al. 2014 ), B. halodurans TSEV1 (Kumar and Satyanarayana 2014 ) and Geobacillus thermoleovorans (Verma and Satyanarayana 2012b ) and actinomycetes such as Actinomadura sp. Cpt20 (Taibi et al. 2012 ) and Streptomyces althioticus LMZM (Luo et al. 2016 ).
Fungal sources of xylanases
The mesophilic fungi of genera Aspergillus and Trichoderma are well known to be potent xylanase producer and most widely used for commercial production. Thielavia terrestris, (Garcia-Huante et al. 2017 ) , Talaromyces thermophilus (Maalej et al. 2009 ), Paecilomyces thermophile (Fan et al. 2012 ), Achaetomium sp. X2-8 (Chadha et al. 2019 ), Rhizomucor pusillus (Hüttner et al. 2018 ), Rasamsonia emersonii, (Martínez et al. 2016 ) T. Leycettanus (Wang et al. 2017 ) , Melanocarpus albomyces (Gupta et al. 2013 ) and Aspergillus oryzae LC1 (Bhardwaj et al. 2019 ) were found to be producer of hyper-thermophilic active xylanase. Several alkali stable xylanases were obtained from different fungal strains such as Paenibacillus barcinonensis (Valenzuela et al. 2010 ) , Aspergillus fumigatus MA28 (Bajaj and Abbass 2011 ), Cladosporium oxysporum (Guan et al. 2016 ) and Aspergillus oryzae LC1 (Bhardwaj et al. 2019 ).
Strategies employed for xylanase production from different microbial sources
The production of xylanase from microorganisms is affected by the fermentation process employed, choice of substrate and different media components. These components are often regulated by different process optimization for enhanced production of the enzyme for its application at large scale.
Different fermentation process employed for xylanase production: submerged and solid-state fermentation
Xylanases are produced by a different fermentation process using various microorganisms. The better understanding of the physiology and different metabolic processes of the microbial system has led to an improvement in the fermentation process. However, there is still an opportunity to improve the yield of enzymes. The optimization of the xylanase production will be discussed in a later section.
The xylanase production has been carried out under submerged fermentation (SmF) and solid-state fermentation (SSF) (Motta et al. 2013 ). The choice of the fermentation process usually depends on the type of microorganisms used (Table 3 ). Bacteria require a high amount of water during growth; therefore, SmF is preferred whereas fungi due to its mycelia nature require less moisture and can be grown under SSF (Walia et al. 2017 ). Several reports suggest that submerged fermentation using bacteria and fungi is the most preferred method for xylanase production. Statistically speaking approximately 90% of total xylanase is produced globally through SmF. During SmF, the synergistic effect of different xylan degrading enzymes can be observed and even result in better biomass utilization for enhanced xylanase production (Polizeli et al. 2005 ; Bajpai 2014 ). Xylanase production utilizes soybean residues and rice straw as a substrate under SmF by Aspergillus oryzae LC1 and Aspergillus foetidus (Bhardwaj et al. 2017 ; De Queiroz Brito Cunha et al. 2018a , b ). Similarly, Irfan et al. ( 2016 ) suggested the production of xylanase under SmF by B. subtilis BS04 and B. megaterium BM07. Different advantages of the SmF are homogenous condition throughout medium; method is well characterized and can be easily scaled up (Guleria et al. 2013 ). There are some disadvantages to SmF as well which limit its industrial application, i.e., high maintenance cost, energy intensive and complex downstream (Virupakshi et al. 2005 ; Walia et al. 2017 ).
Recent trends suggest that xylanase production by SSF is also gaining popularity (Walia et al. 2014 ). Bacillus sp. was used for the production of thermo-alkalophillic extracellular xylanase under SSF using wheat bran as substrate (Kamble and Jadhav 2012 ). Similarly, SSF of Trichoderma koeningi using corn cob supplemented with pineapple peel powder showed enhanced production of xylanase (Bandikari et al. 2014 ). It has several advantages such as low cultivation, operation and capital cost, a lower rate of contamination, easy enzyme recovery, and high productivity per reactor volume. The disadvantages associated with SSF are not suitable for all microorganisms (preferred for the fungal system) and require proper aeration and humidity control and up-scaling is a tedious process (Mienda et al. 2011 ).
Selection of suitable substrate for xylanase production
Quantity and quality of the fermentation product vary with different substrates. There are various commercially available substrates, i.e., xylan, carboxymethyl cellulose (CMC), pectin, and starch for, i.e., xylanase, cellulase, pectinase, and amylase, respectively (Barman et al. 2015 ; Bhardwaj et al. 2017 ; Kumar et al. 2018a ). Due to the high cost of commercial substrates and considering the economic feasibility of the process, scientists are working from past several years to find alternative substrates for the production of these enzymes.
Agrowastes and other organic wastes (domestic and industrial) are used as a carbon source for the production of xylanase with the focus on sustainability and best utilization of these wastes (Table 3 ). Some of the most commonly used agro-residues for xylanase production are wheat bran, wheat husk (Kumar et al. 2018a , b , c , d ), rice straw (Bhardwaj et al. 2017 ), rice husk, sugarcane bagasse (Suleman and Aujla 2016 ), coconut coir, coconut oil cake (Rosmine et al. 2019 ), groundnut shell (Namasivayam et al. 2015 ), wood pulp (Kalpana and Rajeswari 2015 ), sawdust, chilli post-harvest (Sindhu et al. 2017 ), corncobs, molasses, sugar beet pulp fruit, and vegetable waste (Bandikari et al. 2014 ). Recent studies also showed that wastewater from pulp industry was reused as media for xylanase production (de Queiroz-Fernandes et al. 2017 ).
Role of important media components used for xylanase production
Naturally, xylanolytic enzymes are induced by the different intermediate products generated by their own action. Xylan is found to be best xylanase inducer (Taibi et al. 2012 ; Guleria et al. 2013 ; Walia et al. 2013 , 2014 ). However, xylan being a high-molecular weight polymer cannot stimulate xylanase as it cannot enter the microbial cells. Therefore, a small amount of constitutive enzyme produced in the media results in the generation of low-molecular weight fragments, i.e., xylobiose, xylotriose, xylotetraose, xylose from the breakdown of xylan and further induces the xylanolytic enzymes for enhanced enzyme production (Walia et al. 2017 ). Cellulose, synthetic alkyl, aryl β- d xylosides, and methyl β- d -xyloside also act as an inducer for xylanolytic enzyme production (Thomas et al. 2013 ). Busk and Lange ( 2013 ) observed that poor quality paper can efficiently induce the xylanase production in Thermoascus aurantiacus even in the absence of xylan and xylooligosaccharides.
Nitrogen is an important structural element required for the metabolic processes in the microbial system. Therefore, the choice of nitrogen source is important for the growth of microorganisms that subsequently affect the overall enzyme yield. Peptone, tryptone, soymeal, yeast extract, etc. have found to be suitable nitrogen source. The requirement of these nitrogen sources varies for different microorganisms; therefore, optimizing the type and level of nitrogen source in the media is an important parameter (Seyis and Aksoz 2005 ; Naveen et al. 2014 ; Irfan et al. 2016 ). Trace elements, amino acids, and vitamins are also important parameters for the growth of different microorganisms (Simair et al. 2010 ; Bibra et al. 2018 ). Therefore, regulating their levels in the media is important for regulating the production of xylanase. Also, the addition of biosurfactant such as Tween 80 affected the level of xylanase production (Liu et al. 2006 ; Kumar et al. 2013 ).
Strategies employed for the selection of the method of xylanase production and its optimization
Intially a common minimal media providing essential nutrients to the growth of microorganisms are used. This will allow to check the strains are capable of producing required enzymes/metabolite of desired interest. Then, the process is further optimized for higher production of enzymes from the strain (Walia et al. 2017 ). For the production of desired product, different strategies are used for improving yield such as optimization of media components, regulating physical growth parameters, and improving the strain by use of the different biotechnological tool (Sharma 2017 ). The schematic representation of the methodology adapted for production, purification and characterization of xylanase are shown in Fig. 5 . In this section, the focus will be on the optimization of media and growth parameters and biotechnological tool approach will be discussed in a later section. During SmF for enzyme production, different components which need to be optimized are selection of substrate and microorganisms, regulation of nutrients concentration in media, i.e., carbon, nitrogen, trace elements, vitamins and amino acids, and physical parameters, i.e., temperature, pH, agitation, aeration, inoculum sizes, and incubation period (Motta et al. 2013 ; Walia et al. 2015a , 2017 ).
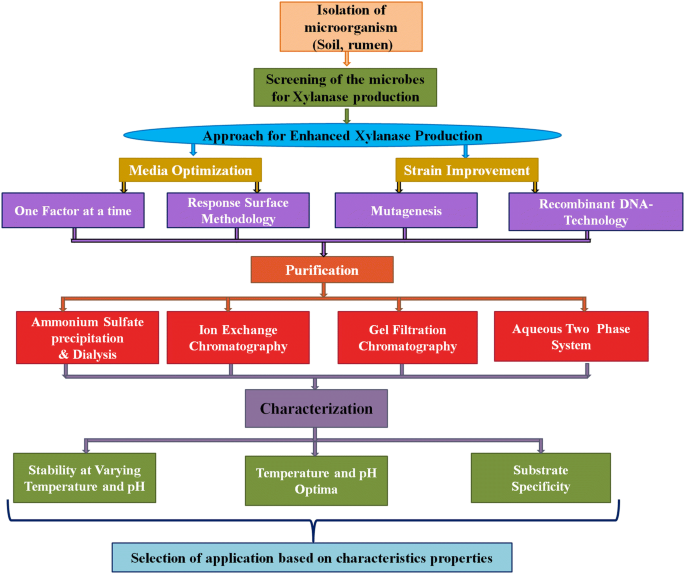
Schematic representation of the methodology for production, purification and characterization of xylanase
During optimization of the SSF, there is requirement of regulating particle size, pretreatment, humidity, water content and water activity (a w ) of substrate, type and size of the inoculums, removal of extra heat generated during microbial metabolism and most importantly maintaining the uniform environment (temperature) and evolution of CO 2 and consumption of O 2 , i.e., gaseous system (Murugan et al. 2011 ; Behera and Ray 2016 ; Behnam et al. 2016 ; Leite et al. 2016 ; Walia et al. 2017 ).
Approach for enhanced xylanase production: one factor at a time (OFAT)
To proceed for the optimization of the xylanase production, one factor at a time (OFAT) approach is used for the selection of important factors affecting the xylanase yield. In the OFAT approach, one factor is kept variable keeping other factors at constant (Bhardwaj et al. 2018 ). The factor may be important physical or nutritional parameters regulating the growth of microorganisms and its enzyme yield. Ramanjaneyulu et al. ( 2017 ) have evaluated several operating parameters for nutritional (different substrates and their concentrations, additional carbon and nitrogen sources) and physical factors (incubation temperature, pH, agitation) along with inoculum size of Fusarium sp. BVKT R2 in a shake flask culture (SmF) by OFAT approach. The high xylanase yield of 4200 U/mL was obtained with birch wood xylan in mineral salt medium with 1.5% sorbitol (additional carbon source), 1.5% yeast extract (nitrogen source) at temperature of 30 °C, pH of 5.0, agitation of 200 rpm and inoculum of agar plugs (6) for only 5 days incubation. Under unoptimized condition, xylanase yield was only 1290 U/mL after 7 days of incubation, thus improving by 3.2-fold. Bhardwaj et al. ( 2018 ) also optimized xylanase production using Aspergillus oryzae LC1 using OFAT approach. The physical parameters (liquid to solid ratio, pH, inoculums size, incubation time and temperature) and nutrient parameters (substrate concentration) were optimized using OFAT approach for enhanced production of xylanase by T. viride -IR05 under SSF (Irfan et al. 2014 ).
Statistical approach for enhanced xylanase production
The OFAT approach is tedious and requires a large set of experiments for optimization. The recent trend suggested the application of the statistical approach to design experiments considering different factors as variable and performing the interaction studies among several physical and nutritional parameters. The statistical-based approach has shown satisfactory results for optimization of xylanase production using fungal and bacterial strains with the minimum number of experimental sets (Guleria et al. 2015 , 2016a ; Walia et al. 2015b ; Bhardwaj et al. 2017 ).
Response surface methodology (RSM) was employed to optimize the fermentation medium constituents and the physical factors affecting xylanase production using Bacillus tequilensis strain ARMATI under SmF (Khusro et al. 2016 ). The experimental design consists of central composite design (CCD) with four (4) independent variable (carbon and nitrogen source, temperature and time) resulting in 30 experimental runs. The central composite design gave an optimum parameter for studied variable (1.5% w/v birchwood xylan, 1% w/v yeast extract, temperature 40 °C, time 24 h) showing 3.7-fold enhanced xylanase production as compared to OFAT. High coefficient of determination (R 2 ) of 0.9978 with p < 0.05 as obtained by analysis of variance (ANOVA) analysis suggested the accuracy of the overall process at a significant level. The R 2 value of 0.9978 represents that sample variation of 99.78% and only 0.21% of the total variation in the response cannot be explained by the model. The xylanase obtained has shown high thermal (60 °C) and alkali stability (pH 9). Bhardwaj et al. ( 2017 ) optimized nutritional components (rice straw, MgSO 4 , and CaCl 2 concentration) and physical parameters (temperature and pH) for enhanced xylanase production with an Aspergillus oryzae LC1 under submerged fermentation using CCD-RSM. The statistical design suggested optimum condition of 1% rice straw (w/v), 1.0 g/L CaCl 2 , and 0.3 g/L MgSO 4 , with pH 5 and 25 °C. It resulted in maximum xylanase activity of 935 ± 2.3 IU/mL which is 3.8-fold higher than the un-optimized Mendel’s Stenberg Basal Salt medium (245 ± 1.9 IU/mL). The enzyme showed thermal (25–60 °C) and pH (3–10) stability. The xylanase also showed potential for efficient enzymatic hydrolysis of different lignocellulosic agro-residues.
Similarly, Tai et al. ( 2019 ) reported the optimization of five physical and two nutritional parameters using the RSM approach for enhanced xylanase production. Indigenous fungus Aspergillus niger DWA8 was grown under SSF on an oil palm frond. One physical (moisture content 75%) and one nutritional parameters (substrate concentration 2.5 g) have significant effect on xylanase production. Under optimum condition, an increase in xylanase yield by 78.5% was observed as compared to an un-optimized condition. The xylanase was efficiently used for saccharification of biomass. The statistical optimization method for enhanced xylanase production has been applied and widely accepted for SSF and SmF that helped in overcoming several limitations of classical empirical (OFAT) methods.
Biotechnological approach for enhanced xylanase production
There is a need of high yield of the enzyme with specific properties such as high stability over a wide range of temperature and pH, high substrate specificity and strong resistance to metal cations and chemicals for the industrial application (Garg et al. 2010 ; Qiu et al. 2010 ). The native enzyme is usually produced in low quantity and also lacks all the characteristics to meet the industrial needs (Ahmed et al. 2009 ). Therefore, different biotechnological approaches are used for improving the yield and imparting characteristic properties to the desired enzyme. These approaches involve genetic manipulation involving mutation and recombinant DNA technology.
Mutagenesis of microorganisms for enhanced xylanase production
Several researchers suggested that the application of physical mutagens such as UV radiation (Rahim et al. 2009 ; Abdel-Aziz et al. 2011 ) and chemical mutagens such as N -methyl N -nitro N -nitroso guanidine (MNNG) (Haq et al. 2004 , 2008 ) resulted in enhanced xylanase production. Burlacu et al. ( 2017 ) demonstrated the improvement of xylanase production in fungal strains, i.e., Aspergillus brasiliensis and Penicillium digitatum by physical mutagenesis (5–50 min, exposure to UV light) and chemical mutagenesis (150 µg/mL of N -methyl- N ′-nitro- N -nitrosoguanidine or ethyl methane sulfonate). The exposure to physical and chemical mutagens has resulted in significant changes in the mutant strain as compared to the wild type. Han et al. ( 2017 ) demonstrated the site-directed mutagenesis of XynCDBFV gene of ruminal fungus Neocallimastix patriciarum for improving the thermostability of XynCDBFV, a glycoside hydrolase (GH) family 11 xylanase. Similar work has also been carried out in different bacterial strains, a rifampin-resistant mutant of Cellulomonas biazotea , designated 7Rf, resulting in elevated levels of xylanases production as compared to the parental strain. After mutation, maximum xylanase and β-xylosidase production of 493 IU/L/h and 30.7 IU/L/h of β-xylosidase were obtained respectively. This increase in xylanase and β-xylosidase yield were 1.21- and 2.29-fold higher respectively as compared to the parental strain (Rajoka et al. 1997 ). Bacillus mojavensis PTCC 1723 when subjected to UV light exposure (280 nm, 30 s) resulted in the xylanase yield 330.6 IU/mL which is 3.45 times higher as compared to 95.7 IU/mL for wild strain (Ghazi et al. 2014 ). Lu et al. ( 2016 ) demonstrated mutation of XynHB, alkaline stable xylanase from Bacillus pumilus HBP8 at N188A. The mutant XynHBN188A is expressed in E. coli and Pichia pastoris with improved xylanase yield by 1.5- and 7.5-fold, respectively. The codon-based optimization and high-density fermentation using Pichia pastoris system were utilized for improving the xylanase yield.
Gene cloning and expression of xylanase genes using recombinant DNA
The recombinant xylanases are designed to have equivalent or better properties than the wild-type enzymes with high yield in the expression system which can be employed in the fermentation industry. The highly thermo-alkalophilic xylanase producing strains can be directly employed during simultaneous saccharification and fermentation for ethanol generation using lignocellulosic biomass. The inherited stability will enable the xylanase to work efficiently even at high temperature and varying pH range of the fermentation system.
Several reports suggests that desired xylanase gene was cloned into the suitable vector followed by its expression in the suitable microbial systems such as bacteria, yeasts, and fungus (Belancic et al. 1995 ; Goswami et al. 2014 ; Jhamb and Sahoo 2012 ; Juturu and Wu 2012 ; Motta et al. 2013 ; Nevalainen and Peterson 2014 ; Verma et al. 2013 ).
Expression in bacteria
Goswami et al. ( 2014 ) demonstrated the expression of a xylanase gene from Bacillus brevis in E. coli BL21. The recombinant strain predominantly secreted xylanase in the culture medium with 30 IU/mL xylanase activity. The culture filtrate is free from cellulase activity and found to be active in a wide range of pH and temperature. A thermo-alkali stable xylanase encoding gene (Mxyl) was retrieved from compost-soil metagenome library construct and cloned into pET28a vector expressed in E. coli BL21(DE3). The recombinant xylanase has shown half-life of 2 h and 15 min at 80 °C and 90 °C, respectively. The recombinant xylanase has pH and temperature optima of 9.0 and 90 °C, respectively (Verma et al. 2013 ).
Escherichia coli is preferred and most widely used expression host due to its inexpensive growth conditions, easy manipulation, simple transformation techniques requirement, high level of product accumulation in the cell cytoplasm (Jhamb and Sahoo 2012 ). However, efficient and functional expression of many xylanase genes is not possible with E. coli which may be due to repetitive appearance of rare codons and the requirement for specific translational modifications (disulfide-bond formation and glycosylation) (Belancic et al. 1995 ; Jhamb and Sahoo 2012 ; Juturu and Wu 2012 ; Motta et al. 2013 ). One of the other important concerns associated with E. coli is the presence of endotoxins (lipopolysaccharide) which makes the protein purification process very tedious. Lactobacillus and Bacillus species are used for heterologous expression of xylanase than in E. coli . It is capable of performing N-glycosylation, generally regarded as safe (GRAS) due to the absence of endotoxins and their secretory production is beneficial in industries (Bron et al. 1998 ; Subramaniyan and Prema 2002 ; Upreti et al. 2003 ; Juturu and Wu 2012 ). Zhang et al. ( 2010a , b ) demonstrated the expression and characterization of the xylanase gene (xynB) from Dictyoglomus thermophilum Rt46B.1 in Bacillus subtilis system. The pH and temperature optima for the purified recombinant enzyme were 6.5 and 85 °C, respectively. The xylanase was stable up to 95 °C and retained its activity in surfactants such as EDTA, DTT, Tween-20 and Triton X-100.
Expression in yeast
The heterologous protein expression in yeast system is highly attractive due to its ability to perform eukaryotic post-translational modifications and can grow to very higher cell densities with the ability to secrete enzyme into the fermentation system. Most of the yeasts are considered as GRAS organisms and do not produce toxins (Juturu and Wu 2012 ). Saccharomyces cerevisiae already established as an industrial microorganism, thus, can be conveniently used for xylanase production (Ahmed et al. 2009 ).
The application of Pichia pastoris as expression system has gained impetuous because it can promote the expression of the protein on their own using alcohol oxidase as promoter using methanol utilization pathway (Ahmed et al. 2009 ; Juturu and Wu 2012 ; Motta et al. 2013 ). Pichia pastoris as expression system is preferred as it can grow to very high cell densities, inherit strong and tightly regulated promoters, and produce high titer of recombinant protein (g/L) both intracellularly and in the secretory manner (Ahmad et al. 2014 ). Basit et al. ( 2018 ) demonstrated the cloning of two GH11 xylanase genes, MYCTH_56237 and MYCTH_49824 , from thermophilic fungus Myceliophthora thermophila and its expression in Pichia pastoris . The specific activities of purified recombinant xylanase were observed at 1533.7 U/mg and 1412.5 U/mg for MYCTH_56237 and MYCTH_49824 , respectively. The recombinant xylanase showed stability under harsh condition (high pH and temperature) and high efficiency for biomass saccharification. However, the application of Pichia pastoris at large scale is limited due to health and fire hazards of methanol (Ahmed et al. 2009 ). In the case of P. pastoris as expression system, lower protein yield was obtained while expressing membrane-attached protein or proteolytic degradation prone protein and complex protein such as hetero-oligomers (Ahmad et al. 2014 ).
Expression in filamentous fungi
Filamentous fungi can be efficiently used for heterologous and homologous gene expression resulting in high yield of recombinant gene products (Su et al. 2012 ; Motta et al. 2013 ; Nevalainen and Peterson 2014 ; Nevalainen et al. 2018 ). Similar to yeast, it can regulate expression yields with their own promoters and can provide eukaryotic style post-translational modification of proteins such as N-glycosylation, proteolytic processing, or formation of multiple disulfide bonds (Ahmed et al. 2009 ; Fleissner and Dersch 2010 ; Landowski et al. 2015 ).
The application of fungi as an expression system also has advantages associated with cost-effectiveness of the overall process due to low-cost substrate and downstream processing. Further, fungi have already been subjected to many strain improvement procedures for enhanced production of xylanase. Therefore, the native xylanase expressing machinery can be efficiently used for functional expression of a foreign xylanase gene from other sources. Xyn2 xylanase gene was expressed in T. reesei by homologous expression resulting in the 1.61 g/L of xylanase 2 on glucose-containing medium (Li et al. 2012 ). Godlewski et al. ( 2009 ) demonstrated xylanase B(XynB) gene expression in T. reesei. Similarly, the expression of xylanase 2 (XYN2) and xylanase gene from the thermophilic fungus Humicola grisea var. thermoidea and P. griseofulvum was expressed in Trichoderma reesei and Aspergillus oryzae , respectively (De Faria et al. 2002 ; Motta et al. 2013 ). Nevalainen and Peterson ( 2014 ) presented a comprehensive review on application of filamentous fungus as expression system and suggested that research is now focused on understanding the cellular mechanisms for better internal protein quality control and secretion stress. The better utilization of “omics” tools can help in improving the regulation of xylanase production using filamentous fungus as an expression system.
Strategies for enhanced purification and characterization of xylanase for industrial application
The microbial system produces a wide range of biochemical’s during different growth and development of the microorganisms. These biochemical’s are enzymes, secondary metabolites, etc. which are of great importance to human applications. Similarly, enzymes are produced by microorganisms along with other enzymes or metabolites. Therefore, purification is prerequisites for obtaining pure enzyme with minimum or no impurities (Zhang et al. 2012 ). The characterization of the purified enzyme such as evaluation of temperature and pH optimum, thermal and acid/alkali stability, role of metal ions and inhibitors in regulation of enzyme activity, and substrates specificity was performed for selecting the suitable industrial process (Bhardwaj et al. 2019 ). There are different enzyme purification strategies for the xylanase such as ammonium sulfate precipitation (salting in) followed by dialysis (salting out), gel permeation chromatography, ion exchange chromatography, recently developed techniques aqueous phase chromatography and ultrafiltration (Walia et al. 2014 ; Guleria et al. 2016b ; Bhardwaj et al. 2019 ).
Ammonium sulfate precipitation followed by dialysis
The crude xylanase preparation is subjected to different ranges of ammonium sulfate concentration (30–90%) for selection of suitable salt concentration for precipitation of the enzyme. The precipitated enzyme is then subjected to dialysis for removal of the salt. The crude xylanase obtained from Streptomyces P12-137 was subjected to ammonium sulfate precipitation (40–90%) followed by dialysis. The purification fold of 4.18 was observed with two different endoxylanase observed as F5 (65%) and F6 (80%) with the specific activity of 45.4 U/mg and 36.5 U/mg, respectively. This was also confirmed by HPLC analysis. The purified enzyme was further characterized by incubating at different temperature and pH followed by analyzing the enzyme for xylanase activity. The optimum pH and temperature of pH 7.0, 60 °C and 6.5, 60 °C, for F5 and F6 xylanase, respectively, were obtained (Coman et al. 2013 ).
Bhardwaj et al. ( 2017 ) performed partial purification of the crude xylanase obtained from Aspergillus oryzae LC1 using ammonium sulfate (60%) precipitation followed by dialysis against 50 mM acetate buffer (pH 5.0). The partially purified enzyme was further characterized which showed stability over a wide pH range of 3 to 10 and thermal stability over the temperature range of 25 to 60 °C. Similarly, Kumar et al. ( 2018d ) have demonstrated the purification and characterization of xylanase obtained from sea sediment bacteria using a combination of ammonium sulfate precipitation and dialysis. The improvement in specific activity and characteristic properties of xylanase was observed. The major limitations of the precipitation are needed to remove salt from protein sample so further processing in the form of dialysis or chromatography is required. Further, for dialysis, there is a need to have a better understanding of the protein solubility. It is also stated that ammonium precipitation concentrates the protein rather than purifying it. Thus, contaminant present in the crude sample may also be present along with the protein sample even after precipitation and dialysis (Biosciences 2019 ). The xylanase is also concentrated or precipitated using trichloroacetic acid (TCA) and acetone. However, the TCA may denature the protein; therefore, it is not advisable to use TCA when the protein is required in the folded state (for activity assay) and the toxicity of TCA also limits its applications (Koontz 2014 ).
Chromatography techniques for enhanced xylanase yield employed for purification
Usually, it has been observed that xylanase purification was performed by the multi-step process where the concentration of protein using ammonium acetate/TCA/acetone precipitation or ultracentrifugation was followed by a single step or series of chromatography techniques. Yadav et al. ( 2018 ) demonstrated the purification and characterization of extracellular xylanase obtained from A. kamchatkensis NASTPD13 cultures. The crude xylanase was subjected to ammonium sulfate (80%) precipitation followed by dialysis. The dialyzed sample was further subjected to Sephadex G100 column chromatography. The fractions collected showing maximum xylanase activity were concentrated and analyzed by SDS-PAGE (MW obtained was 37 kDa). The two-step purification has led to increased xylanase activity by 11-fold with a 33 U/mg specific activity. The characterization of purified protein showed pH and temperature optimum of 9.0 and 65 °C, respectively, and also retained more than 50% of its activity over a wide range of 6–9 pH and 30–65 °C temperature. An insight into several purification strategies employed for xylanase from different microorganisms along with the process efficiency in terms of recovery potential and kinetics property is tabulated in Table 4 .
Purification of endoxylanase obtained from Bacillus pumilus B20 was performed in three steps (Geetha and Gunasekaran 2017 ). The first step was ammonium sulfate precipitation (60–80%) followed by FPLC using DEAE Sepharose column as the second step and further subjecting the eluted sample onto a Sephacryl S-200 column as the third purification step. At each step, the specific activity was improved as compared to the crude enzyme by 5 to 14.8-fold with maximum 755.8 U/mg specific activity at the end of all the three purification steps. After the purification, the fractions showing maximum xylanase activity were subjected to xylanase assay and other characterization studies such as SDS-PAGE, zymography, temperature and pH stability. The SDS-PAGE and zymography analysis showed the purified enzyme of ~ 85 kDa, i.e., endoxylanase (XylB). The purified enzyme was stable in a pH range of pH 6.5 to 7.5 and the temperature range of 20 to 50 °C. The purified enzyme was highly specific to different commercial and natural xylan substrate and has the potential to generate xylooligosaccharides.
Aqueous two-phase system employed for purification of xylanase
The conventional multistep purification techniques are time consuming, which increases the cost of the overall process and also results in loss of protein at each step (Iqbal et al. 2016 ; Ramakrishnan et al. 2016 ). The 60–70% of total processing cost in enzyme downstream process comes from the purification step (Loureiro et al. 2017 ; Bhardwaj et al. 2019 ). Therefore, several scientists suggested a single step liquid–liquid fractionation technique, i.e., aqueous two-phase system (Naganagouda and Mulimani 2008 ; Yasinok et al. 2010 ; Glyk et al. 2015 ).
Garai and Kumar ( 2013 ) purified alkaline xylanase from Aspergillus candidus using aqueous two-phase system (ATPS) composed of PEG 4000/NaH 2 PO 4 system. The critical factors of ATPS such as PEG molecular weight, PEG and phosphate salt concentration using Box–Behnken design approach were used for the optimization of enhanced xylanase purification. The optimum condition was PEG 4000 at 8.66% w/w with a high salt concentration of 22.4 w/w that resulted in 8.41% purification fold. The enzyme was stable at alkaline pH and activity is enhanced with Mn 2+ ions. Ng et al. ( 2018 ) demonstrated the recovery of xylanase from Bacillus subtilis fermentation broth with an alcohol/salt ATPS. The ATPS system consists of 26% (w/w) 1-propanol and 18% (w/w) ammonium sulfate resulting in 5.74 ± 0.33 purification fold and yield of 71.88% ± 0.15.
Gómez-garcía et al. ( 2018 ) demonstrated purification of xylanase by Trichoderma harzianum using ATPS with PEG/salt system. The PEG molecular weight, PEG, phosphate salt concentration, and salt conditions were optimized. The best enzyme recovery and purification fold of 62.5% and 10% respectively was obtained using 20.2% PEG 8000, 14.8% K 2 HPO 4 , and tie to a length of 45% w/w. Bhardwaj et al. ( 2019 ) subjected crude xylanase from Aspergillus oryzae LC1 to four different single-step purification by ammonium sulfate precipitation, ion exchange, gel filtration chromatography and ATPS PEG/Salt system. The xylanase purification using single-step ATPS system resulted in highest purification yield (PY) of 86.8% and 13-fold purification fold (PF) which was much higher than other purification strategy, i.e., ammonium precipitation (PY-21%, PF-4.1), anion exchange (PY-31.9%, PF-3) and gel filtration (PY-78.7%, PF-6.6).
Therefore, ATPS exhibits several advantages over traditional purification techniques, i.e., it requires low-cost materials, low energy consumption with high yield and better resolution (Naganagouda and Mulimani 2008 ; Yasinok et al. 2010 ; Glyk et al. 2015 ). The ATPS method is independent of protein concentration and does not affect the native property of protein (Iqbal et al. 2016 ; Ramakrishnan et al. 2016 ).
Structural properties of xylanase responsible for thermal and pH stability required for industrial application
The high stability of xylanase was due to the presence of intrinsic structural properties. The presence of extra disulfide and salt bridges, hydrophobic side chains, and N-terminal proline residues helps in reduction of conformational freedom of the protein structure. Thus, it help in providing more stability to protein at the higher temperature (Turunen et al. 2001 ; Chen et al. 2015 ). Different structural modifications such as high Thr/Ser ratio and high charged residues, i.e., Arg, cause enhanced polar interaction and improved stabilization of the alpha-helix region and secondary structures (Hakulinen et al. 2003 ). The xylanase protein has a large number of ion pairs/aromatic residues on the surface of protein resulting in enhanced interactions (Polizeli et al. 2005 ; Chen et al. 2015 ). The low average protein rigidity i.e. low B factor, low flexibility results in high rigidity at extreme physical conditions (Xie et al. 2014 ). The presence of divalent metal ions and removal of N or C terminal disordered residues protect xylanase from heat and protease inactivation (Andrews et al. 2004 ; Chen et al. 2015 ). The presence of carbohydrate-binding modules (CBM22 and CBM9) at N or C terminal often imparts heat stability to xylanase. The pH stability of the xylanases is often affected by the presence of several amino acids near the catalytic residues (Singh et al. 2019 ).
Cost estimation of the xylanase production
Polizeli et al. ( 2005 ) suggested that 20% of the total global enzyme production is from biomass hydrolysis enzymes, i.e., xylanase, cellulase and pectinases. An extensive study on cost involved in each step of xylanase production at industrial scale is unavailable on public domain. Klein-Marcuschamer et al. ( 2012 ) performed a study on the cost analysis of application of enzymes during the lignocellulosic biomass based biofuel production and suggested breakdown of the operating cost (annual) in their enzyme production facility. They suggested percentage of cost involved for each component, i.e., raw materials (28%), labor (7%), transportation (1%), consumables (4%), utilities (10%), facility dependent (48%), and waste treatment (2%). This clearly shows that maximum contribution of 48% comes from the capital investment followed by cost of substrate (28%). Klein-Marcuschamer et al. ( 2012 ) also suggested the baseline production cost of hydrolysis enzyme as $10.14/kg.
Da Gama Ferreira et al. ( 2018 ) performed techno-economic analysis of the β-glucosidase enzyme production from E. coli on industrial scale. They showed major cost during industrial production are facility dependent (45%) followed by raw materials (25%) and consumables (23%), that are similar to observations made by Klein-Marcuschamer et al. ( 2012 ). Capital investment/facility-dependent cost is required for development of infrastructure (i.e., equipments), insurance, maintenance and depreciation. This upstream and downstream process during enzyme production involves the cost on part of capital investment along with the cost of consumables and utilities. da Gama Ferreira et al. ( 2018 ) performed sensitivity analyses of process scale, inoculation volume with respect to volumetric productivity, which suggested that remarkable reduction in cost of enzyme production may be observed under optimized conditions. Thus, based on the above studies, we can suggest that xylanase production can be based on the cost of substrate and consumable, along with the cost of each step involved in upstream and downstream processing. Therefore, utilizing cheap raw materials, less number of steps during upstream and downstream process (such as single step purification instead of multistep process) can help in keeping the enzyme production cost as low as possible.
Xylanase employed as a greener tool in different industries
Xylanase with such unique characteristics of thermo-alkali tolerant nature has a diverse range of application in different industries such as paper and pulp, deinking, biomass utilization and food feed industries (Fig. 6 ).
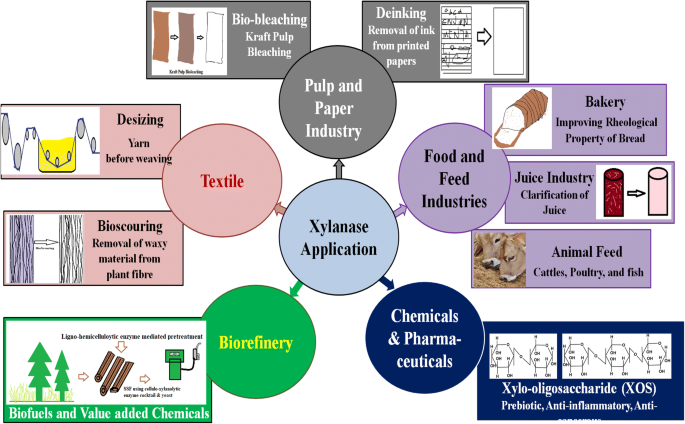
Xylanase as a greener tool in different industries
Xylanase employed in the food and feed industry
The xylanase finds application in food industries such as bakery. The bread is made up of wheat consisting of hemicelluloses such as arabinoxylan. The xylanase can solubilize the water unextractable arabinoxylan into water-extractable arabinoxylan (Courtin and Delcour 2002 ). This help in uniform water distribution and improvement in gluten network formation throughout the dough. The addition of xylanase improves the rheological properties of dough such as softness, extensibility, and elasticity along with bread-specific volume and crumb firmness (Harbak and Thygesen 2002 ; Camacho and Aguilar 2003 ; Butt et al. 2008 ). The breakdown products of arabinoxylan, i.e., arabino-xylooligosaccharides in bread have its health benefits (Polizeli et al. 2005 ; Bajpai 2014 ).
Butt et al. ( 2008 ) demonstrated the role of GH11 endoxylanases from B. subtilis in solubilizing the arabinoxylan. This increases the viscosity and volume of dough and decreases gluten agglomeration and dough firmness resulting in the development of uniform and fine crumbs. GH11 xylanase (0.12 U/g flour) from Penicillium occitanis Pol6 resulted in improvement of bread-making process such as the decrease in water absorption (8%) and an increase in dough rising (36.8%), volume (17.8%), specific volume (34.9%) and cohesiveness. The bread has improved rheological and sensory properties (texture, taste, flavor, softness, and overall acceptability). Low springiness and gumminess were observed in the bread prepared using xylanase (Driss et al. 2013 ). Partially purified microbial xylanase was used by Ghoshal et al. ( 2013 ) to produce whole‐wheat bread with better sensory properties (brighter color). The addition of xylanase also resulted in increased specific volume, and shelf life, with lower firmness and reduced staling during storage. Panzea, new generation xylanase obtained from Bacillus Licheniformis , can help in improving dough properties at low enzyme dosage. It helps in achieving the desired texture, appearance, loaf volume and crumb structure (Bajpai 2014 ). Similarly, recombinant xylanase (r-XynBS27) obtained from Pichia pastoris ( xynBS27 gene from Streptomyces sp. S27) used as an additive during bread-making process. The recombinant xylanase resulted in improvement in a specific volume and reducing sugar content with a decrease in firmness, consistency, and stiffness (De Queiroz Brito Cunha et al. 2018a , b ).
Fruit juice clarification
The enzymatic process in fruit juice extraction and clarification is widely used. Raw juices of fruit contain polysaccharides such as cellulose, hemicellulose, starch pectin and surface-bound lignin and decrease the quality of the juice, e.g., hazy color and high viscosity (Danalache et al. 2018 ). The use of enzymes decreases the viscosity and avoids the formation of clusters, by removing the suspended and undissolved solid using centrifugation and filtration methods. This increases the clarity, aroma, and color of the juice (Danalache et al. 2018 ). Xylanase from Streptomyces sp was used for the clarification of orange, mousambi, and pineapple with 20.9%, 23.6% and 27.9% clarity, respectively (Rosmine et al. 2017 ). Immobilized xylanase obtained from Bacillus pumilus VLK-1 was used for orange (29%) and grape juice (26%) enrichment (Kumar et al. 2014 ). Xylanase immobilized on 1,3,5-triazine-functionalized silica-encapsulated magnetic nanoparticles was reported to clarify the three different types of fruit juices after five hours of incubation at 50 °C (Shahrestani et al. 2016 ). Partially purified xylanase from Streptomyces sp AOA40 was used in fruit juice industry for increased clarity of juices from apple (17.8%), orange (18.4%) and grape (17.9%) (Adigüzel and Tunçer 2016 ). Glutaraldehyde-activated immobilized xylanase was used for the clarification of tomato juice. Xylanase from P. acidilactici GC25 was used to treat the kiwi, apple, peach, orange, apricot, grape, and pomegranate in which increase in the amount of reducing sugar was observed along with the decrease in turbidity of the juice (Adiguzel et al. 2019 ).
Animal feed
Xylanases plays an important role in animal feed by breaking the feed ingredient arabinoxylan and reduces the raw material viscosity. Aspergillus japonicus C03 with good endoxylanase and cellulase production ability with high stability in the presence of goat ruminal environment showed ruminant feed applications (Facchini et al. 2011 ). A number of studies reported the availability of distillers dried grains with soluble (DDGS) to be utilized in animal feeds and use of exogenous xylanase in poultry diets to treat the higher fiber content (Pirgozliev et al. 2016 ; Whiting et al. 2019 ). The exogenous enzymes effectively improved the nutritional value of co-products of bioethanol as reported previously with DDGS obtained from corn (Liu et al. 2011 ). Xylanases have been involved in animal feed over decades, as it reduces the viscosity of digesta in poultry. Xylanase addition showed the weight gain improvement and enhanced feed conversion ratio because of the improvement in the arabinoxylan digestibility in monogastric animal diets (Paloheimo et al. 2010 ; Van Dorn et al. 2018 ). Xylanase utilized as a dietary supplement for the nutrients digestibility, digesta viscosity growing pigs fed corn intestinal morphology diet based on soybean meal was reported by Passos et al. ( 2015 ). ECONASE XT a well-known commercial endo -1,4-β-xylanase which has been used as feed additives for chicken fattening, weaned piglets and fattening for pigs (Rychen et al. 2018 ).
Xylanase in paper and pulp industries
Bio-bleaching.
The process of removal of lignin from wood pulp to produce bright and completely white finished paper is known as bleaching (Beg et al. 2001 ). Traditionally, chemical bleaching agents (such as chlorine) were used for bleaching (Subramaniyan and Prema 2002 ). The use of ligno-hemicellulolytic enzymes for bleaching has gained impetuous all over the world. Xylanases are capable of hydrolyzing xylan which is linked to the cellulose and lignin of the pulp fiber. Thus, xylan disruption will eventually lead to the separation between these components, enhance swelling in the fiber wall, and improve lignin extraction from the pulp (Thomas et al. 2015 ). Thus, xylanase in combination with lignin-degrading enzyme help in increasing the brightness of pulp (Viikari et al. 1994 ; Sunna and Antranikian 1997 ; Pérez et al. 2002 ; Motta et al. 2013 ). The exposures of the cellulose fiber to enzymatic pulping enhance the bonding forces of paper and improve paper strength via degradation of xylan and removal of lignin during enzymatic treatment (Lin et al. 2018 ). The enzymatic system has been highly selective, non-toxic, environmentally friendly approach for bio-bleaching (Bajpai 2012 ).
Paper and biomass pulp processing takes place at varying pH and temperature. Therefore, thermo-alkali stable xylanases are required for the bio-bleaching. An alkaliphilic Bacillus strain produced thermoactive cellulase-free xylanase using agro-residues active at high temperature 60 °C and pH 6–10 and was utilized for bio-bleaching of kraft pulp (Azeri et al. 2010 ). Paenibacillus campinasensis BL11 xylanase pretreatments showed the increased brightness and viscosity of hardwood kraft pulp (Ko et al. 2010 ). S. thermophilum xylanase active at high temperature (50–70 °C) was used for the bleaching of bagasse pulp (Joshi and Khare 2011 ). T. lanuginosus VAPS24 xylanase was stable at wide range pH that can be useful in both alkaline and acidic bioprocesses (Kumar and Shukla 2018 ). An alkaliphilic Bacillus liceniformis Alk-1 xylanase was utilized in a purified form for the enzymatic pretreatment on eucalyptus kraft pulp bleaching (Raj et al. 2018 ). The xylanase preparation obtained from white-rot fungi, S. commune ARC-11 was capable of ethanol soda pulp pre-bleaching from Eulaliopsisbinata (Gautam et al. 2018 ). The paper manufacturing units of various countries, i.e., Japan. South America, North America and Europe are slowly replacing chemical pulp bleaching by xylanase mediated pulp bleaching. Canada is known to be the leading producer of pulp and they are bleaching more than 10% its pulp via xylanase (Dhiman et al. 2008a ). In addition to that, several reports also suggest that the xylanase enzyme-mediated pretreatment can help in generation of cellulosic nanofibres (CNF) with improved crystallinity from unbleached bagasse and eucalyptus pulp (Nie et al. 2018 ; Zhang et al. 2018 ; Tao et al. 2019 ). Zhang et al. ( 2018 ) suggested that application of commercial Novozyme X2753 can simplify the CNF’s production and purification process. Tao et al. ( 2019 ) demonstrated that xylanase can directly act on the unbleached pulp, where it acts on the covalent bond between hemicellulose molecule and hydrogen bond between hemicelluloses and cellulose. The presence of a small amount of hemicelluloses in cellulose nanofibrils increases light blockage efficiency and subsequently the energy storage capacity of solar cells. Thus, xylanase-mediated bio-bleached pulp acts as a potential substrate for flexible solar cells.
Deinking of waste paper
The dislodgement of ink from the waste used paper is required for its recycling and reuse. Chemical-based methods involving chlorine or chlorine-based derivatives, ClO − , NaOH, NaCO 3 , H 2 O 2 , Na 2 SiO 2 , have been used for removing ink from the paper. This resulted in generation of hazardous effluents and required tedious treatment before disposal to the environment (Maity et al. 2012 ). The enzyme-based methods utilizing xylanase and laccase have been suggested for the removal of ink from paper and pulp industries effluents (Chandra and Singh 2012 ; Dhiman et al. 2014 ).
Virk et al. ( 2013 ) explored the deinking efficiencies of bacterial alkalophilic laccase and xylanase along with physical deinking methods such as microwave and ultrasound for recycling of waste paper. The combination of xylanase and laccase enzymes showed an increase in brightness of different waste pulp old newsprint pulp (21.6%), inkjet print pulp (4.1%), laser print pulp (3.1%), magazine pulp (8.3%), and xerox paper pulp (1.9%) only. Gupta et al. ( 2015 ) reported that synergistic action of xylanase and laccase enzyme (co-cultivation of Bacillus sp. and B. halodurans FNP135) resulted in improvement of physical properties like freeness, breaking length, burst factor and tear factor by 17.8%, 34.8%, 2.77%, and 2.4%, respectively, of old newspaper. The appearance of the newspaper was also improved with an increase in 11.8% brightness and 39% whiteness. The effective dose of commercial cellulase and xylanase from Bacillus halodurans TSEV1 for removal of ink was determined at 1.2. U/mg (each enzyme) by Kumar and Satyanarayana ( 2014 ). The cellulase and xylanase complex obtained from Escherichia coli SD5 facilitated the reduction in hexenuronic acid (Hex A) and kappa number, increase in brightness (10%) and tear strength of recycled paper (Kumar et al. 2018c ).
Xylanase employed in textile industries
The textile processing can be broadly divided into desizing, scouring and bleaching. Desizing involves removal of adhesive material from plant fibers and scouring to remove the inhibitory material from desized fibers (Hartzell et al. 1998 ; Dhiman et al. 2008b ). The conventional method used for desizing and scouring involves the application of high temperature under the influence of oxidizing agents in the alkaline system. This method is not only chemical intensive but also non-specific that causes hamper to the useful cellulosic fractions compromising the overall strength of the textile fibers. Therefore, application of highly thermo-alkali stable cellulase-free xylanolytic enzyme can efficiently be used for desizing and scouring (Csiszár et al. 2001 ; Losonczi et al. 2005 ; Dhiman et al. 2008b ; Bajpai 2014 ).
Dhiman et al. ( 2008b ) demonstrated the application of alkalo-thermophilic xylanase from Bacillus stearothermophilus SDX for processing of cotton and microply fabrics. The desizing and bioscouring treatments were performed using 5 g/L of xylanase at 70 °C, pH 9.5, for 90 min. This resulted in weight loss for 0.91% in microply and 0.83% in cotton with overall whiteness index of 11.81% for cotton and 52.15% for micropoly. The processed fabric has increased tensile strength (1.1–1.2%) and tearness value (1.6–2.4%) as compared to control.
Garg et al. ( 2013 ) demonstrated the application of alkalo-thermostable xylanase from Bacillus pumilus ASH in bioscouring of jute fabric. The oven-dried jute fabric when incubated with a small dose of 5 IU/g xylanase at 55 °C for 2 h resulted in an increase in 4.3% whiteness and 10.7% brightness of fabric. Further, it also helped in decreasing in yellowness of fabric by 5.57%. Similarly, xylanase from Bacillus pumilus was studied for enzymatic desizing of cotton and micropoly fabrics (Battan et al. 2012 ). The enzymatic desizing with enzyme load of 5 IU/g at pH 7.0, temperature 60 °C for 90 min resulted in improved whiteness of 0.9% with respect to the chemical process. The addition of surfactant such as EDTA improved the desizing and bioscouring efficiency (Losonczi et al. 2005 ; Battan et al. 2012 ; Garg et al. 2013 ).
The synergistic action of xylanase and pectinase enzyme was used for scouring of cotton fabrics. The bioscouring was performed with 5.0 IU xylanase and 4.0 IU pectinase from Bacillus pumilus strain AJK (MTCC 10414) along with surfactants such as 1.0 mM EDTA and 1% Tween-80 at high pH 8.5 for 1 h at 50 °C. They observed improvement in whiteness, brightness, and reduction in yellowness by 1.2%, 3.2%, and 4.2% respectively that is better in comparison to chemical-based alkaline scouring method (Singh et al. 2018 ). El et al. ( 2018 ) reported improvement in desizing, bioscouring and bio-finishing efficiency using xylanase obtained from T. longibrachiatum KT693225 without any requirement of additives.
Xylanase employed in chemical and pharmaceutical industries
The non-digestible sugar molecules together form oligomers known as xylooligosaccharides, which are made up of xylose monomers (Vazquez et al. 2000 ). XOS has various applications in biotechnology, pharmaceutical, food and feed industries (Chang et al. 2017 ). XOS plays a vital role as prebiotic as it is not hydrolyzed or absorbed in the gastrointestinal tract. Thus, XOS selectively stimulates the growth of important gastrointestinal microorganisms regulating the human digestive health (Roberfroid 1997 ; Collins and Gibson 1999 ; Vazquez et al. 2000 ). The potential of XOS as an efficient feed alternative is established by the fact that it help in cholesterol reduction, inhibit starch retro-gradation, improve the bioavailability of calcium thus improving the nutritional and sensory properties of food (Voragen 1998 ; Motta et al. 2013 ). XOS has shown the application in pharmaceutical sectors due to its immunomodulatory (Chen et al. 2012 ), anti-cancerous (Gupta et al. 2018 ), anti-microbial, antioxidant (Kallel et al. 2015b ), anti-allergy, anti-inflammatory (Aachary and Prapulla 2011 ), and anti-hyperlipidemic activity (Li et al. 2010 ). XOS have also shown phyto-pharmaceutical and feed applications such as growth regulatory activity in aquaculture and poultry. These properties may be due to the presence of uronic substituents in acidic oligosaccharides.
The process of XOS synthesis involves the physical (autohydrolysis), chemical (hydronium ions generated by water autoionization and in situ organic acids) or enzymatic hydrolysis (xylanase or β-xylosidase) of hemicellulose-rich agricultural wastes (Aachary and Prapulla 2011 ). Several reports suggested that XOS can be enzymatically produced from different agro-residues such as hardwoods (Huang et al. 2016 ), straws (Gullón et al. 2008 ; Kallel et al. 2015a ; Moniz et al. 2016 ) corn cobs (Chapla et al. 2013 ; Gowdhaman and Ponnusami 2015 ), bran (Otieno and Ahring 2012 ), sugarcane bagasse (Jayapal et al. 2013 ) and bamboo (Xiao et al. 2013 ) using microbial xylanases.
Alkaline xylanase from Bacillus mojavensis A21 utilized corncob xylan for the release of xylotriose and xylobiose (Haddar et al. 2012 ). Bacillus aerophilus KGJ2 xylanase showed efficiency toward XOS synthesis, e.g., xylobiose, xylotriose, and xylose after hydrolysis of xylan (Gowdhaman et al. 2014 ). A cellulase free xylanase (EX624) from Streptomyces sp. CS624 produced xylose, xylobiose and xylotriose with commercial beech wood xylan and wheat bran (Mander et al. 2014 ). Using deoiled Jatropha curcas seed cake as substrate, Sporotrichum thermophile xylanase was produced which showed the efficiency to produce XOS by the hydrolysis of oat spelt xylan, with 73% xylotetraose, 15.4% xylotriose and 10% xylobiose (Sadaf and Khare 2014 ). Xylanase obtained from the mixed microbial culture of Cellulomonas uda NCIM 2523 and Acetobacter xylinum NCIM 2526 using Prosopis juliflora showed the potential to produce XOS with probiotic activity from beech wood xylan (Anthony et al. 2016 ). A xylanase gene PbXyn10A isolated from Paenibacillus barengoltaii cloned in E. coli showed 75% XOS yield from xylan extracted from raw corncobs (Liu et al. 2018 ). The hydrolysis of xylan using xylanase from Pichia stipitis produced 2% XOS consisting of xylotetraose 14%, xylotriose 49% and xylobiose 29% (Ding et al. 2018 ). Bhardwaj et al. ( 2019 ) demonstrated the partially purified xylanase obtained from Aspergillus oryzae LC1 resulted in the generation of xylobiose, xylotriose, and xylotetraose.
Xylanase employed in biorefinery
Efficient conversion of lignocellulosic biomass (LCB) into fuel-grade ethanol has become a world priority for producing environmentally friendly renewable energy at a reasonable price for the transportation sector. The process of bioconversion of lignocellulosic biomass requires hydrolysis of the pretreated biomass for the conversion of complex carbohydrate polymer of LCB to the simple monomers which will be further converted to ethanol by fermentation. The xylanolytic enzyme in combination with cellulolytic enzyme plays an important role in the hydrolysis process.
Several reports suggest that xylanase obtained from several microorganisms plays an important role in saccharification of LCB for lignocellulosic-based biorefinery (Hu et al. 2011 ; Choudhary et al. 2014 ; Ramanjaneyulu et al. 2017 ; Basit et al. 2018 ). Hydrolysis and fermentation are important steps in biomass to bioethanol generation. Initially, several groups demonstrated separate hydrolysis of biomass followed by fermentation (SHF). SHF is a time-consuming process and thus increases the overall cost of the process. Later on, different integrated process (combined hydrolysis and fermentation) have been developed such as simultaneous saccharification and co-fermentation (SSCF), simultaneous saccharification and fermentation (SSF), and consolidated bioprocessing (CB) (Malhotra and Chapadgaonkar 2018 ). These strategies resulted in an enhancement in reaction rates and ethanol yields (Eklund and Zacchi 1995 ; Sun and Cheng 2002 ). Bibra et al. ( 2018 ) showed thermostable xylanase production using Geobacillus sp. DUSELR13, which is applied further for ethanol generation from LCB. SSF Geobacillus sp. DUSELR13 and Geobacillus thermoglucosidasius are co-cultured for SSF of prairie cord grass (PCG), and corn stover (CS). The SSF resulted in 3.53 and 3.72 g/L ethanol from PCG and CS, respectively.
Hu et al. ( 2011 ) suggested that xylanase causes fiber swelling improving porosity that helps in improving the accessibility of cellulose. To ferment both cellulose-derived hexoses (C6) and xylan-derived pentoses (C5), simultaneous saccharification and co-fermentation (SSCF) was introduced which causes ethanol production using single microorganisms co-cultured with cellulase and xylanase producing strain. Yasuda et al. ( 2014 ) demonstrated bioethanol generation by SSCF of anhydrous ammonia-pretreated Pennisetum purpureum Schumach (Napier grass) using Escherichia coli KO11 and Saccharomyces cerevisiae cellulase, and xylanase. SSCF for 96 h was reported to have a maximum 74% ethanol yield as compared to theoretical yield calculated based on glucan and xylan yield of 397 mg/g and 214 mg/g, respectively.
Bondesson and Galbe ( 2016 ) designed experimental setup of SSCF for ethanol production from steam-pretreated, acetic acid-impregnated wheat straw using a pentose fermenting S. cerevisiae KE6-12b strain. The highest 37.5 g/L ethanol concentration with 0.32 g/g ethanol yield was obtained based on the glucose–xylose available in the pretreated wheat straw. Shariq and Sohail ( 2018 ) demonstrated that yeast strain Candida tropicalis MK-160 can help in xylanase and endoglucanase production as well as ethanol production. Therefore, it can be potentially used for SSCF involves single microorganism.
The consolidated processing or simultaneous delignification, saccharification, and fermentation involve the cultivation of ligno-hemicellulolytic enzyme-producing strains along with ethanol-producing strain in a reactor. It may be monoculture or co-culture of different microorganisms. It will help to decrease the overall process cost required for bioreactor and operation of different enzyme production and ethanol generation (Chadha et al. 1995 ).
To design a monoculture-based consolidated processing, different engineered microorganisms are used having ligno-hemicellulolytic enzymes having the capability along with ethanol generating potential. Shen et al. ( 2012 ) engineered a thermostable self-splicing bacterial intein-modified xylanase for consolidated lignocellulosic biomass processing. Sun et al. ( 2012 ) demonstrated expression of recombinant Saccharomyces cerevisiae strain having an engineered minihemicellulosome. It has the capability of converting xylan directly to ethanol. Horisawa et al. ( 2019 ) suggested direct ethanol production from lignocellulosic materials by consolidated bioprocessing using the mixed culture of wood rot fungi, i.e., Schizophyllum commune, Bjerkandera adusta , and Fomitopsis palustris .
Commercial xylanase enzyme and their application
The xylanase enzyme is commercially employed in several industries such as pulp bleaching, food, feed, and brewing. The major application of the xylanase is in pulp bleaching and is produced by different companies around the world with various trade name such as Bleachzyme (Biocon, India), Cartzyme (Sandoz, US), Cartzyme MP (Clarient, UK), Ecozyme (Thomas Swan, UK), Irgazyme 10 A & Irgazyme 40–4× (Genercor, Finland), Ecopulp (Alko Rajamaki, Finland), VAI Xylanase (Voest Alpine, Austria), and Rholase 7118 (Rohm, Germany). Xylanase is commercially produced by several international industries for its application in food and feed industries. Sankyo from Japan, Ciba Giegy from Switzerland produces xylanase as trade name Sanzyme, and Albazyme-10A, respectively, and has been used commercially in food and baking industries. A Danish firm Novo Nordisk, produces three commercial xylanases namely Pulpzyme (HA, HB, HC), Biofeed (Beta, plus) and Ceremix and used in pulp bleaching, feed and brewing industries, respectively (Walia et al. 2017 ). An American firm Alltech, Inc., commercially produces xylanase with trade name Allzym PT and Fibrozyme and has been used for the upgrading the animal feed. A Japanese firm named Amano Pharmaceutical Co, Ltd. produced xylanase enzyme named Amano 90 and it has been used in food, feed and pharmaceutical industries. Most of the commercial xylanases are produced by fungal source due to its high production potential.
Challenges and future trends in commercial production, purification and application of xylanase
The search of super xylanase is still on, therefore, searching for new microbial source with the ability to produce highly active and stable xylanase is going on around the world. The strains isolated from different extreme habitat can be of potential applications as these strains already possesses the ability to withstand different stress such as high temperature and pH variations. The selection of such thermal and pH tolerant strains and subjecting them to different optimization strategies for enhanced xylanase production can be one alternative. The advancement in biotechnological tools and techniques (Recombinant DNA technology or genetic engineering) provides an opportunity to select the gene responsible for xylanase production that can be isolated and efficiently transferred to the expression system. These expression systems can be regulated for enhanced production of xylanase with desired property for specific industrial applications. The availability of a high amount of genomics, proteomics and metabolomics data can be used via different bioinformatics tools to develop different approaches for enhanced xylanase production. The combination of new technology such as synthetic biology (DNA oligo-synthesis) and conventional recombinant DNA technology can be used for attaining the objective of high xylanase production with desired industrial properties (Fig. 7 ). However, limitations associated with mimicking the natural system into synthetic system need to be taken care before full-scale applications. Also, the ethical, socio-economic and health concerns need to taken care before commercial exploitation of the developed strategies.
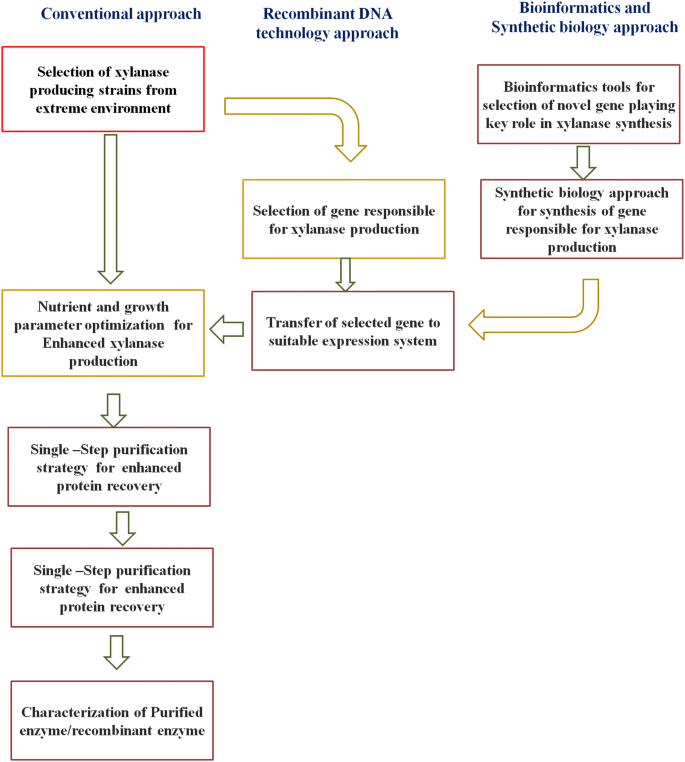
Future prospect for development in area of xylanase production using conventional and advanced approaches
The enzymatic breakdown of the xylan into its constituent component requires the synergistic action of xylanases and other debranching enzymes. The key enzyme used for xylan hydrolysis is endo -1,4-β-xylanase that cleaves β-1,4-glycoside linkage of xylan. The xylanase can be grouped under different GH families with major xylanases from GH10 or GH11 families, followed by GH5, GH7, GH8 and GH43. The xylanase enzyme acts as a “Green” alternative to already existing industrial processes for processing of xylan to different industrially important product such as paper, textile, food, feed, pharmaceuticals, and biofuels. The application of xylanases in the production of the above-mentioned products can regulate the overall economics of the process. Therefore, already existing method can be further improved or new strategies may be developed for enhanced and cost-efficient production of xylanase with desired characteristics. Further, it is often observed that native enzyme cannot meet the industrial process requirement; thus, the combination of already existing technology with new technology such as synthetic biology (DNA oligo-synthesis), rational engineering and directed evolution can be used for attaining the objective of high xylanase production with desired industrial properties.
Availability of data and materials
Not applicable.
Abbreviations
lignocellulosic biomass
enzyme commission
high-molecular weight with low isoelectric point
low-molecular weight with high isoelectric point
carbohydrate-active enzyme database
National Centre for Biotechnological Information
glycoside hydrolase
xylooligosaccharides
xylotetraose
catalytic nucleophile/base
catalystic proton donor
aspartic acid
gluctamic acid
submerged fermentation
solid-state fermentation
carboxymethyl cellulose
one factor at a time
central composite design
analysis of variance
response surface methodology
generally regarded as safe
trichloroacetic acid
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
diethylaminoethyl
fast protein liquid chromatography
aqueous two-phase system
polyethylene glycol
distillers dried grains with soluble
simultaneous saccharification and co-fermentation
simultaneous saccharification and fermentation
consolidated bioprocessing
prairie cord grass
corn stover
Aachary AA, Prapulla SG (2011) Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr Rev Food Sci Food Saf 10:2–16
Article CAS Google Scholar
Abdel-Aziz MS, Talkhan FN, Fadel M et al (2011) Improvement of xylanase production from Streptomyces pseudogriseolus via UV mutagenesis. Aust J Basic Appl Sci 5:1045–1050
CAS Google Scholar
Abou Hachem M, Nordberg Karlsson E, Bartonek-Roxâ E et al (2000) Carbohydrate-binding modules from a thermostable Rhodothermus marinus xylanase: cloning, expression and binding studies. Biochem J 345(Pt 1):53–60
Article CAS PubMed PubMed Central Google Scholar
Adesina FC, Onilude AA, Oluboyede OA et al (2017) Production and application of xylanase by Fusarium spp. using wood shavings as substrate. EC Microbiol 6:4–13
Google Scholar
Adhyaru DN, Bhatt NS, Modi HA, Divecha J (2017) Cellulase-free-thermo-alkali-solvent-stable xylanase from Bacillus altitudinis DHN8: over-production through statistical approach, purification and bio-deinking/bio-bleaching potential. Biocatal Agric Biotechnol 12:220–227
Article Google Scholar
Adigüzel AO, Tunçer M (2016) Production, characterization and application of a xylanase from Streptomyces sp. AOA40 in fruit juice and bakery industries. Food Biotechnol 30:189–218
Adigüzel AO, Tunçer M (2017) Production and characterization of partially purified thermostable endoxylanase and endoglucanase from novel Actinomadura geliboluensis and the biotechnological applications in the saccharification of lignocellulosic biomass. BioResources 12:2528–2547. https://doi.org/10.15376/biores.12.2.2528-2547
Adiguzel G, Faiz O, Sisecioglu M et al (2019) A novel endo-β-1,4-xylanase from Pediococcus acidilactici GC25; purification, characterization and application in clarification of fruit juices. Int J Biol Macromol 129:571–578
Article CAS PubMed Google Scholar
Ahmad M, Hirz M, Pichler H, Schwab H (2014) Protein expression in Pichia pastoris : recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol 98:5301–5317. https://doi.org/10.1007/s00253-014-5732-5
Ahmed S, Jabeen A, Jamil A (2007) Xylanase from Trichoderma harzianum : enzyme characterization and gene isolation. J Chem Soc Pak 29:176–182
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanases: an overview. Appl Microbiol Biotechnol 84:19–35
Akila G, Chandra TS (2003) A novel cold-tolerant Clostridium strain PXYL1 isolated from a psychrophilic cattle manure digester that secretes thermolabile xylanase and cellulase. FEMS Microbiol Lett 219:63–67
Al Balaa B, Wouters J, Dogne S et al (2006) Identification, cloning, and expression of the Scytalidium acidophilum XYL1 gene encoding for an acidophilic xylanase. Biosci Biotechnol Biochem 70:269–272. https://doi.org/10.1271/bbb.70.269
Alam M, Gomes I, Mohiuddin G, Hoq MM (1994) Production and characterization of thermostable xylanases by Thermomyces lanuginosus and Thermoascus aurantiacus grown on lignocelluloses. Enzyme Microb Technol 16:298–302. https://doi.org/10.1016/0141-0229(94)90170-8
Amore A, Parameswaran B, Kumar R et al (2015) Application of a new xylanase activity from Bacillus amyloliquefaciens XR44A in brewer’s spent grain saccharification. J Chem Technol Biotechnol 90:573–581. https://doi.org/10.1002/jctb.4589
Andrews SR, Taylor EJ, Pell G et al (2004) The use of forced protein evolution to investigate and improve stability of family 10 xylanases the production of Ca2 + -independent stable xylanases. J Biol Chem 279:54369–54379
Ang SK, Ariyani LP et al (2013) Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem 48:1293–1302. https://doi.org/10.1016/j.procbio.2013.06.019
Anthony P, Harish BS, Jampala P et al (2016) Statistical optimization, purification and applications of xylanase produced from mixed bacteria in a solid liquid fermentation using Prosopis juliflora. Biocatal Agric Biotechnol 8:213–220
Azeri C, Tamer UA, Oskay M (2010) Thermoactive cellulase-free xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleaching of kraft pulp. Afr J Biotechnol 9:63
Azin M, Moravej R, Zareh D (2007) Production of xylanase by Trichoderma longibrachiatum on a mixture of wheat bran and wheat straw: optimization of culture condition by Taguchi method. Enzyme Microb Technol 40:801–805. https://doi.org/10.1016/j.enzmictec.2006.06.013
Bae H-J, Kim HJ, Kim YS (2008) Production of a recombinant xylanase in plants and its potential for pulp biobleaching applications. Bioresour Technol 99:3513–3519
Bajaj BK, Abbass M (2011) Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28. 3 Biotech 1:161–171. https://doi.org/10.1007/s13205-011-0020-x
Article PubMed PubMed Central Google Scholar
Bajaj BK, Manhas K (2012) Production and characterization of xylanase from Bacillus licheniformis P11(C) with potential for fruit juice and bakery industry. Biocatal Agric Biotechnol 1:330–337. https://doi.org/10.1016/j.bcab.2012.07.003
Bajpai P (2012) Biobleaching. In Bajpai P (eds) Biotechnology for pulp and paper processing. Springer, Berlin, pp 93–138
Chapter Google Scholar
Bajpai P (2014) Sources, production, and classification of xylanases. Xylanolytic Enzyme Academic Press (Imprint Elsevier), Tokyo, pp 43–52
Bandikari R, Poondla V, Obulam VSR (2014) Enhanced production of xylanase by solid state fermentation using Trichoderma koeningi isolate: effect of pretreated agro-residues. 3 Biotech 4:655–664. https://doi.org/10.1007/s13205-014-0239-4
Banka AL, Guralp SA, Gulari E (2014) Secretory expression and characterization of two hemicellulases, xylanase, and β-xylosidase, isolated from Bacillus subtilis M015. Appl Biochem Biotechnol 174:2702–2710
Barman S, Sit N, Badwaik LS, Deka SC (2015) Pectinase production by Aspergillus niger using banana ( Musa balbisiana ) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol 52:3579–3589. https://doi.org/10.1007/s13197-014-1413-8
Basit A, Liu J, Miao T et al (2018) Characterization of two endo-β-1,4-xylanases from Myceliophthora thermophila and their saccharification efficiencies, synergistic with commercial cellulase. Front Microbiol 9:1–11. https://doi.org/10.3389/fmicb.2018.00233
Battan B, Dhiman SS, Ahlawat S et al (2012) Application of thermostable xylanase of Bacillus pumilus in textile processing. Indian J Microbiol 52:222–229
Beg Q, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Behera SS, Ray RC (2016) Solid state fermentation for production of microbial cellulases: recent advances and improvement strategies. Int J Biol Macromol 86:656–669
Behnam S, Karimi K, Khanahmadi M, Salimian Z (2016) Optimization of xylanase production by Mucor indicus , Mucor hiemalis , and Rhizopus oryzae through solid state fermentation. Biol J Microorg 4:1–11
Belancic A, Scarpa J, Peirano A et al (1995) Penicillium purpurogenum produces several xylanases: purification and properties of two of the enzymes. J Biotechnol 41:71–79
Béra-Maillet C, Devillard E, Cezette M et al (2005) Xylanases and carboxymethylcellulases of the rumen protozoa Polyplastron multivesiculatum , Eudiplodinium maggii and Entodinium sp. FEMS Microbiol Lett 244:149–156. https://doi.org/10.1016/j.femsle.2005.01.035
Berka RM, Grigoriev IV, Otillar R et al (2011) Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris . Nat Biotechnol 29:922
Betini JHA, Michelin M, Peixoto-Nogueira SC et al (2009) Xylanases from Aspergillus niger , Aspergillus niveus , and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess Biosyst Eng 32:819–824. https://doi.org/10.1007/s00449-009-0308-y
Bhardwaj N, Chanda K, Kumar B et al (2017) Statistical optimization of nutritional and physical parameters for xylanase production from newly isolated Aspergillus oryzae LC1 and its application in the hydrolysis of lignocellulosic agro-residues. BioResources 12:8519–8538
Bhardwaj N, Verma VK, Chaturvedi V, Verma P (2018) GH10 XynF1 and Xyn11A: the predominant xylanase identified in the profiling of extracellular proteome of Aspergillus oryzae LC1. Ann Microbiol 68:731–742
Bhardwaj N, Kumar B, Agarwal K et al (2019) Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in xylo-oligosaccharides production from lignocellulosic agricultural wastes. Int J Biol Macromol 122:1191–1202. https://doi.org/10.1016/j.ijbiomac.2018.09.070
Bibi Z, Ansari A, Zohra RR et al (2014) Production of xylan degrading endo-1, 4-β-xylanase from thermophilic Geobacillus stearothermophilus KIBGE-IB29. J Radiat Res Appl Sci 7:478–485. https://doi.org/10.1016/j.jrras.2014.08.001
Bibra M, Kunreddy V, Sani R (2018) Thermostable xylanase production by Geobacillus sp. strain DUSELR13, and its application in ethanol production with lignocellulosic biomass. Microorganisms 6:93. https://doi.org/10.3390/microorganisms6030093
Article CAS PubMed Central Google Scholar
Biely P, Vršanská M, Tenkanen M, Kluepfel D (1997) Endo-β-1, 4-xylanase families: differences in catalytic properties. J Biotechnol 57:151–166
Biosciences G (2019) Ammonium sulfate protein precipitation-the key to salting-out. https://info.gbiosciences.com/blog/ammonium-sulfat
Bondesson PM, Galbe M (2016) Process design of SSCF for ethanol production from steam-pretreated, acetic-acid—impregnated wheat straw. Biotechnol Biofuels 222:1–12. https://doi.org/10.1186/s13068-016-0635-6
Boonchuay P, Takenaka S, Kuntiya A et al (2016) Journal of molecular catalysis b: enzymatic purification, characterization, and molecular cloning of the xylanase from Streptomyces thermovulgaris TISTR1948 and its application to xylooligosaccharide production. J Mol Catal B Enzyme 129:61–68. https://doi.org/10.1016/j.molcatb.2016.03.014
Brennan Y, Callen WN, Christoffersen L et al (2004) Unusual microbial xylanases from insect guts. Appl Environ Microbiol 70:3609–3617
Bron S, Bolhuis A, Tjalsma H et al (1998) Protein secretion and possible roles for multiple signal peptidases for precursor processing in bacilli. J Biotechnol 64:3–13
Burlacu A, Israel-Roming F, Cornea CP et al (2017) Fungal strains improvement for xylanase over production through physical and chemical mutagenesis. AgroLife Sci J 6:40–47
Busk PK, Lange L (2013) Cellulolytic potential of thermophilic species from four fungal orders. AMB Express 3:47
Article PubMed PubMed Central CAS Google Scholar
Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Camacho NA, Aguilar OG (2003) Production, purification, and characterization of a low-molecular-mass xylanase from Aspergillus sp. and Its application in baking. Appl Biochem Biotechnol Part A Enzyme Eng Biotechnol 104:159–171. https://doi.org/10.1385/ABAB:104:3:159
Camas M, Sazak A, Spröer C et al (2013) Nonomuraea jabiensis sp. nov., isolated from arid soil. Int J Syst Evol Microbiol 63:212–218. https://doi.org/10.1099/ijs.0.039362-0
Article PubMed Google Scholar
Carvalho EA, dos Santos Góes LM, Uetanabaro APT et al (2017) Thermoresistant xylanases from Trichoderma stromaticum : application in bread making and manufacturing xylo-oligosaccharides. Food Chem 221:1499–1506. https://doi.org/10.1016/j.foodchem.2016.10.144
Chadha BS, Kanwar SS, Garcha HS (1995) Simultaneous saccharification and fermentation of rice straw into ethanol. Acta Microbiol Immunol Hung 42:71–75
CAS PubMed Google Scholar
Chadha BS, Kaur B, Basotra N et al (2019) Thermostable xylanases from thermophilic fungi and bacteria: current perspective. Bioresour Technol 277:195–203
Chakdar H, Kumar M, Pandiyan K et al (2016) Bacterial xylanases: biology to biotechnology. 3 Biotech 6:1–15. https://doi.org/10.1007/s13205-016-0457-z
Chandra R, Singh R (2012) Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem Eng J 61:49–58
Chang S, Chu J, Guo Y et al (2017) An efficient production of high-pure xylooligosaccharides from corncob with affinity adsorption-enzymatic reaction integrated approach. Bioresour Technol 241:1043–1049
Chanwicha N, Katekaew S, Aimi T, Boonlue S (2015) Purification and characterization of alkaline xylanase from Thermoascus aurantiacus var. levisporus KKU-PN-I2-1 cultivated by solid-state fermentation. Mycoscience 56:309–318. https://doi.org/10.1016/j.myc.2014.09.003
Chapla D, Dholakiya S, Madamwar D, Shah A (2013) Characterization of purified fungal endoxylanase and its application for production of value added food ingredient from agroresidues. Food Bioprod Process 91:682–692
Chávez R, Bull P, Eyzaguirre J (2006) The xylanolytic enzyme system from the genus Penicillium. J Biotechnol 123:413–433
Article PubMed CAS Google Scholar
Chen HH, Chen YK, Chang HC, Lin SY (2012) Immunomodulatory effects of xylooligosaccharides. Food Sci Technol Res 18:195–199
Chen C-C, Ko T-P, Huang J-W, Guo R-T (2015) Heat- and alkaline-stable xylanases: application, protein structure and engineering. ChemBioEng Rev 2:95–106. https://doi.org/10.1002/cben.201400035
Chen Z, Zaky AA, Liu Y et al (2019) Purification and characterization of a new xylanase with excellent stability from Aspergillus flavus and its application in hydrolyzing pretreated corncobs. Protein Expr Purif 154:91–97. https://doi.org/10.1016/j.pep.2018.10.006
Choudhary J, Saritha M, Nain L, Arora A (2014) Enhanced saccharification of steam-pretreated rice straw by commercial cellulases supplemented with xylanase. J Bioprocess Biotechnol 4:1
Collins MD, Gibson GR (1999) Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69:1052s–1057s
Collins T, Meuwis M-A, Stals I et al (2002) A novel family 8 xylanase, functional and physicochemical characterization. J Biol Chem 277:35133–35139
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Coman G, Georgescu L, Bahrim G (2013) Streptomyces p12-137 endoxylanases characteristics evaluation in order to obtain xylo-oligosaccharides. Rom Biotechnol Lett 18:8086–8096
Courtin CM, Delcour JA (2002) Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci 35:225–243. https://doi.org/10.1006/jcrs.2001.0433
Csiszár E, Urbánszki K, Szakács G (2001) Biotreatment of desized cotton fabric by commercial cellulase and xylanase enzymes. J Mol Catal B Enzyme 11:1065–1072. https://doi.org/10.1016/S1381-1177(00)00149-1
Cunha L, Martarello R, de Souza PM et al (2018) Optimization of Xylanase Production from Aspergillus foetidus in Soybean Residue. Enzyme Res 2018:1–7. https://doi.org/10.1155/2018/6597017
da Costa AC, Cavalheiro GF, de Queiroz Vieira ER et al (2019) Catalytic properties of xylanases produced by Trichoderma piluliferum and Trichoderma viride and their application as additives in bovine feeding. Biocatal Agric Biotechnol 19:101161. https://doi.org/10.1016/j.bcab.2019.101161
Da Gama Ferreira R, Azzoni AR, Freitas S (2018) Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli : the case of recombinant β-glucosidase. Biotechnol Biofuels 11(81):1–13
da Silva R, Yim DK, Park YK (1994) Application of thermostable xylanases from Humicola sp. for pulp improvement. J Ferment Bioeng 77:109–111. https://doi.org/10.1016/0922-338X(94)90221-6
da Silva LA, Terrasan CRF, Carmona EC (2015) Purification and characterization of xylanases from Trichoderma inhamatum . Electron J Biotechnol 18:307–313. https://doi.org/10.1016/j.ejbt.2015.06.001
Danalache F, Mata P, Alves VD, Moldão-martins M (2018) Enzyme-assisted extraction of fruit juices. Elsevier Inc., New York
Book Google Scholar
Davies GJ, Wilson KS, Henrissat B (1997) Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem J 321:557
de Amo GS, Bezerra-Bussoli C, da Silva RR et al (2019) Heterologous expression, purification and biochemical characterization of a new xylanase from Myceliophthora heterothallica F.2.1.4. Int J Biol Macromol 131:798–805. https://doi.org/10.1016/j.ijbiomac.2019.03.108
De Faria FP, Te’o VSJ, Bergquist PL et al (2002) Expression and processing of a major xylanase (XYN2) from the thermophilic fungus Humicola grisea var. thermoidea in Trichoderma reesei. Lett Appl Microbiol 34:119–123
de Guimaraes NCA, Sorgatto M, de Peixoto-Nogueira SC et al (2013) Bioprocess and biotechnology: effect of xylanase from Aspergillus niger and Aspergillus flavus on pulp biobleaching and enzyme production using agroindustrial residues as substract. Springerplus 2:1–7. https://doi.org/10.1186/2193-1801-2-380
De Queiroz Brito Cunha CC, Gama AR, Cintra LC et al (2018) Improvement of bread making quality by supplementation with a recombinant xylanase produced by Pichia pastoris . PLoS ONE 13:1–14. https://doi.org/10.1371/journal.pone.0192996
de Queiroz-Fernandes GM, Martins BL, Rustiguel CB (2017) Reuse of wastewater from pulp industry for the optimization of fungal xylanase production. Acta Sci Biol Sci 39:21–26. https://doi.org/10.4025/actascibiolsci.v39i1.34459
de Sousa Gomes K, Maitan-Alfenas GP, de Andrade LGA et al (2017) Purification and characterization of xylanases from the fungus Chrysoporthe cubensis for production of xylooligosaccharides and fermentable sugars. Appl Biochem Biotechnol 182:818–830. https://doi.org/10.1007/s12010-016-2364-5
Devillard E, Newbold CJ, Scott KP et al (1999) A xylanase produced by the rumen anaerobic protozoan Polyplastron multivesiculatum shows close sequence similarity to family 11 xylanases from Gram-positive bacteria. FEMS Microbiol Lett 181:145–152. https://doi.org/10.1016/S0378-1097(99)00525-X
Dhiman SS, Sharma J, Battan B (2008a) Industrial applications and future prospects of microbial xylanases: a Review. BioResources 3(4):1377–1402
Dhiman SS, Sharma J, Battan B (2008b) Pretreatment processing of fabrics by alkalothermophilic xylanase from Bacillus stearothermophilus SDX. Enzyme Microb Technol 43:262–269. https://doi.org/10.1016/j.enzmictec.2008.03.016
Dhiman SS, Garg G, Sharma J et al (2014) Reduction in acute ecotoxicity of paper mill effluent by sequential application of xylanase and laccase. PLoS ONE 9:e102581
Dhulappa A, Lingappa K (2013) Xylanase-screening and biosynthesis from Aspergillus tamarii . Int J Curr Microbiol Appl Sci 2:79–83
Ding C, Li M, Hu Y (2018) High-activity production of xylanase by Pichia stipitis : purification, characterization, kinetic evaluation and xylooligosaccharides production. Int J Biol Macromol 117:72–77
Dodd D, Cann IKO (2009) Enzymatic deconstruction of xylan for biofuel production. Gcb Bioenergy 1:2–17
Dornez E, Verjans P, Arnaut F et al (2011) Use of psychrophilic xylanases provides insight into the xylanase functionality in bread making. J Agric Food Chem 59:9553–9562
Driss D, Bhiri F, Elleuch L et al (2011) Purification and properties of an extracellular acidophilic endo-1, 4-β-xylanase, naturally deleted in the “thumb”, from Penicillium occitanis Pol6. Process Biochem 46:1299–1306
Driss D, Bhiri F, Siela M et al (2013) Improvement of breadmaking quality by xylanase GH11 from Penicillium occitanis Pol6. J Texture Stud 44:75–84. https://doi.org/10.1111/j.1745-4603.2012.00367.x
Du Y, Shi P, Huang H et al (2013) Characterization of three novel thermophilic xylanases from Humicola insolens Y1 with application potentials in the brewing industry. Bioresour Technol 130:161–167. https://doi.org/10.1016/j.biortech.2012.12.067
Eklund R, Zacchi G (1995) Simultaneous saccharification and fermentation of steam-pretreated willow. Enzyme Microb Technol 17:255–259
El AAAA, Saleh SA, Eid EB et al (2018) Thermodynamics characterization and potential textile applications of Trichoderma longibrachiatum KT693225 xylanase. Biocatal Agric Biotechnol 1:129–137. https://doi.org/10.1016/j.ultramic.2015.06.008
El-Naghy MA, El-Ktatny MS, Attia AA (1991) Factors affecting activity of CMC-ase and xylanase produced by Sporotrichum thermophile and fractionation of the enzymes. Zentralbl Mikrobiol 146:379–385. https://doi.org/10.1016/S0232-4393(11)80172-1
Ezeilo UR, Lee CT, Huyop F et al (2019) Raw oil palm frond leaves as cost-effective substrate for cellulase and xylanase productions by Trichoderma asperellum UC1 under solid-state fermentation. J Environ Manage 243:206–217. https://doi.org/10.1016/j.jenvman.2019.04.113
Facchini FDA, Vici AC, Reis VRA et al (2011) Production of fibrolytic enzymes by Aspergillus japonicus C03 using agro-industrial residues with potential application as additives in animal feed. Bioprocess Biosyst Eng 34:347–355
Fan G, Katrolia P, Jia H et al (2012) High-level expression of a xylanase gene from the thermophilic fungus Paecilomyces thermophila in Pichia pastoris . Biotechnol Lett 34:2043–2048
Faulds CB, Mandalari G, Lo Curto RB et al (2006) Synergy between xylanases from glycoside hydrolase family 10 and family 11 and a feruloyl esterase in the release of phenolic acids from cereal arabinoxylan. Appl Microbiol Biotechnol 71:622–629
Fernandes AC, Fontes CMGA, Gilbert HJ et al (2015) Homologous xylanases from Clostridium thermocellum : evidence for bi-functional activity, synergism between xylanase catalytic modules and the presence of xylan-binding domains in enzyme complexes. Biochem J 342:105–110. https://doi.org/10.1042/bj3420105
Fleissner A, Dersch P (2010) Expression and export: recombinant protein production systems for Aspergillus . Appl Microbiol Biotechnol 87:1255–1270
Gandhi JP, Rao KK, Dave PJ (1994) Characterization of extracellular thermostable xylanase from Chaetomium globosum . J Chem Technol Biotechnol 60:55–60. https://doi.org/10.1002/jctb.280600109
Garai D, Kumar V (2013) Aqueous two phase extraction of alkaline fungal xylanase in PEG/phosphate system: optimization by Box–Behnken design approach. Biocatal Agric Biotechnol 2:125–131
Garcia-Huante Y, Cayetano-Cruz M, Santiago-Hernández A et al (2017) The thermophilic biomass-degrading fungus Thielavia terrestris Co3Bag1 produces a hyperthermophilic and thermostable β-1, 4-xylanase with exo-and endo-activity. Extremophiles 21:175–186
Garg N, Kumar A, Mahatman KK (2010) Xylanase: applications and biotechnological aspects: biotechnological aspects of xylanase. LAP LAMBERT Academic Publ, Lambert
Garg G, Dhiman SS, Gautam R et al (2013) Bioscouring of jute fabric by cellulase-free alkalo-thermostable xylanase from Bacillus pumilus ASH. J Mol Catal B Enzym 85–86:43–48. https://doi.org/10.1016/j.molcatb.2012.08.002
Gascoigne JA, Gascoigne MM (2019) The Xylanases of Fusarium roseum . J Gen Microbiol 1:242–248
Gautam A, Kumar A, Bharti AK, Dutt D (2018) Rice straw fermentation by Schizophyllum commune ARC-11 to produce high level of xylanase for its application in pre-bleaching. J Genet Eng Biotechnol 16:693–701
Gawande PV, Kamat MY (1999) Production of Aspergillus xylanase by lignocellulosic waste fermentation and its application. J Appl Microbiol 87:511–519. https://doi.org/10.1046/j.1365-2672.1999.00843.x
Geetha K, Gunasekaran P (2017) Purification of endoxylanase from Bacillus pumilus B20 for production of prebiotic xylooligosaccharide syrup; an in vitro study. Iran J Biotechnol 15:232-240
Gerasimova J, Kuisiene N (2012) Characterization of the novel xylanase from the thermophilic Geobacillus thermodenitrificans JK1. Microbiology 81:418–424. https://doi.org/10.1134/s0026261712040066
Gessesse A, Mamo G (1998) Purification and characterization of an alkaline xylanase from alkaliphilic Micrococcus sp AR-135. J Ind Microbiol Biotechnol 20:210–214. https://doi.org/10.1038/sj.jim.2900503
Ghazi S, Sepahy AA, Azin M et al (2014) Uv mutagenesis for the overproduction of xylanase from Bacillus mojavensis ptcc 1723 and optimization of the production condition. Iran J Basic Med Sci 17:844–853
PubMed PubMed Central Google Scholar
Ghosh A, Sutradhar S, Baishya D (2019) Delineating thermophilic xylanase from Bacillus licheniformis DM5 towards its potential application in xylooligosaccharides production. World J Microbiol Biotechnol 35:1–18. https://doi.org/10.1007/s11274-019-2605-1
Ghoshal G, Shivhare US, Banerjee UC (2013) Effect of xylanase on quality attributes of whole-wheat bread. J Food Qual 36:172–180. https://doi.org/10.1111/jfq.12034
Glyk A, Scheper T, Beutel S (2015) PEG-salt aqueous two-phase systems: an attractive and versatile liquid–liquid extraction technology for the downstream processing of proteins and enzymes. Appl Microbiol Biotechnol 99:6599–6616
Godlewski M, Kautto L, Nevalainen H (2009) The elusive pulse in protein production in Trichoderma reesei . Poster Light Life Sci Conf Melbourne, Aust November, p 2
Gomez LD, Steele-King CG, McQueen-Mason SJ (2008) Sustainable liquid biofuels from biomass: the writing’s on the walls. New Phytol 178:473–485
Gómez-garcía R, Medina-morales MA, Farruggia B et al (2018) Production of a xylanase by Trichoderma harzianum ( Hypocrea lixii ) in solid-state fermentation and its recovery by an aqueous two-phase system. Can J Biotechnol 2:108–115
Goswami GK, Krishnamohan M, Nain V et al (2014) Cloning and heterologous expression of cellulose free thermostable xylanase from Bacillus brevis . Springerplus 3:1–6. https://doi.org/10.1186/2193-1801-3-20
Gowdhaman D, Ponnusami V (2015) Production and optimization of xylooligosaccharides from corncob by Bacillus aerophilus KGJ2 xylanase and its antioxidant potential. Int J Biol Macromol 79:595–600
Gowdhaman D, Manaswini VS, Jayanthi V et al (2014) Xylanase production from Bacillus aerophilus KGJ2 and its application in xylooligosaccharides preparation. Int J Biol Macromol 64:90–98
Gray KA, Zhao L, Emptage M (2006) Current opinion in chemical biology. Bioethanol Curr Opin Chem Biol 10:141–146
Guan G-Q, Zhao P-X, Zhao J et al (2016) Production and partial characterization of an alkaline xylanase from a novel fungus Cladosporium oxysporum . Biomed Res Int 2016:1–7. https://doi.org/10.1155/2016/4575024
Guleria S, Walia A, Chauhan A, Shirkot CK (2013) Optimization of cultural conditions for cellulase-free xylanase production by mutant strain of alkalophilic Cellulosimicrobium sp. CKMX1 in submerged fermentation. Appl Biol Res 15:137–144
Guleria S, Walia A, Chauhan A et al (2015) Mutagenesis of alkalophilic Cellulosimicrobium sp. CKMX1 for hyper-production of cellulase-free xylanase in solid state fermentation of apple pomace. Proc Natl Acad Sci India Sect B Biol Sci 85:241–252
Guleria S, Walia A, Chauhan A, Shirkot CK (2016a) Optimization of milk-clotting enzyme production by Bacillus amyloliquefaciens SP1 isolated from apple rhizosphere. Bioresour Bioprocess 3:30
Guleria S, Walia A, Chauhan A, Shirkot CK (2016b) Purification and characterization of detergent stable alkaline protease from Bacillus amyloliquefaciens SP1 isolated from apple rhizosphere. J Basic Microbiol 56:138–152
Gullón P, Moura P, Esteves MP et al (2008) Assessment on the fermentability of xylooligosaccharides from rice husks by probiotic bacteria. J Agric Food Chem 56:7482–7487
Guo B, Chen XL, Sun CY et al (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl Microbiol Biotechnol 84:1107–1115. https://doi.org/10.1007/s00253-009-2056-y
Guo B, Li PY, Yue YS et al (2013) Gene cloning, expression and characterization of a novel xylanase from the marine bacterium, Glaciecola mesophila KMM241. Mar Drugs 11:1173–1187. https://doi.org/10.3390/md11041173
Gupta S, Bhushan B, Hoondal GS (2000) Isolation, purification and characterization of xylanase from Staphylococcus sp. SG-13 and its application in biobleaching of kraft pulp. J Appl Microbiol 88:325–334. https://doi.org/10.1046/j.1365-2672.2000.00974.x
Gupta G, Sahai V, Gupta RK (2013) Optimization of xylanase production from Melanocarpus albomyces using wheat straw extract and its scale up in stirred tank bioreactor. Indian J Chem Technol 20:282–289
Gupta V, Garg S, Capalash N et al (2015) Production of thermo-alkali-stable laccase and xylanase by co-culturing of Bacillus sp. and B. halodurans for biobleaching of kraft pulp and deinking of waste paper. Bioprocess Biosyst Eng 38:947–956
Gupta PK, Agrawal P, Hedge P, Akhtar MS (2018) Xylooligosaccharides and their anticancer potential: an update. Anticancer plants: natural products and biotechnological implements. Springer, New York, pp 255–271
Haddar A, Driss D, Frikha F et al (2012) Alkaline xylanases Bacillus mojavensis A21: production and generation of xylooligosaccharides. Int J Biol Macromol 51:647–656
Hakulinen N, Turunen O, Jänis J et al (2003) Three-dimensional structures of thermophilic β-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa : comparison of twelve xylanases in relation to their thermal stability. Eur J Biochem 270:1399–1412
Ham S-J, Kim HJ, Kim J et al (2012) Novel modular endo- β -1,4-xylanase with transglycosylation activity from Cellulosimicrobium sp. strain HY-13 that is homologous to inverting GH family 6 enzymes. Bioresour Technol 107:25–32
Han N, Miao H, Ding J et al (2017) Improving the thermostability of a fungal GH11 xylanase via site-directed mutagenesis guided by sequence and structural analysis. Biotechnol Biofuels 10:133
Haq I, Tasneem M, Raana K et al (2004) Optimization of cultural conditions for the production of xylanase by chemically mutated strain of Aspergillus niger GCBCX-20. Int J Agric Biol 6:1115–1118
Haq I, Hussain R, Hameed U, Javad M (2008) Selection of Aspergillus niger mutant using antimetabolite 2-deoxy d -glucose after N-methyl N-nito N-nitroso guanidine (MNNG) treatment. Pak J Bot 40:2613–2623
Harbak L, Thygesen HV (2002) Safety evaluation of a xylanase expressed in Bacillus subtilis . Food Chem Toxicol 40:1–8. https://doi.org/10.1016/S0278-6915(01)00092-8
Hartzell MM, Hsieh Y (1998) Enzymatic scouring to improve cotton fabric wettability. Text Res J 68:233–241. https://doi.org/10.1177/004051759806800401
Heinen PR, Bauermeister A, Ribeiro LF et al (2018) GH11 xylanase from Aspergillus tamarii Kita: purification by one-step chromatography and xylooligosaccharides hydrolysis monitored in real-time by mass spectrometry. Int J Biol Macromol 108:291–299
Heinze S, Mechelke M, Kornberger P et al (2017) Identification of endoxylanase XynE from Clostridium thermocellum as the first xylanase of glycoside hydrolase family GH141. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-11598-y
Henrissat B, Coutinho PM (2001) Classification of glycoside hydrolases and glycosyltransferases from hyperthermophiles. Methods in enzymology. Elsevier, New York, pp 183–201
Hong P-Y, Iakiviak M, Dodd D et al (2014) Two New xylanases with different substrate specificities from the human gut bacterium bacteroides intestinalis DSM 17393. Appl Environ Microbiol 80:2084–2093. https://doi.org/10.1128/aem.03176-13
Horisawa S, Inoue A, Yamanaka Y (2019) Direct ethanol production from lignocellulosic materials by mixed culture of wood rot fungi Schizophyllum commune, Bjerkandera adusta , and Fomitopsis palustris . Fermantation 5:21. https://doi.org/10.3390/fermentation5010021
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 36:1–13. https://doi.org/10.1186/1754-6834-4-36
Huang C, Jeuck B, Du J et al (2016) Novel process for the coproduction of xylo-oligosaccharides, fermentable sugars, and lignosulfonates from hardwood. Bioresour Technol 219:600–607
Humphry DR, George A, Black GW, Cummings SP (2001) Flavobacterium frigidarium sp. nov., an aerobic, psychrophilic, xylanolytic and laminarinolytic bacterium from Antarctica. Int J Syst Evol Microbiol 51:1235–1243
Hunt CJ, Tanksale A, Haritos VS (2016) Biochemical characterization of a halotolerant feruloyl esterase from Actinomyces spp.: refolding and activity following thermal deactivation. Appl Microbiol Biotechnol 100:1777–1787
Hüttner S, Granchi Z, Nguyen TT et al (2018) Genome sequence of Rhizomucor pusillus FCH 5.7, a thermophilic zygomycete involved in plant biomass degradation harbouring putative GH9 endoglucanases. Biotechnol Rep 20:e00279. https://doi.org/10.1016/j.btre.2018.e00279
Iloduba MI, Milala MA, Ali A (2016) Isolation and partial characterization of crude cellulase-free xylanase from Pseudomonas aeruginosa and Staphylococcus aureus for possible use in paper industry. Int J Microbiol 3:8
Imad M, Arabi E, Bakri Y, Jawhar M (2011) Extracellular xylanase production by Fusarium species in solid state fermentation. Polish J Microbiol 60:209–212
Iqbal M, Tao Y, Xie S et al (2016) Aqueous two-phase system (ATPS): an overview and advances in its applications. Biol Proced Online 18:18
Irfan M, Nadeem M, Syed Q (2014) One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride -IR05 in solid-state fermentation. J Radiat Res Appl Sci 7:317–326. https://doi.org/10.1016/j.jrras.2014.04.004
Irfan M, Asghar U, Nadeem M et al (2016) Optimization of process parameters for xylanase production by Bacillus sp. in submerged fermentation. J Radiat Res Appl Sci 9:139–147. https://doi.org/10.1016/j.jrras.2015.10.008
Irwin D, Jung ED, Wilson DB (1994) Characterization and sequence of a Thermomonospora fusca xylanase. Appl Environ Microbiol 60:763–770
CAS PubMed PubMed Central Google Scholar
Izumi Y, Koga T, Matsumoto T, Kato T (1997) Purification and some properties of endo-1,4-beta- d -xylanase from a fresh-water mollusc, Pomacea insularus (de Ordigny). Biosci Biotechnol Biochem 61:615–620
Jayapal N, Samanta AK, Kolte AP et al (2013) Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Ind Crops Prod 42:14–24
Jeffries TW (1996) Biochemistry and genetics of microbial xylanases. Curr Opin Biotechnol 7:337–342
Jensen JK, Busse-Wicher M, Poulsen CP et al (2018) Identification of an algal xylan synthase indicates that there is functional orthology between algal and plant cell wall biosynthesis. New Phytol 218:1049–1060
Jhamb K, Sahoo DK (2012) Production of soluble recombinant proteins in Escherichia coli : effects of process conditions and chaperone co-expression on cell growth and production of xylanase. Bioresour Technol 123:135–143
Joshi C, Khare SK (2011) Utilization of deoiled Jatropha curcas seed cake for production of xylanase from thermophilic Scytalidium thermophilum . Bioresour Technol 102:1722–1726
Joshi C, Khare SK (2012) Induction of xylanase in thermophilic fungi Scytalidium thermophilum and Sporotrichum thermophile . Brazilian Arch Biol Technol 55:21–27. https://doi.org/10.1590/S1516-89132012000100003
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30:1219–1227
Juturu V, Wu JC (2014) Microbial exo-xylanases: a mini review. Appl Biochem Biotechnol 174:81–92
Kallel F, Driss D, Bouaziz F et al (2015a) Production of xylooligosaccharides from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK and their in vitro evaluation as prebiotics. Food Bioprod Process 94:536–546. https://doi.org/10.1016/j.fbp.2014.07.012
Kallel F, Driss D, Chaabouni SE, Ghorbel R (2015b) Biological activities of xylooligosaccharides generated from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK. Appl Biochem Biotechnol 175:950–964
Kalpana VN, Rajeswari VD (2015) Production of xylanase from various lignocellulosic waste materials by Streptomyces sp. and its potential role in deinking of newsprint. Asian J Biochem 10:222–229
Kamble RD, Jadhav AR (2012) Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. Int J Microbiol 2012:683193. https://doi.org/10.1155/2012/683193
Kamra P, Satyanarayana T (2004) Xylanase production by the thermophilic mold Humicola lanuginosa in solid-state fermentation. Appl Biochem Biotechnol Part A Enzym Eng Biotechnol 119:145–157. https://doi.org/10.1385/ABAB:119:2:145
Karlsson EN, Hachem MA, Ramchuran S et al (2004) The modular xylanase Xyn10A from Rhodothermus marinus is cell-attached, and its C-terminal domain has several putative homologues among cell-attached proteins within the phylum Bacteroidetes. FEMS Microbiol Lett 241:233–242. https://doi.org/10.1016/j.femsle.2004.10.026
Katsimpouras C, Dedes G, Thomaidis NS, Topakas E (2019) A novel fungal GH30 xylanase with xylobiohydrolase auxiliary activity. Biotechnol Biofuels 12:1–14. https://doi.org/10.1186/s13068-019-1455-2
Khandeparkar RDS, Bhosle NB (2006) Isolation, purification and characterization of the xylanase produced by Arthrobacter sp. MTCC 5214 when grown in solid-state fermentation. Enzyme Microb Technol 39:732–742. https://doi.org/10.1016/j.enzmictec.2005.12.008
Khusro A, Kaliyan BK, Al-Dhabi NA et al (2016) Statistical optimization of thermo-alkali stable xylanase production from Bacillus tequilensis strain ARMATI. Electron J Biotechnol 22:16–25. https://doi.org/10.1016/j.ejbt.2016.04.002
Kiddinamoorthy J, Anceno AJ, Haki GD, Rakshit SK (2008) Production, purification and characterization of Bacillus sp. GRE7 xylanase and its application in eucalyptus Kraft pulp biobleaching. World J Microbiol Biotechnol 24:605–612. https://doi.org/10.1007/s11274-007-9516-2
Kim KK, Park HY, Park W et al (2005) Microbacterium xylanilyticum sp. nov., a xylan-degrading bacterium isolated from a biofilm. Int J Syst Evol Microbiol 55:2075–2079. https://doi.org/10.1099/ijs.0.63706-0
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109(4):1083–1087
Knob A, Beitel SM, Fortkamp D et al (2013) Production, purification, and characterization of a major Penicillium glabrum xylanase using Brewer’s spent grain as substrate. Biomed Res Int 2013:1–8. https://doi.org/10.1155/2013/728735
Ko C-H, Lin Z-P, Tu J et al (2010) Xylanase production by Paenibacillus campinasensis BL11 and its pretreatment of hardwood kraft pulp bleaching. Int Biodeterior Biodegrad 64:13–19
Kocabacs DS, Güder S, Özben N (2015) Purification strategies and properties of a low-molecular weight xylanase and its application in agricultural waste biomass hydrolysis. J Mol Catal B Enzyme 115:66–75
Kohli U, Nigam P, Singh D, Chaudhary K (2001) Thermostable, alkalophilic and cellulase free xylanase production by Thermoactinomyces thalophilus subgroup C. Enzyme Microb Technol 28:606–610. https://doi.org/10.1016/S0141-0229(01)00320-9
Koontz L (2014) TCA precipitation. Methods in enzymology. Elsevier, New York, pp 3–10
Krishnamurthy S, Vithayathil PJ (1989) Purification and characterization of endo-1,4-β-xylanase from Paecilomyces variotii Bainier. J Ferment Bioeng 67:77–82. https://doi.org/10.1016/0922-338X(89)90183-9
Kumar V, Satyanarayana T (2014) Production of endoxylanase with enhanced thermostability by a novel polyextremophilic Bacillus halodurans TSEV1 and its applicability in waste paper deinking. Process Biochem 49:386–394
Kumar V, Shukla P (2018) Extracellular xylanase production from T. lanuginosus VAPS24 at pilot scale and thermostability enhancement by immobilization. Process Biochem 71:53–60. https://doi.org/10.1016/j.procbio.2018.05.019
Kumar L, Nagar S, Kumar D et al (2013) Production of an alkali tolerant extracellular xylanase from Bacillus pumilus VLK-1 in solid state fermentation and its use in tomato juice clarification. Int J Cell Sci Biotechnol 2:1–10
Kumar L, Nagar S, Mittal A et al (2014) Immobilization of xylanase purified from Bacillus pumilus VLK-1 and its application in enrichment of orange and grape juices. J Food Sci Technol 51:1737–1749
Kumar B, Bhardwaj N, Alam A et al (2018a) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Express 8:1–16. https://doi.org/10.1186/s13568-018-0696-y
Kumar BA, Amit K, Alok K, Dharm D (2018b) Wheat bran fermentation for the production of cellulase and xylanase by Aspergillus niger NFCCI 4113. Res J Biotechnol 13:5
Kumar NV, Rani ME, Gunaseeli R, Kannan ND (2018c) Paper pulp modification and deinking efficiency of cellulase-xylanase complex from Escherichia coli SD5. Int J Biol Macromol 111:289–295
Kumar PS, Yaashikaa PR, Saravanan A (2018d) Isolation, characterization and purification of xylanase producing bacteria from sea sediment. Biocatal Agric Biotechnol 13:299–303
Landowski CP, Huuskonen A, Wahl R et al (2015) Enabling low cost biopharmaceuticals: a systematic approach to delete proteases from a well-known protein production host Trichoderma reesei . PLoS ONE 10:e0134723
Lange L (2017) Fungal enzymes and yeasts for conversion of plant biomass to bioenergy and high-value products. Microbiol Spectr. 5:1. https://doi.org/10.1128/microbiolspec.FUNK-0007-2016.10.1128/microbiolspec.FUNK-0007-2016
Latif F, Asgher M, Saleem R et al (2006) Purification and characterization of a xylanase produced by Chaetomium thermophile NIBGE. World J Microbiol Biotechnol 22:45–50. https://doi.org/10.1007/s11274-005-5745-4
Lee JH, Kim YG, Lee J (2018) Thermostable xylanase inhibits and disassembles Pseudomonas aeruginosa biofilms. Biofouling 34:346–356. https://doi.org/10.1080/08927014.2018.1440551
Leite P, Salgado JM, Venâncio A et al (2016) Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour Technol 214:737–746
Li T, Li S, Du L et al (2010) Effects of haw pectic oligosaccharide on lipid metabolism and oxidative stress in experimental hyperlipidemia mice induced by high-fat diet. Food Chem 121:1010–1013
Li J, Wang J, Wang S et al (2012) Achieving efficient protein expression in Trichoderma reesei by using strong constitutive promoters. Microb Cell Fact 11:84
Lin C, Shen Z, Zhu T, Qin W (2017) Bacterial xylanase in Pseudomonas boreopolis LUQ1 is highly induced by xylose. Can J Biotechnol 1:73–79. https://doi.org/10.24870/cjb.2017-000112
Lin X, Wu Z, Zhang C, Liu S, Nie S (2018) Enzymatic pulping of lignocellulosic biomass. Ind crop prod 120:16–24
Lisov AV, Belova OV, Lisova ZA et al (2017) Xylanases of Cellulomonas flavigena : expression, biochemical characterization, and biotechnological potential. AMB Express. 5:7. https://doi.org/10.1186/s13568-016-0308-7
Liu X, Kokare C (2017) Chapter 11—microbial enzymes of use in industry. In: Brahmachari G (ed) Biotechnology of microbial enzymes. Academic Press, Cambridge, pp 267–298
Liu J, Yuan X, Zeng G et al (2006) Effect of biosurfactant on cellulase and xylanase production by Trichoderma viride in solid substrate fermentation. Process Biochem 41:2347–2351
Liu N, Ru YJ, Tang DF et al (2011) Effects of corn distillers dried grains with solubles and xylanase on growth performance and digestibility of diet components in broilers. Anim Feed Sci Technol 163:260–266
Liu X, Liu Y, Jiang Z et al (2018) Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chem 264:310–318. https://doi.org/10.1016/j.foodchem.2018.05.023
Lombard V, Golaconda Ramulu H, Drula E et al (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:490–495. https://doi.org/10.1093/nar/gkt1178
Losonczi A, Csiszár E, Szakács G, Bezúr L (2005) Role of the EDTA chelating agent in bioscouring of cotton. Text Res J 75:411–417. https://doi.org/10.1177/0040517505053812
Loureiro DB, Braia M, Romanini D, Tubio G (2017) Partitioning of xylanase from Thermomyces lanuginosus in PEG/NaCit aqueous two-phase systems: structural and functional approach. Protein Expr Purif 129:25–30
Lu Y, Fang C, Wang Q et al (2016) High-level expression of improved thermo-stable alkaline xylanase variant in Pichia Pastoris through codon optimization, multiple gene insertion and high-density fermentation. Sci Rep 6:1–10. https://doi.org/10.1038/srep37869
Luo L, Cai J, Wang C et al (2016) Purification and characterization of an alkaliphilic endo-xylanase from Streptomyces althioticus LMZM and utilization in the pulp paper industry. J Chem Technol Biotechnol 91:1093–1098
Maalej I, Belhaj I, Masmoudi NF, Belghith H (2009) Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus : purification and characterization. Appl Biochem Biotechnol 158:200–212
Maity C, Ghosh K, Halder SK et al (2012) Xylanase isozymes from the newly isolated Bacillus sp. CKBx1D and optimization of its deinking potentiality. Appl Biochem Biotechnol 167:1208–1219
Malhotra G, Chapadgaonkar SS (2018) Production and applications of xylanases—an overview. BioTechnologia 99:59–72
Mander P, Choi YH, Pradeep GC et al (2014) Biochemical characterization of xylanase produced from Streptomyces sp. CS624 using an agro residue substrate. Process Biochem 49:451–456
Martínez PM, Appeldoorn MM, Gruppen H, Kabel MA (2016) The two Rasamsonia emersonii α-glucuronidases, ReGH67 and ReGH115, show a different mode-of-action towards glucuronoxylan and glucuronoxylo-oligosaccharides. Biotechnol Biofuels 9:1–10. https://doi.org/10.1186/s13068-016-0519-9
Maslen SL, Goubet F, Adam A et al (2007) Structure elucidation of arabinoxylan isomers by normal phase HPLC–MALDI-TOF/TOF-MS/MS. Carbohydr Res 342:724–735
Mewis K, Lenfant N, Lombard V, Henrissat B (2016) Dividing the large glycoside hydrolase family 43 into subfamilies: a motivation for detailed enzyme characterization. Appl Environ Microbiol 82:1686–1692
Mienda BS, Idi A, Umar A (2011) Microbiological features of solid state fermentation and its applications—an overview. Res Biotechnol 2:21–26
Mittal A, Nagar S, Gupta VK (2013) Production and purification of high levels of cellulase-free bacterial xylanase by Bacillus sp. SV-34S using agro-residue. Ann Microbiol 63:1157–1167. https://doi.org/10.1007/s13213-012-0574-9
Mmango-Kaseke Z, Okaiyeto K, Nwodo UU et al (2016) Optimization of cellulase and xylanase production by Micrococcus species under submerged fermentation. Sustain 8:1–15. https://doi.org/10.3390/su8111168
Mohana S, Shah A, Divecha J, Madamwar D (2008) Xylanase production by Burkholderia sp. DMAX strain under solid state fermentation using distillery spent wash. Bioresour Technol 99:7553–7564. https://doi.org/10.1016/j.biortech.2008.02.009
Moniz P, Ho AL, Duarte LC et al (2016) Assessment of the bifidogenic effect of substituted xylo-oligosaccharides obtained from corn straw. Carbohydr Polym 136:466–473
Monti R, Cardello L, Custódio MF et al (2003) Production and purification of an endo-1,4-β-xylanase from Humicola grisea var. thermoidea by electroelution. Brazilian J Microbiol 34:124–128
Motta FL, Andrade CCP, Santana MHA (2013) A review of xylanase production by the fermentation of xylan: classification, characterization and applications. Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization. InTechOpen, New York, pp 251–275
Murugan P, Jampala P (2015) Production of xylanase from A mixed culture system of Acetobacter xylinum and Cellulomonas uda in submerged fermentation. Biosci Biotechnol Res Asia 12:1615–1622
Murugan S, Arnold D, Pongiya UD, Narayanan PM (2011) Production of xylanase from Arthrobacter sp. MTCC 6915 using saw dust as substrate under solid state fermentation. Enzyme Res 2011:696942
Naganagouda K, Mulimani VH (2008) Aqueous two-phase extraction (ATPE): an attractive and economically viable technology for downstream processing of Aspergillus oryzae α-galactosidase. Process Biochem 43:1293–1299
Namasivayam S, Babu M, Bharani RS (2015) Evaluation of lignocellulosic agro wastes for the enhanced production of extracellular cellulase and xylanase by Trichoderma harzianum . Nat Environ Pollut Technol 14:47–52
Nathan VK, Rani ME, Rathinasamy G, Dhiraviam KN (2017) Low molecular weight xylanase from Trichoderma viride VKF3 for bio-bleaching of newspaper pulp. BioResources 12:5264–5278. https://doi.org/10.15376/biores.12.3.5264-5278
Naveen M, Saroj Y, Pramod T et al (2014) Role of nitrogen source for the production of xylanase from Aspergillus sp. J Drug Deliv Ther 4:147–149
Nevalainen H, Peterson R (2014) Making recombinant proteins in filamentous fungi-are we expecting too much ? Front Microbiol 5:1–10. https://doi.org/10.3389/fmicb.2014.00075
Nevalainen H, Peterson R, Curach N (2018) Overview of gene expression using filamentous fungi. Curr Protoc Protein Sci 92:e55
Ng HS, Chai CXY, Chow YH et al (2018) Direct recovery of Bacillus subtilis xylanase from fermentation broth with an alcohol/salt aqueous biphasic system. J Biosci Bioeng. 125:585–589. https://doi.org/10.1016/j.jbiosc.2017.12.010
Nie S, Zhang K, Lin X, Zhang C, Yan D, Liang H, Wang S (2018) Enzymatic pretreatment for the improvement of dispersion and film properties of cellulose nanofibrils. Carbohyd Polym 181:1136–1142
Notenboom V, Birsan C, Warren RAJ et al (1998) Exploring the cellulose/xylan specificity of the β-1,4-glycanase Cex from Cellulomonas fimi through crystallography and mutation. Biochemistry 37:4751–4758. https://doi.org/10.1021/bi9729211
Otieno DO, Ahring BK (2012) A thermochemical pretreatment process to produce xylooligosaccharides (XOS), arabinooligosaccharides (AOS) and mannooligosaccharides (MOS) from lignocellulosic biomasses. Bioresour Technol 112:285–292
Paloheimo M, Mäntylä A, Kallio J et al (2007) Increased production of xylanase by expression of a truncated version of the xyn11A gene from Nonomuraea flexuosa in Trichoderma reesei . Appl Environ Microbiol 73:3215–3224. https://doi.org/10.1128/AEM.02967-06
Paloheimo M, Piironen J, Vehmaanperä J et al (2010) Xylanases and cellulases as feed additives. Enzymes in farm animal nutrition. Nutr Finnfeeds Malborough, Wiltshire, pp 12–53
Passos AA, Park I, Ferket P et al (2015) Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim Nutr 1:19–23
Paulo RH, Caroline H, Rosane MP et al (2014) Xylanase from Fusarium heterosporum : properties and influence of thiol compounds on xylanase activity. African J Biotechnol 13:1047–1055. https://doi.org/10.5897/AJB2013.13282
Pérez J, Munoz-Dorado J, la Rubia T, Martinez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63
Ping L, Wang M, Yuan X et al (2018) Production and characterization of a novel acidophilic and thermostable xylanase from Thermoascus aurantiacu . Int J Biol Macromol 109:1270–1279. https://doi.org/10.1016/j.ijbiomac.2017.11.130
Pirgozliev V, Whiting I, Rose SP et al (2016) Variability between wheat dry distillers grains with solubles samples influence the effectiveness of exogenous enzymes when fed to broiler chickens. Vet Med Anim Stud 6:61–69
Pohlschroder M, Leschine SB, Canale-Parola E (1994) Multicomplex cellulase–xylanase system of Clostridium papyrosolvens C7. J Bacteriol 176:70–76. https://doi.org/10.1128/jb.176.1.70-76.1994
Polizeli M, Rizzatti ACS, Monti R et al (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Puchart V, Fravnová L, Krogh KBRM et al (2018) Action of different types of endoxylanases on eucalyptus xylan in situ. Appl Microbiol Biotechnol 102:1725–1736
Purkan P, Huruniawati E, Sumarsih S (2017) Xylanase enzyme from a local strain of Pseudomonas stutzeri . J Chem Technol Metall 52:1079–1085
Qiu Z, Shi P, Luo H et al (2010) A xylanase with broad pH and temperature adaptability from Streptomyces megasporus DSM 41476, and its potential application in brewing industry. Enzyme Microb Technol 46:506–512
Raghothama S, Simpson PJ, Szabó L et al (2000) Solution structure of the CBM10 cellulose binding module from Pseudomonas xylanase A. Biochemistry 39:978–984. https://doi.org/10.1021/bi992163+
Rahim T, Ray AL, Beauty SP, Gomes DJ (2009) Induction of mutation in Neurospora crassa with ultraviolet radiation and evaluation of cellulase and xylanase activities. Bangladesh J Bot 38:201–203
Rahmani N, Kahar P, Lisdiyanti P et al (2018) Xylanase and feruloyl esterase from actinomycetes cultures could enhance sugarcane bagasse hydrolysis in the production of fermentable sugars. Biosci Biotechnol Biochem 82:904–915. https://doi.org/10.1080/09168451.2018.1438169
Rahmani N, Kahar P, Lisdiyanti P et al (2019) GH-10 and GH-11 Endo-1,4-β-xylanase enzymes from Kitasatospora sp. produce xylose and xylooligosaccharides from sugarcane bagasse with no xylose inhibition. Bioresour Technol 272:315–325. https://doi.org/10.1016/j.biortech.2018.10.007
Raj A, Kumar S, Singh SK (2013) A highly thermostable xylanase from Stenotrophomonas maltophilia : purification and partial characterization. Enzyme Res 2013:429305
Raj A, Kumar S, Singh SK, Prakash J (2018) Production and purification of xylanase from alkaliphilic Bacillus licheniformis and its pretreatment of eucalyptus kraft pulp. Biocatal Agric Biotechnol 15:199–209
Rajoka MI, Bashir A, Malik KA (1997) Mutagenesis of Cellulomonas biazotea for enhanced production of xylanases. Bioresour Technol 62:99–108
Ramakrishnan V, Goveas LC, Suralikerimath N et al (2016) Extraction and purification of lipase from Enterococcus faecium MTCC5695 by PEG/phosphate aqueous-two phase system (ATPS) and its biochemical characterization. Biocatal Agric Biotechnol 6:19–27
Ramanjaneyulu G, Sridevi A, Seshapani P et al (2017) Enhanced production of xylanase by Fusarium sp. BVKT R2 and evaluation of its biomass saccharification efficiency. 3 Biotech 7:1–17. https://doi.org/10.1007/s13205-017-0977-1
Ray S, Vigouroux J, Bouder A et al (2019) Functional exploration of Pseudoalteromonas atlantica as a source of hemicellulose-active enzymes: evidence for a GH8 xylanase with unusual mode of action. Enzyme Microb Technol. 127:6–16. https://doi.org/10.1016/j.enzmictec.2019.04.007
Roberfroid MB (1997) Health benefits of non-digestible oligosaccharides. Dietary fiber in health and disease. Springer, New York, pp 211–219
Romero-Fernández M, Moreno-Perez S, Orrego AH et al (2018) Designing continuous flow reaction of xylan hydrolysis for xylooligosaccharides production in packed-bed reactors using xylanase immobilized on methacrylic polymer-based supports. Bioresour Technol 266:249–258
Rosmine E, Sainjan NC, Silvester R et al (2017) Statistical optimisation of xylanase production by estuarine Streptomyces sp. and its application in clarification of fruit juice. J Genet Eng Biotechnol 15:393–401
Rosmine E, Edassery Sainjan NC, Silvester R, Varghese SA (2019) Utilisation of agrowaste xylan for the production of industrially important enzyme xylanase from aquatic Streptomyces sp. and potential role of xylanase in deinking of newsprint. Int J Curr Microbiol Appl Sci 8:2061–2076. https://doi.org/10.20546/ijcmas.2019.801.216
Rychen G, Aquilina G, Azimonti G et al (2018) Safety and efficacy of ECONASE ® XT (endo-1, 4-$β$-xylanase) as a feed additive for pigs for fattening. EFSA J 16:e05217
Rytioja J, Hildén K, Yuzon J et al (2014) Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol Mol Biol Rev 78:614–649. https://doi.org/10.1128/MMBR.00035-14
Sadaf A, Khare SK (2014) Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiled Jatropha curcas seed cake and its application in xylooligosaccharide synthesis. Bioresour Technol 153:126–130
Saha BC (2002) Production, purification and properties of xylanase from a newly isolated Fusarium proliferatum . Process Biochem 37:1279–1284. https://doi.org/10.1016/S0032-9592(02)00012-2
Sánchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194
Sanjivkumar M, Silambarasan T, Palavesam A, Immanuel G (2017) Biosynthesis, purification and characterization of β-1,4-xylanase from a novel mangrove associated actinobacterium Streptomyces olivaceus (MSU3) and its applications. Protein Expr Purif 130:1–12. https://doi.org/10.1016/j.pep.2016.09.017
Selim S (2016) Molecular characterization of thermostable xylanase producing Thermoactinomyces vulgaris HSB4. J Pure Appl Microbiol 10:1965–1969
Seyis I, Aksoz N (2005) Effect of carbon and nitrogen sources on xylanase production by Trichoderma harzianum 1073 D3. Int Biodeterior Biodegradation 55:115–119
Shahrestani H, Taheri-Kafrani A, Soozanipour A, Tavakoli O (2016) Enzymatic clarification of fruit juices using xylanase immobilized on 1, 3, 5-triazine-functionalized silica-encapsulated magnetic nanoparticles. Biochem Eng J 109:51–58
Shariq M, Sohail M (2018) Application of Candida tropicalis MK-160 for the production of xylanase and ethanol. J King Saud Univ. https://doi.org/10.1016/j.jksus.2018.04.009
Sharma M, Chadha BS, Kaur M et al (2008) Molecular characterization of multiple xylanase producing thermophilic/thermotolerant fungi isolated from composting materials. Lett Appl Microbiol 46:526–535. https://doi.org/10.1111/j.1472-765X.2008.02357.x
Sharma M, Mehta S, Kumar A (2013) Purification and characterization of alkaline xylanase secreted from Paenibacillus macquariensis . Adv Microbiol 03:32–41. https://doi.org/10.4236/aim.2013.31005
Sharma PK (2017) Xylanases current and future perspectives : a review Pawan Kumar Sharma. New Biol Rep 6:122
Shen B, Sun X, Zuo X et al (2012) Engineering a thermoregulated intein-modified xylanase into maize for consolidated lignocellulosic biomass processing. Nat Biotechnol 30:1131–1136
Shi P, Tian J, Yuan T et al (2010) Paenibacillus sp. Strain E18 bifunctional xylanase–glucanase with a single catalytic domain. Appl Environ Microbiol 76:3620–3624. https://doi.org/10.1128/AEM.00345-10
Shi H, Zhang Y, Li X et al (2013) A novel highly thermostable xylanase stimulated by Ca 2+ from Thermotoga thermarum : cloning, expression and characterization. Biotechnol Biofuels 6:1–9. https://doi.org/10.1186/1754-6834-6-26
Shi H, Zhang Y, Zhong H et al (2014) Cloning, over-expression and characterization of a thermo-tolerant xylanase from Thermotoga thermarum . Biotechnol Lett 36:587–593. https://doi.org/10.1007/s10529-013-1392-2
Shi P, Du Y, Yang H et al (2015) Molecular characterization of a new alkaline-tolerant xylanase from Humicola insolens Y1. Biomed Res Int 2015:1–7. https://doi.org/10.1155/2015/149504
Shibuya H, Kaneko S, Hayashi K (2000) Enhancement of the thermostability and hydrolytic activity of xylanase by random gene shuffling. Biochem J 349:651–656
Simair AA, Dahot MU, Mangrio GS et al (2010) Enhancing effect of amino acids and vitamins on xylanase production by Pleurotus Eryngii through submerged fermentation. Pak J Biotechnol 7:123–126
Sindhu R, Binod P, Mathew AK et al (2017) Development of a novel ultrasound-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post harvest residue. Bioresour Technol 242:146–151
Singh A, Kaur A, Patra AK, Mahajan R (2018) A sustainable and green process for scouring of cotton fabrics using xylano-pectinolytic synergism: switching from noxious chemicals to eco-friendly catalysts. 3 Biotech 8:184
Singh S, Sidhu GK, Kumar V et al (2019) Fungal xylanases: sources, types, and biotechnological applications. Recent advancement in white biotechnology through fungi. Springer, New York, pp 405–428
Singla A, Paroda S, Dhamija SS et al (2012) Bioethanol production from xylose: problems and possibilities. J Biofuels 3:1–17
Sizova MV, Izquierdo JA, Panikov NS, Lynd LR (2011) Cellulose-and xylan-degrading thermophilic anaerobic bacteria from biocompost. Appl Environ Microbiol 77:2282–2291
Song HY, Lim HK, Kim DR et al (2014) A new bi-modular endo-β-1,4-xylanase KRICT PX-3 from whole genome sequence of Paenibacillus terrae HPL-003. Enzyme Microb Technol 54:1–7. https://doi.org/10.1016/j.enzmictec.2013.09.002
Srinivasan S, Kim MK, Sathiyaraj G et al (2010) Microbacterium soli sp. nov., an α-glucosidase-producing bacterium isolated from soil of a ginseng field. Int J Syst Evol Microbiol 60:478–483. https://doi.org/10.1099/ijs.0.012526-0
St. John FJ, Rice JD, Preston JF (2006) Characterization of XynC from Bacillus subtilis subsp. subtilis strain 168 and analysis of its role in depolymerization of glucuronoxylan. J Bacteriol 188:8617–8626. https://doi.org/10.1128/JB.01283-06
Stutzenberger F (1994) Extracellular enzyme production by Thermomonospora curvata grown on bagasse. J Ind Microbiol 13:35–42. https://doi.org/10.1007/BF01569660
Su X, Schmitz G, Zhang M et al (2012) Heterologous gene expression in filamentous fungi. In: Gadd GM, Sariaslani S (eds) Advances in applied microbiology. Academic Press, Cambridge, pp 1–61
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol 22:33–64
Sukhumsirichart W, Deesukon W, Kawakami T et al (2014) Expression and characterization of recombinant GH11 xylanase from thermotolerant Streptomyces sp. SWU10. Appl Biochem Biotechnol 172:436–446
Suleman M, Aujla MI (2016) Production and characterization of xylanase from Aspergillus niger using wheat bran, corn cobs, and sugar cane bagasse as carbon sources with different concentrations. J Bioresourc Mang. 3:1-9. https://doi.org/10.35691/JBM.5102.0040
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Sun J, Wen F, Si T et al (2012) Direct conversion of xylan to ethanol by recombinant Saccharomyces cerevisiae strains displaying an engineered mini-hemicellulosome. Appl Environ Microbiol. 78:3837–3845. https://doi.org/10.1128/AEM.07679-11
Sunna A, Antranikian G (1997) Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol 17:39–67
Suzuki M, Yoshida K, Ashida K (1991) Purification and characterization of xylanase from the mid-gut gland of the apple snail ( Pomacea canaliculata ). Agric Biol Chem 55:693–700
Syuan KY, Ai LOG, Suan TK (2018) Evaluation of cellulase and xylanase production from Trichoderma harzianum using acid-treated rice straw as solid substrate. Mater Today Proc 5:22109–22117. https://doi.org/10.1016/j.matpr.2018.07.077
Tai WY, Tan JS, Lim V, Lee CK (2019) Comprehensive studies on optimization of cellulase and xylanase production by a local indigenous fungus strain via solid state fermentation using oil palm frond as substrate. Biotechnol Prog. 35:e2781. https://doi.org/10.1002/btpr.2781
Taibi Z, Saoudi B, Boudelaa M et al (2012) Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp. strain Cpt20 isolated from poultry compost. Appl Biochem Biotechnol 166:663–679
Tang F, Chen D, Yu B et al (2017) Improving the thermostability of Trichoderma reesei xylanase 2 by introducing disulfide bonds. Electron J Biotechnol 26:52–59. https://doi.org/10.1016/j.ejbt.2017.01.001
Tao P, Wu Z, Xing C, Zhang Q, Wei Z, Nie S (2019) Effect of enzymatic treatment on the thermal stability of cellulose nanofibrils. Cellulose 26:7717–7725
Terrone CC, de Freitas C, Terrasan CRF et al (2018) Agroindustrial biomass for xylanase production by Penicillium chrysogenum : purification, biochemical properties and hydrolysis of hemicelluloses. Electron J Biotechnol 33:39–45. https://doi.org/10.1016/j.ejbt.2018.04.001
Thanh VN, Thuy NT, Huong HTT et al (2019) Surveying of acid-tolerant thermophilic lignocellulolytic fungi in Vietnam reveals surprisingly high genetic diversity. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-40213-5
Thomas L, Joseph A, Arumugam M, Pandey A (2013) Production, purification, characterization and over-expression of xylanases from actinomycetes. Indian J Exp Biol 51:875–884
Thomas L, Ushasree MV, Pandey A (2014) An alkali-thermostable xylanase from Bacillus pumilus functionally expressed in Kluyveromyces lactis and evaluation of its deinking efficiency. Bioresour Technol 165:309–313
Thomas L, Sindhu R, Binod P, Pandey A (2015) Production of an alkaline xylanase from recombinant Kluyveromyces lactis (KY1) by submerged fermentation and its application in bio-bleaching. Biochem Eng J 102:24–30
Topakas E, Katapodis P, Kekos D et al (2003) Production and partial characterization of xylanase by Sporotrichum thermophile under solid-state fermentation. World J Microbiol Biotechnol 19:195–198. https://doi.org/10.1023/A:1023207429410
Turunen O, Etuaho K, Fenel F et al (2001) A combination of weakly stabilizing mutations with a disulfide bridge in the α-helix region of Trichoderma reesei endo-1,4-β-xylanase II increases the thermal stability through synergism. J Biotechnol 88:37–46
Upreti RK, Kumar M, Shankar V (2003) Bacterial glycoproteins: functions, biosynthesis and applications. Proteomics 3:363–379
Urbániková L, Vršanská M, Krogh KBRM et al (2011) Structural basis for substrate recognition by Erwinia chrysanthemi GH30 glucuronoxylanase. FEBS J 278:2105–2116
Valenzuela SV, Díaz P, Javier Pastor FI (2010) Recombinant expression of an alkali stable GH10 Xylanase from Paenibacillus barcinonensis . J Agric Food Chem 58:4814–4818. https://doi.org/10.1021/jf9045792
van den Brink J, van Muiswinkel GCJ, Theelen B et al (2013) Efficient plant biomass degradation by thermophilic fungus Myceliophthora heterothallica . Appl Environ Microbiol 79:1316–1324
Van Dorn R, Shanahan D, Ciofalo V (2018) Safety evaluation of xylanase 50316 enzyme preparation (also known as VR007), expressed in Pseudomonas fluorescens , intended for use in animal feed. Regul Toxicol Pharmacol 97:48–56
Van Petegem F, Collins T, Meuwis M-A et al (2002) Crystallization and preliminary X-ray analysis of a xylanase from the psychrophile Pseudoalteromonas haloplanktis . Acta Crystallogr Sect D 58:1494–1496. https://doi.org/10.1107/S0907444902011666
Vardakou M, Flint J, Christakopoulos P et al (2005) A family 10 Thermoascus aurantiacus xylanase utilizes arabinose decorations of xylan as significant substrate specificity determinants. J Mol Biol 352:1060–1067. https://doi.org/10.1016/j.jmb.2005.07.051
Vazquez MJ, Alonso JL, Dominguez H, Parajo JC (2000) Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol 11:387–393
Velikodvorskaya TV, Iy Volkov, Vasilevko VT et al (1997) Purification and some properties of Thermotoga neapolitana thermostable xylanase B expressed in E. coli cells. Biochemistry (Mosc) 62:66–70
Verma D, Satyanarayana T (2012a) Molecular approaches for ameliorating microbial xylanases. Bioresour Technol 117:360–367
Verma D, Satyanarayana T (2012b) Cloning, expression and applicability of thermo-alkali-stable xylanase of Geobacillus thermoleovorans in generating xylooligosaccharides from agro-residues. Bioresour Technol 107:333–338
Verma D, Kawarabayasi Y, Miyazaki K, Satyanarayana T (2013) Cloning, expression and characteristics of a novel alkalistable and thermostable xylanase encoding gene (Mxyl) retrieved from compost-soil metagenome. PLoS ONE 8:e52459
Viikari L, Kantelinen A, Sundquist J, Linko M (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev 13:335–350
Virk AP, Puri M, Gupta V et al (2013) Combined enzymatic and physical deinking methodology for efficient eco-friendly recycling of old newsprint. PLoS ONE 8:e72346
Virupakshi S, Babu KG, Gaikwad SR, Naik GR (2005) Production of a xylanolytic enzyme by a thermoalkaliphilic Bacillus sp. JB-99 in solid state fermentation. Process Biochem 40:431–435
Voragen AGJ (1998) Technological aspects of functional food-related carbohydrates. Trends Food Sci Technol 9:328–335
Walia A, Mehta P, Chauhan A, Shirkot CK (2013) Optimization of cellulase-free xylanase production by alkalophilic Cellulosimicrobium sp. CKMX1 in solid-state fermentation of apple pomace using central composite design and response surface methodology. Ann Microbiol 63:187–198. https://doi.org/10.1007/s13213-012-0460-5
Walia A, Mehta P, Chauhan A et al (2014) Purification and characterization of cellulase-free low molecular weight endo β-1,4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J Microbiol Biotechnol 30:2597–2608
Walia A, Mehta P, Guleria S, Shirkot CK (2015a) Modification in the properties of paper by using cellulase-free xylanase produced from alkalophilic Cellulosimicrobium cellulans CKMX1 in biobleaching of wheat straw pulp. Can J Microbiol 61:671–681
Walia A, Mehta P, Guleria S, Shirkot CK (2015b) Improvement for enhanced xylanase production by Cellulosimicrobium cellulans CKMX1 using central composite design of response surface methodology. 3 Biotech 5:1053–1066
Walia A, Guleria S, Mehta P et al (2017) Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech 7:1–12. https://doi.org/10.1007/s13205-016-0584-6
Wang SY, Hu W, Lin XY et al (2012) A novel cold-active xylanase from the cellulolytic myxobacterium Sorangium cellulosum So9733-1: gene cloning, expression, and enzymatic characterization. Appl Microbiol Biotechnol 93:1503–1512. https://doi.org/10.1007/s00253-011-3480-3
Wang X, Ma R, Xie X et al (2017) Thermostability improvement of a Talaromyces leycettanus xylanase by rational protein engineering. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-12659-y
Waters DM, Ryan LAM, Murray PG et al (2011) Characterisation of a Talaromyces emersonii thermostable enzyme cocktail with applications in wheat dough rheology. Enzyme Microb Technol 49:229–236. https://doi.org/10.1016/j.enzmictec.2011.04.006
Whiting IM, Rose SP, Mackenzie AM et al (2019) Effect of wheat distillers dried grains with solubles and exogenous xylanase on laying hen performance and egg quality. Poult Sci 98:3756–3762
Wong KK, Tan LU, Saddler JN (1988) Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Xiao X, Bian J, Peng X-P et al (2013) Autohydrolysis of bamboo ( Dendrocalamus giganteus Munro) culm for the production of xylo-oligosaccharides. Bioresour Technol 138:63–70
Xie Y, An J, Yang G et al (2014) Enhanced enzyme kinetic stability by increasing rigidity within the active site. J Biol Chem 289:7994–8006. https://doi.org/10.1074/jbc.M113.536045
Xu ZH, Bai YL, Xu X et al (2005) Production of alkali-tolerant cellulase-free xylanase by Pseudomonas sp. WLUN024 with wheat bran as the main substrate. World J Microbiol Biotechnol 21:575–581. https://doi.org/10.1007/s11274-004-3491-7
Yadav P, Maharjan J, Korpole S et al (2018) Production, purification, and characterization of thermostable alkaline xylanase from Anoxybacillus kamchatkensis NASTPD13. Front Bioeng Biotechnol 6:65
Yang J, Han Z (2018) Understanding the positional binding and substrate interaction of a highly thermostable GH10 xylanase from Thermotoga maritima by molecular docking. Biomolecules 8:64. https://doi.org/10.3390/biom8030064
Yasinok AE, Biran S, Kocabas A, Bakir U (2010) Xylanase from a soil isolate, Bacillus pumilus : gene isolation, enzyme production, purification, characterization and one-step separation by aqueous-two-phase system. World J Microbiol Biotechnol 26:1641–1652
Yasuda M, Nagai H, Takeo K, et al (2014) Bio-ethanol production through simultaneous saccharification and co-fermentation (SSCF) of a low-moisture anhydrous ammonia (LMAA)-pretreated napiegrass ( Pennisetum purpureum Schumach). SpringerPlus 3:333
Yoon H, Han NS, Kim CH (2014) E. coli and characterization of the recombinant enzyme. Microb Cell Fact 13:45
Yoshioka H, Chavanich S, Nilubol N, Hayashida S (1981) Production and characterization of thermostable xylanase from Talaromyces byssochlamydoidesyh -50. Agric Biol Chem 45:579–586. https://doi.org/10.1080/00021369.1981.10864586
Zhang M, Jiang Z, Yang S et al (2010a) Cloning and expression of a Paecilomyces thermophila xylanase gene in E. coli and characterization of the recombinant xylanase. Bioresour Technol 101:688–695. https://doi.org/10.1016/j.biortech.2009.08.055
Zhang W, Lou K, Li G (2010b) Expression and characterization of the Dictyoglomus thermophilum Rt46B.1 xylanase gene (xynB) in Bacillus subtilis . Appl Biochem Biotechnol 160:1484–1495. https://doi.org/10.1007/s12010-009-8634-8
Zhang F, Hu S-N, Chen J-J et al (2012) Purification and partial characterisation of a thermostable xylanase from salt-tolerant Thermobifida halotolerans YIM 90462T. Process Biochem 47:225–228
Zhang D, Wang Y, Zheng D et al (2016a) New combination of xylanolytic bacteria isolated from the lignocellulose degradation microbial consortium XDC-2 with enhanced xylanase activity. Bioresour Technol 221:686–690
Zhang Y, An J, Yang G et al (2016b) Structure features of GH10 xylanase from Caldicellulosiruptor bescii : implication for its thermophilic adaption and substrate binding preference. Acta Biochim Biophys Sin (Shanghai) 48:948–957. https://doi.org/10.1093/abbs/gmw086
Zhang K, Zhang Y, Yan D, Zhang C, Nie S (2018) Enzyme-assisted mechanical production of cellulose nanofibrils: thermal stability. Cellulose 25(9):5049–5061
Download references
Acknowledgements
The authors are thankful to the Department of Biotechnology, Government of India for providing the financial support (Grant Nos. BT/304/NE/TBP/2012 and BT/PR7333/PBD/26/373/2012). NB is thankful to the University Grants Commission for providing fellowship for doctoral studies. BK is thankful to Jawaharlal Nehru memorial Fund and CSIR for providing scholarship for doctoral studies.
The authors thank Department of Biotechnology, Government of India (Grant Nos. BT/304/NE/TBP/2012 and BT/PR7333/PBD/26/373/2012).
Author information
Nisha Bhardwaj and Bikash Kumar contributed equally to this work
Authors and Affiliations
Bioprocess and Bioenergy Laboratory, Department of Microbiology, Central University of Rajasthan, NH-8, Bandarsindri, Kishangarh, Ajmer, 305817, India
Nisha Bhardwaj, Bikash Kumar & Pradeep Verma
You can also search for this author in PubMed Google Scholar
Contributions
NB and BK developed the manuscript and wrote the manuscript under guidance of PV. PV provided time to time scientific and technical comments to enhance the quality of manuscript. All authors read and approved the final manuscript
Corresponding author
Correspondence to Pradeep Verma .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and permissions
About this article
Cite this article.
Bhardwaj, N., Kumar, B. & Verma, P. A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 6 , 40 (2019). https://doi.org/10.1186/s40643-019-0276-2
Download citation
Received : 18 July 2019
Accepted : 09 October 2019
Published : 18 October 2019
DOI : https://doi.org/10.1186/s40643-019-0276-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Glycoside hydrolase
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 22 October 2022
Optimization, purification, and characterization of xylanase production by a newly isolated Trichoderma harzianum strain by a two-step statistical experimental design strategy
- Priyashini Dhaver 1 ,
- Brett Pletschke 2 ,
- Bruce Sithole 3 , 4 &
- Roshini Govinden 1
Scientific Reports volume 12 , Article number: 17791 ( 2022 ) Cite this article
5959 Accesses
16 Citations
9 Altmetric
Metrics details
- Biotechnology
- Microbiology
Xylanases are hydrolytic enzymes with a wide range of applications in several industries such as biofuels, paper and pulp, food, and feed. The objective of this study was to optimize the culture conditions and medium components for maximal xylanase production from a newly isolated Trichoderma harzianum strain using the Plackett–Burman Design (PBD) and Box Behnken Design (BBD) experimental strategies. Xylanase production was enhanced 4.16-fold to 153.80 U/ml by BBD compared to a preliminary one-factor-at-a-time (OFAT) activity of 37.01 U/ml and 2.24-fold compared to the PBD (68.70 U/ml). The optimal conditions for xylanase production were: 6 days of fermentation, incubation temperature of 70 °C, pH 5.0, agitation of 160 rpm, and 1.2% wheat bran and ammonium sulphate. The experimental design effectively provided conditions for the production of an acidic-thermostable enzyme with exciting potential for application in animal feed improvement. The acidic-thermostable xylanase was purified from the submerged culture and SDS-PAGE analysis revealed a molecular weight of 72 kDa. This protein had maximum xylanolytic activity at pH 6.0 and 65 °C and was stable for 4 h retaining > 70% activity and exhibited substrate specificity for beechwood xylan with a K m of 5.56 mg/ml and V max of 1052.63 µmol/min/mg. Enzyme activity was enhanced by Fe 2+ , Mg 2+ , and Zn 2+ . There was an absence of strong inhibitors of xylanase activity. Overall, these characteristics indicate the potential for at least two industrial applications.
Similar content being viewed by others
Enhanced production of a recombinant xylanase (XT6): optimization of production and purification, and scaled-up batch fermentation in a stirred tank bioreactor

Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through Response Surface Methodology
Optimizing alpha-amylase from Bacillus amyloliquefaciens on bread waste for effective industrial wastewater treatment and textile desizing through response surface methodology
Introduction.
After cellulose, hemicellulose is the second most abundant terrestrial polysaccharide composed of ß-1,4- d -xylopyranoside residues and ß-1,4-xylan as main constituents with arabinosyl and acetyl side chains 1 . Hemicellulose is a short crosslinked polymer compared to cellulose, which is a long straight-chain homopolymer. Xylans have a β-(1,4) linked backbone made of d -xylose and there are three subtypes of xylan based on the side chain. The subtypes are homoxylan, glucuronoxylan, and arabinoxylan. Homoxylan is only found in two or three types of plants and is mostly cross-linked by β-(1,4)-glycosidic bonds. Xylan is a renewable biomass resource that has potential as a substrate in many production processes. However, it must be hydrolysed to xylose and xylo-oligosaccharides which can be accomplished by xylanolytic enzymes. Among them, xylanases deserve special attention as they degrade the major hemicellulosic polysaccharides by catalyzing the hydrolysis of xylopyranosyl linkages of ß-1,4-xylan 2 . The main enzymes involved are endo-1,4-β-xylanases which make random cuts in the xylan backbone and β-xylosidases which are exoglycosidases with the ability to degrade the non-reducing ends of xylooligosaccharides into xylose. The side groups in xylans are cleaved by α- l -arabinofuranosidases (EC 3.2.1.55), α-glucuronidases (EC 3.2.1.139), and acetyl xylan esterases (EC 3.1.1.72) 3 . The most common microbial xylanases that would hydrolyze all types of xylan are grouped based on amino acid similarities and structural characteristics, into glycoside hydrolase (GH) families 10 or 11 in the Carbohydrate-Active enzyme (CAZy) database ( http://www.cazy.org ) 4 . GH10 families are able to catalyze the hydrolysis of a wide range of xylans, while GH11 families are known to cleave unsubstituted regions of arabinoxylan 4 . However, both GH10 and GH11 xylanases have applications in various industries such as food and feed 5 , biofuel production 6 , paper and pulp 7 , and medical and pharmaceutical 8 . Xylanases are also found in GH families 30, 8, and 5. The complete degradation of heterogeneous xylan into simple sugars requires the synergistic action of several inducible hemicellulases 9 .
Microbes such as bacteria, fungi, and actinomycetes are ubiquitous in nature 10 and several endogenous and exogenous microbial enzymes have been widely explored, resulting in a variety of microorganisms commonly regarded as the most significant and convenient producers of large quantities of enzymes in a short period on inexpensive feedstock. Xylanases are produced by microbial biosynthesis for industrial applications 11 , 12 . Thermophilic fungi in particular are promising candidates for biotechnological applications due to their strong ability to degrade plant polysaccharide components and their robustness under harsh environmental conditions 2 . Many of the filamentous fungi that have been studied produce several xylanases and have high xylan-degrading ability. Trichoderma sp. and Aspergillus sp. are most frequently employed for industrial applications 9 including the bioconversion of plant biomass into animal feed 5 , plant fertilizers, and chemicals for the food industry 9 .
The production of enzymes is costly, thus, to meet industrial demand, a low-cost growth medium is required for microbial growth and enzyme production. There are two possible cultivation methods for microbial xylanase production: solid-state and submerged fermentation 13 . Submerged fermentation technology has the advantage of short production periods to achieve high yields at low costs. Both the nutrient medium composition and culture conditions have a strong influence on xylanase production. The physical and chemical factors known to influence xylanase production are temperature, pH, incubation period, carbon and nitrogen sources and concentration, and agitation speed 14 . Temperature effects on enzyme production are predominantly related to the growth of the organism (mesophilic, psychrophilic, or thermophilic). The pH is one of the most important factors governing microbial growth due to their sensitivity to the hydrogen ion concentration in the medium 15 . It is also key to enzyme activity as it can alter the ionic charges on the molecule, which could cause changes to the enzyme’s shape (they may denature), and that usually leads to a reduction or loss of the catalytic properties of the enzymes and cessation of metabolic activity 16 .
Supplementation of the growth medium with carbon and nitrogen sources usually increases enzyme production as this provides an enriched environment for microbial growth 17 . Therefore, screening of the most influential factors and optimization of the growth conditions are essential to ensure maximal enzyme production, potentially significantly reducing production costs for xylanases 18 . There are two approaches to optimize the fermentation process classical and statistical. The classical approach is based on the testing of “one-factor-at-a-time (OFAT)” and the statistical approach includes the Plackett–Burman design (PBD) and response surface methodologies (RSM).
The OFAT approach is a conventional single-dimensional investigation that involves changing one independent variable at a time while the others remain at their optimal level 19 , 20 . This is the main strategy used for selecting optimal conditions, which continues to be widely used in preliminary optimization studies 21 . The main disadvantages of OFAT are the partial explanation regarding the effect of the factors on the response and the absence of the interaction effects between the variables 22 . This method also involves a relatively high number of experiments, which makes it laborious and time-consuming 21 . Moreover, it may lead to unreliable results and inaccurate conclusions.
To resolve this problem, optimization studies can be carried out by using multivariate statistical methods 21 , PBD and RSM can potentially eliminate the limitations of the OFAT optimization process 23 . PBD is a powerful statistical technique for screening medium components in shake flask fermentation and reduces the total number of experiments 24 . This technique is useful and has been widely used as the first step of an optimization procedure, however, it cannot determine the interaction effects 23 but allows the evaluation of the importance of each factor in moderately few experiments 25 . RSM using a Box–Behnken Design (BBD) is an effective optimization tool. The RSM design can provide the dependence of enzyme production on independent variables, predicted results for responses, and levels for independent variables in the form of mathematical models 26 , 27 .
Hydrolysis of xylan and hemicellulosic materials to various xylooligosaccharides has been accomplished using crude xylanases. However, to meet the desired requirements of some applications, robust xylanases (resistant to metal ions and chemicals, displaying pH, and thermostability) with specific biochemical properties for pH and temperature optima as well as, high specific activity are required, which would require purification of appropriate candidate enzymes 28 . Purification of xylanases is necessary to remove contamination by proteins and other enzymes in the culture medium, such as cellulases, as well as compounds derived from hydrolysis of the substrate. These contaminants can complicate activity assays, protein quantification, and physicochemical assays 29 . Purification and physicochemical characterization (activity and stability at various pH and temperatures) of pure xylanases provide information on the enzyme's structural and functional features, which may be used to assess its application potential 29 . Purification should be centred on attaining the highest yield while retaining the highest possible enzymatic activity and purity 30 .
Xylanases are required in large quantities for industrial-level applications because they not only have application in several processes but possess the necessary characteristics to withstand harsh conditions during these industrial processes. As a result, there is a need to select microorganisms that produce high levels of xylanases with appropriate properties, followed by optimization of growth which would lead to higher levels of enzymes 19 . There are several reports available on the optimization of media components and the physical growth parameters for the production and purification of xylanases for various applications using different substrates 8 , 12 , 21 . Xylanase production by Trichoderma reesei SAF3 31 and Trichoderma stromaticum AM7 32 was increased by optimization. While there are reports on multiple other species, there were very few reports on thermostable Trichoderma harzianum xylanases in literature.
Therefore, in the present study, a recently isolated and characterized fungal strain, T. harzianum , producing a thermophilic and acidic xylanase was the subject of study. The major focus of this study was to employ statistical design strategies to optimize xylanase production. Although this is not a novel approach, its application to a novel thermophilic and acidic xylanase is. We also report on the purification and characterization of the T. harzianum xylanase, to determine its applicability for future studies in animal feed improvement.
Materials and methods
Microbial strain.
The T. harzianum strain was selected from a previous screening study as the candidate for xylanase production 33 . Fungal cultures were streaked on the PDA plates and slants, grown for 5 days at 30 °C followed by the addition of sterile mineral oil to cover the fungal mycelium and storage at 4 °C. Long-term stocks were prepared by washing fungal spores from the 5-day PDA plates with distilled water and adding 50% glycerol in a 1:1 ratio to the spore suspension and storing at − 20 °C and − 80 °C.
Medium and cultivation
Nutrient salt solution (NSS) used for xylanase production comprised [(g/L): (0.005 g) CaCl, (0.23 g) KH 2 PO 4 , (0.05 g) MgSO 4 , (0.005 g) NaNO 3 , (0.002 g) ZnSO 4 , (0.009 g) FeSO 4 , (0.23 g) KCl, (7 g) peptone, and (20 g) wheat bran]. Erlenmeyer flasks (250 ml) containing 50 ml of the medium were each inoculated with two 5 mm fungal plugs from a 5-day-old plate culture and incubated at 30 °C at 200 rpm for 7 days in a shaker (New Brunswick Innova 44, Germany). Cultured media were removed after the incubation period and the cell-free supernatant was recovered by centrifuging samples at 16,873 × g for 10 min (Eppendorf centrifuge 5418, Germany). Xylanase activity was determined as described below in the xylanase assay method (“ Xylanase assay ”).
Xylanase assay
Xylanase activity was quantified using the 3,5-dintrosalicylic acid (DNS) assay for reducing sugars according to the method by Miller 34 . The reaction included 600 µl of 1% (w/v) of beechwood xylan (1 g in 100 ml of 50 mM citrate buffer pH 5.0) in 15 ml test tubes to which 66.67 µl of the culture supernatant was added. The reaction mixture was incubated in a water bath at 55 °C for 15 min and terminated by the addition of 1 ml DNS reagent followed by heating for 5 min at 100 °C in a water bath. One unit (U) of xylanase was defined as the amount of enzyme that released 1 µmol xylose as reducing sugar equivalents per min under the specified assay conditions.
Optimization of xylanase production: one factor at a time (OFAT)
To optimize the growth parameters, OFAT was used to evaluate the effect of a single parameter at a time performed in earlier study 33 . The enzyme activity was obtained to determine the optimal yield and was reported in previous studies 33 .
Statistical optimization, experimental design, and data analysis
Plackett–burman design (pbd).
Six variables were selected for this study: Incubation temperature (X 1 ), Incubation time (X 2 ), pH (X 3 ), Agitation (X 4 ), Wheat bran (X 5 ), and Ammonium sulphate (X 6 ) (Table 1 ). The total number of experimental runs carried out for the six variables was twelve 35 . Each variable was represented by a high level denoted by ‘+’ and a low level denoted by ‘−’. The high level of each variable was sufficiently far from the low level so that any significant effect would be observed. The experimental runs were performed in duplicate and an average of the results was reported Table 2 represents the PBD based on the first-order polynomial model Eq. ( 1 ):
where Y is defined as the response (peak area and retention factor), β 0 is the model intercept, β i is the linear coefficient and Χ i is the level of the independent variable. The PBD was analyzed using R studio software 36 to estimate the significant factors. ANOVA was performed to determine the p -values as well as the R coefficients to check the significance and fit of the regression model. Screened parameters were represented on a Pareto chart of standardized effects. The effect of each variable was analyzed and the ones with the highest influence on the production of xylanase were selected for the second level optimization by BBD of RSM.
Optimization of the significant variables using response surface methodology (RSM)
The BBD was used to elucidate the main interaction and quadratic effects of the three significant variables arising from the PBD, with replicated centre points 21 . The experimental design and statistical analysis were performed using R Studio 36 . A three-level three-factor BBD was used to evaluate the combined effect of the three significant independent variables, Incubation time (X 2 ), pH (X 3 ), and wheat bran (X 5 ) (Table 3 ). The design consisted of 16 combinations, including three replicates of the centre point as shown in Table 4 . After the experimental runs were completed, the average xylanase activities were taken as the response (Y). A multiple regression analysis of the data was carried out to obtain an empirical model that relates the response measured to the independent variables 37 . The second-order polynomial Eq. ( 2 ) is shown below:
where Y represents the response variable (peak area), β 0 is the interception coefficient, β 1 is the coefficient for the linear effects, β 2 is the coefficient for the quadratic effect, β 12 are interaction coefficient and Χ 1 Χ 2 is the coded independent variables that influence the response variable Y. The response in each run was the average of duplicates. In this experimental design, data were analyzed by one-way ANOVA with Tukey’s multiple comparison test (p ≤ 0.05) using R studio 36 , and ggplot2 was used for the generation of 3D response surface and contour plots 38 .
Scaled-up production in the optimized medium
The optimized parameters for each factor from the statistical design experiments were implemented for the scaled-up production of the xylanases. The nutrient salt solution was prepared as previously described (“ Medium and cultivation ”) and supplemented with the optimized wheat bran and ammonium sulphate concentrations. Erlenmeyer flasks (2 l) containing 400 ml of the medium were each inoculated with two 5 mm fungal plugs from a 5-day-old plate culture and incubated at the optimized parameters in a shaker (New Brunswick Innova 44, Germany). Cultured media were removed after the incubation period and the cell-free supernatant was recovered by centrifuging samples at 16,873 × g for 10 min (Eppendorf centrifuge 5418, Germany). Xylanase activity was determined as described in “ Xylanase assay ”.
Purification of xylanase
All purification steps were carried out at 4 °C. Partial purification of the xylanase was carried out by ammonium sulphate precipitation (20–80%). The pellets were dissolved in 50 mM citrate buffer pH 5.0 and subjected to dialysis overnight in the same buffer. The fraction that resulted in the highest activity, was concentrated in 3 kDa Amicon centrifugal tubes, the protein precipitate dissolved in 50 mM citrate buffer pH 5.0 buffer and loaded onto an anion exchange column (HiTrap Q FF 5 ml) which was connected to the AKTA Purifier (AKTA Purifier, GE Healthcare Bio-Science, AB75184, Uppsala Sweden). Before loading the sample, the column was equilibrated with 20 mM Tris buffer, (pH 8.0). The enzyme was eluted using a 0–2 M sodium chloride gradient at a flow rate of 1.5 ml/min. Fractions were collected and those displaying xylanase activity were pooled, concentrated, and dialyzed against a 50 mM citrate buffer (pH 5.0), to be used for further characterization of the enzyme. Protein concentration was measured by the Bradford method 39 using bovine serum albumin as the standard. The samples were separated on a 12% SDS-polyacrylamide gel according to Laemmli 40 . Native PAGE was performed with 1% xylan as the substrate. Once electrophoresis was completed, the gel was incubated in 50 mM citrate buffer pH 5.0 at the optimum temperature (70 °C) for 20 min and thereafter stained with 0.1% Congo red solution for 30 min and destained in 1 M NaCl until clearance bands representing xylanase activity were obtained.
Characterization of purified xylanase
Effect of ph and temperature on xylanase activity.
The pH optimum was determined by measuring enzyme activity between pH 4.0 and 10.0 The following buffers were used: 0.1 M sodium citrate buffer (pH 3.0–5.0), 0.1 M potassium phosphate buffer (pH 6.0–8.0) and 0.1 M Glycine NaOH buffer (pH 9.0–10.0) 41 . Enzyme assays were conducted as previously described (“ Xylanase assay ”). For determination of the optimum temperature, the reactions were carried out at the optimum pH between 40 to 80 °C with intervals of 5 °C.
pH and thermostability
The pH stability of the enzyme was determined by incubating the enzyme in the optimal pH buffer for 4 h at 55 °C with aliquots removed every 30 min. A substrate control was also incubated for 4 h. Thereafter, xylanase activity was assayed using the DNS method and reported as residual activity (%). Temperature stability was determined by incubating the enzyme in the optimal pH buffer at optimal temperature for 4 h with aliquots collected every 30 min. The activity was assayed and reported as residual activity (%).
Effect of metallic ions and different solvents on xylanase activity
The effect of metallic ions (CaCl 2 , CoCl 2 , FeSO 4 , MgSO 4 , MnSO 4 , and ZnSO 4 ) and chemical agents (SDS, DMSO, and EDTA) on enzyme activity was evaluated at two concentrations: 2 mM and 10 mM. The residual activity was measured using the standard assay conditions. The activity in the absence of metallic ions or inhibitors was taken as the control (100%) 42 .
Substrate specificity
The specificity of the purified xylanase was verified by assaying the activity using various substrates, viz., beechwood xylan, birchwood xylan, xylan from Larchwood, wheat arabinoxylan (soluble and insoluble), carboxymethylcellulose (CMC) and Avicel. Substrates (1% w/v) were suspended in 50 mM citrate buffer (pH 6.0) and incubated with the purified enzyme at 65 °C for 15 min and thereafter the xylanase activity was determined by the DNS method as described previously (“ Xylanase assay ”) 43 .
Kinetic parameters
The K m and V max values for the xylanase were determined by measuring the enzymatic activity using different concentrations of the xylan substrate (1–20 mg/ml). The activity was measured under standard assay conditions as described previously. The Michaelis-Menton and Lineweaver- Burk plots were acquired to determine K m and V max .
Equipment and settings
Neither image acquisition tools nor image processing software packages were used for the figures in this study. For Fig. 7 , processing such as changing brightness and contrast was applied equally across the entire image and applied equally to the controls. The contrast does not allow for any data to disappear. There were no excessive manipulations, such as processing to emphasize one region in the image at the expense of others.
Results and discussion
Screening of significant medium constituents for xylanase production.
The rows in Table 2 represent the twelve different experiments. The data obtained from the PBD runs indicate a wide variation in xylanase activity from 9.8 to 68.7 U/ml across the twelve runs. This variation demonstrated that the effect of the medium and culture conditions on the production of xylanase was significant (p < 0.05). The R 2 , or coefficient of determination, is the percentage of response variance that can be ascribed to the model rather than a random error 44 . According to Xie et al. 45 , R 2 should be at least 90% for a model to fit well. The determination coefficient ( R 2 ) indicates that the independent variables were responsible for 97 percent of the sample variance in xylanase output, and just roughly 3% of the overall variation was not explained by the model. The greater the correlation between experimental and anticipated values, the closer R (correlation coefficient) is to 1. The value of R (0.97) indicated that the experimental data and the theoretical values predicted by the model equation were in close agreement. As indicated in Table 5 , the p -value was used to verify the significance of each of the coefficients. The incubation period (X 2 ), pH (X 3 ), and wheat bran (X 5 ) were all shown to have a significant ( p < 0.05) effect on xylanase activity. The Pareto chart of standardization (Fig. 1 ) confirmed that these three factors significantly influenced xylanase production ( p < 0.05), as they crossed the p -line. However, the other independent factors ( p > 0.05) were generally considered insignificant.

Pareto chart of standardized effects for the production of xylanase. Incubation temperature (X 1 ), incubation time (X 2 ), pH (X 3 ), agitation (X 4 ), wheat bran (X 5 ), ammonium sulphate (X 6 ).
There is a 97% chance that the model explains the measured variations in response. The magnitude and direction of the factor coefficient in the equation clarify the influence of the six variables for xylanase production. The higher magnitude indicated a large effect on the response. The corresponding response of xylanase activity was expressed in terms of the following regression Eq. ( 3 ) derived from the Unstandardized Beta values (Table 6 ):
where Y is defined as the peak area, X 1 refers to the incubation temperature, X 2 is the incubation time, X 3 is the pH, X 4 is the agitation, X 5 is the wheat bran and X 6 is the ammonium sulphate.
Optimization of significant variables for xylanase production
Box behnken design.
A total of 16 runs were performed to determine the conditions for optimal xylanase production by T. harzianu m. A matrix was run with the three significant variables that emerged from the PBD experiments. The results for the BBD runs (Table 4 ) show that the lowest activity of 27.38 U/ml was obtained under zero-level conditions (5 days, pH 5.0, and 1% wheat bran) in run 15 while run 8 resulted in the highest xylanase activity of 153.80 U/ml under the following conditions: 6 days of incubation, pH 5.0, and 1.2% wheat bran. This was significantly and markedly (over four-fold)_higher (p ≥ 0.05) than the highest enzyme activities obtained during OFAT (38.50 U/ml). Long et al. 21 confirmed a similar but lower influence of optimized parameters on xylanase production (174.46–266.70 U/ml) by Trichoderma orientalis . Using the quadratic equation, the predicted values were determined (Table 4 ). The R 2 or coefficient of determination (0.9647, close to 1) confirmed the validity of the model, i.e., that 96.47% of the variability of the response can be expressed by the model. The value of the coefficient of adjusted determination, adjusted R 2 , was 0.9112 confirming that the actual values were close to the predicted values 46 , 47 . The correlation was confirmed by plotting the actual value curve as a function of the predicted values (Fig. 2 ) which shows the points distributed around the regression line. Figure 2 shows that the actual response values agreed well with the predicted response values, thus the predicted xylanase production is within the limits of the experimental factors. Therefore, the model is considered of sufficient quality 46 with a 96.47% chance that it explains the measured variations in response.

Graphical representation of the minimal difference between the actual (straight line) and predicted responses (circles) for the Response Surface Methodology Design for optimal xylanase activity.
Maximum xylanase production (153.80 U/ml) by the T. harzianum strain occurred in BBD run 8 under acidic conditions (pH 5.0), the higher wheat bran (1.2%), and a 5-day incubation period. Lightly lower activity can be observed for run 12 (116.74 U/ml) where the incubation period was 5 days, the wheat bran was 1.2% and the pH was 6.0. Even lower but similar enzyme activities were obtained for runs 6 (101.32 U/ml) and 7 (103.37 U/ml) where either the incubation time (4 or 6 days) or wheat bran (0.8 or 1.2%) was at their low or high levels, respectively compared to run 8 where both these parameters were at their high levels (6 days and 1.2%). This may be due to the presence of two isoforms that are maximally produced under acidic conditions. The presence of isoforms requires different periods of incubation for maximal xylanase activity and various wheat bran concentrations. In the presence of xylan, most microorganisms can produce multiple types of xylanases. Fungi are well-known for producing a wide range of xylanases (up to 30 multiple forms) 5 , 48 . Zhang et al. 49 reported that three xylanase isoforms were produced by Aspergillus fumigatus . Multiple forms of xylanases differ in stability, catalytic efficiency, absorption, and activity on substrates 50 . Okafor et al. 51 isolated a strain of Penicillium chrysogenum PCL501 from wood wastes and found that after 4 days of fermentation, wheat bran produced the highest xylanase activity of 6.47 U/ml. Abdel-Sater et al. 52 obtained maximum xylanase production from T. harzianum after 8 days of fermentation whereas, Thomas et al. 53 achieved maximum xylanase production in 4 days of fermentation by an Aspergillus sp.
The production of multiple forms of xylanases can be influenced by many factors, including the presence of various alleles of the same gene, variable mRNA processing, proteolytic digestion post secretion, and post-translational modifications such as glycosylation and autoaggregation 54 . Because xylanases have varying catalytic efficiencies, the production of several xylanases is particularly beneficial for the complete hydrolysis of hemicellulosic substances 55 . Generally, xylanase production is directly proportional to the duration of the fermentation time up to a certain level and then decreases, thus, incubation time affects xylanase production by fungi 56 .
Second-order regression and prediction
The second-order regression equation provides the xylanase activity produced by the T. harzianum strain as a function of incubation time (X 2 ), pH (X 3 ), and wheat bran (X 5 ) which can be presented in the following Eq. ( 4 ):
where Y is the peak area, X 2 is the incubation time, X 3 is the pH and X 5 is the wheat bran concentration. The statistically insignificant parameters ( p > 0.05) and their interactions were omitted from the equation. The model constants and coefficients were generated using the unstandardized beta values.
ANOVA and Pareto chart
The “Lack of fit p -value” (Table 7 ) was insignificant as the p -value was greater than 0.05, however, literature shows this p -value (> 0.05) is considered acceptable 57 . According to Bezerra et al. 58 , significant regression and a non-significant lack of fit present in the model were well-fitted to the experiments. Based on this, the regression equation can be validated 59 . ANOVA was performed to determine the p -values (Table 7 ). This showed the model, the linear and square terms for X 2 (Incubation time), and the interaction between X 3 (pH) and X 5 (Wheat bran) as well as the linear terms of X 3 (pH) to be significant as the p -values were 0.00001, 0.0001, 0.00005, 0.02042 and 0.01137, respectively. The Pareto chart of standardization histogram graph (Fig. 3 ) also showed that Incubation time (X 2 , X 2 2 ), the interaction between pH and wheat bran (X 3, X 5 ), and pH (X 3 ) was significant ( p < 0.05), as it crosses the p -line.

Pareto chart standardized effects of nine interactive factors affecting the production of xylanase optimization. Incubation temperature (X 1 ), incubation time (X 2 ), pH (X 3 ), agitation (X 4 ), wheat bran (X 5 ), ammonium sulphate (X 6 ).
Interaction of variables
The relationship between the parameters and the responses can be understood by studying the three-dimensional (3D) response surface plots for xylanase activity generated from the quadratic model. The 3D response surface plot can also be used to determine the optimum level of each variable for xylanase activity (Figs. 4 , 5 , 6 ). While maintaining other variables at their optimal level, the Z-axis (referring to xylanase activity) versus any two variables was constructed in the response surface plot.

3D-response surface plots ( a ) and contour plots ( b ) of the combined effects of Incubation time (X 2 ) and pH (X 3 ) on xylanase production by Trichoderma harzianum strain.

3D-response surface plots ( a ) and contour plots ( b ) of the combined effects of Incubation time (X 2 ) and wheat bran (X 5 ) on xylanase production by Trichoderma harzianum strain.

3D-response surface plots ( a ) and contour plots ( b ) of the combined effects of pH (X 3 ) and wheat bran (X 5 ) on xylanase production by Trichoderma harzianum strain.
Figure 4 a,b illustrate the combined effects of incubation time and pH xylanase activity increases at a high pH and shorter incubation time. Figure 4 b illustrates the contour plot, which shows that high enzyme activity was obtained at the shortest (4 days) and longest period (6 days) of incubation in acidic (4.0–6.0) conditions. Yadav et al. 29 , reported similar pH conditions for optimization of xylanse production from Anoxybacillus kamchatkensis NASTPD13.
Figure 5 a,b show that xylanase production is directly proportional to incubation time and wheat bran. This could be due to higher levels of degradation of xylan present in the wheat bran by T. harzianum . In Fig. 5 b, it is apparent that the xylanase activity is highest at high concentrations of wheat bran with the shortest (4 days) and longest period (6 days) of incubation. Previous studies showed the time course during the OFAT approach, being favourable at 4 days and 6 days of incubation with the optimum being at 5 days 33 . The RSM plots correspond with the OFAT results, as the plots show the highest xylanase activity obtained at high wheat bran between 4 and 6 days. Simultaneously, it was highlighted by Beg et al. 60 that wheat bran could effectively induce higher xylanase production by Aspergillus awamori. Li et al. 61 also reported the importance of the substrate concentrations for xylanase production by A. awamori. The facts mentioned here, correspond to the reports by Cui and Zhao 62 , as they mention that the enzymes, which are involved in substrate degradation, were generally mostly inducible. These were formed only when the substrate it correlates with, was present in the nutrient salt solution.
Figure 6 shows the highest xylanase activity obtained with high wheat bran and the entire pH range tested. However, at lower wheat bran concentrations, higher xylanase activity can be observed at the pH extremes tested (at approximately pH 4 and pH 6). Figure 6 b illustrates, at high wheat bran concentration and over a wide pH range with the highest activity obtained at the highest pH and wheat bran concentration. The interaction between the pH and wheat bran (130 U/ml) and between incubation time and wheat bran (130 U/ml) had the highest effect on xylanase activity compared to the interaction between incubation time and pH (120 U/ml).
The study successfully demonstrated a notable increase in enzyme activity using the statistically designed experiments compared to OFAT. It was also demonstrated that multiple forms of xylanase were produced (isoforms) based on variations in the growth and media conditions. Based on Table 4 , there could potentially be 5 different isoforms. High xylanase activity was observed for runs 4, 6, 7, 8, and 12. Supplementary Fig. 6 representing these RSM runs indicates the presence of isoforms by several zones of clearance on the substrate native PAGE gels.
Multiple forms of xylanases with different pH optima could be beneficial for animal feed improvement 13 . Xylanase is used to reduce the viscosity of the feed and improve the absorption of nutrients in the digestive tract of animals. The enzymes could be applied before the pelleting process, which operates between pH 4.0 and 6.0 thus requiring enzymes that are active within this pH range. Most xylanases reported to date are optimally active in the acidic or neutral pH range. Xylanases with acidic pH optima could potentially also be useful for applications containing waste, as a method of waste management, and as a feedstock for fermentable sugars 63 .
Scaled-up fermentation in optimized conditions for further studies
The xylanase enzyme was produced on a larger scale for further studies. The enzyme was produced at pH 5.0 for 6 days of incubation and with 1.2% wheat bran. The enzyme activity was determined in order to compare the activities to the smaller scale production. The enzyme activity obtained was 152.78 U/ml which was similar to the enzyme production on a smaller scale (153.80 U/ml).
Purification of xylanase from the T. harzianum isolate and zymography
The xylanase from T. harzianum was purified using ammonium sulphate precipitation, dialysis, and chromatographic methods in combination. Table 8 summarizes the purification stages. The enzyme was fractionated with the following ammonium sulphate saturations: (0–19%, 20–29%, 30–39%, 40–49%, 50–59%, 60–69% and 70–79%). The 70–79% saturation fraction resulted in significantly high xylanase activity with a recovery of 20.31% enzyme activity. The 50–59%, 60–69%, and fractions also showed relatively high recovery of enzyme activity (18.73%, and 17.48%, respectively) whereas 10.42% enzyme was recovered in the 40–49% fraction (Table 8 ) therefore these fractions were further studied to confirm if they were isoforms. The active fractions were then dialyzed at 4 °C overnight to remove the salts, and the enzyme was loaded onto DEAE Sephadex for further purification. A 0–2 M sodium chloride concentration gradient was used to elute the bound protein. Xylanase activity was measured in both bound and unbound protein fractions. The primary peak eluted at 0.5 M sodium chloride and the corresponding fraction had a specific activity of 254.62 mol/mg, and a 2.52-fold purity. Furthermore, a single band with a molecular weight of 72 kDa was evident on SDS-PAGE gels of the purified enzyme (50%) (Fig. 7 a). The other ammonium sulphate fractions (60–79%) also have the same molecular weight protein (72 kDa). To assess the activity/purity, the purified xylanase was subjected to zymogram analysis by substrate native-PAGE (1% beechwood xylan). The xylanolytic activity of the enzyme was indicated by clear zones in the gel after Congo-red staining (Fig. 7 b). Purified preparations of enzymes are a requisite for their application as well as elucidating their basic characteristics and mechanisms. Based on the high molecular weight of the purified enzyme, it can be tentatively inferred that it may belong to the GH10 family since enzymes belonging to this family feature a larger molecular weight 63 . Enzymes are also classified based on their catalytic reactions. Based on the sequence similarities of amino acids, xylanases are classified into glycosyl hydrolase (GH) families 10 (GH10) and 11 (GH11) 2 . Family GH10 contains xylanases of high molecular mass (> 30 kDa) with a (β/α) 8 barrel structure and acidic pI values, while GH11 include are the low-molecular-weight endoxylanases which are divided into alkaline pI and acidic pI xylanases 2 .
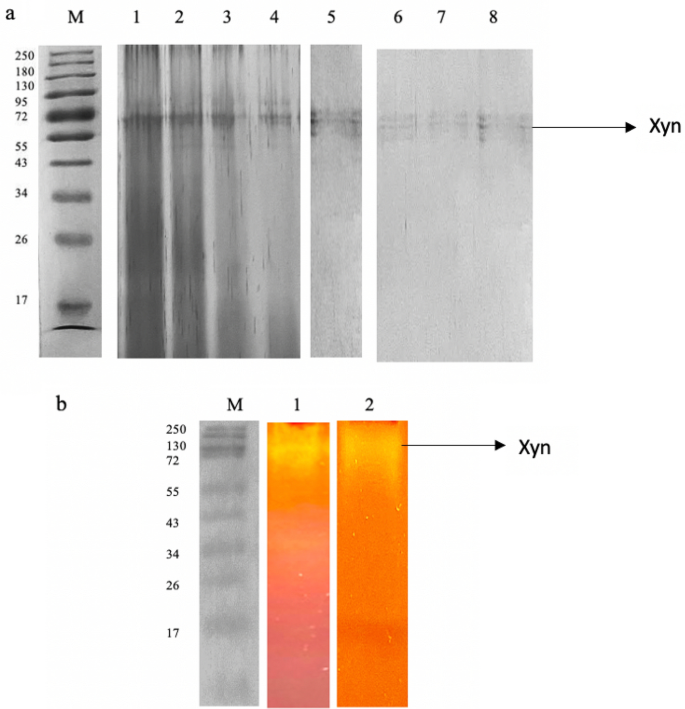
A 12% SDS PAGE ( a ) and Native substrate-PAGE ( b ) analysis of purified xylanase. 12% SDS PAGE (cropped) represents Lane M: Molecular weight marker (Thermoscientific, USA), 1–4: 50, 60, 70, and 80% ammonium sulphate fractions (Coomassie-stained), and 5–8: Purified xylanase from Trichoderma harzianum (Xyn). Native substrate-PAGE (cropped) represents Lane M: Molecular weight marker (Thermoscientific, USA), 1: 50% Ammonium sulphate fraction showing zone of clearance, and Lane 2: Purified xylanase (Xyn) from Trichoderma harzianum on native substrate gel showing zone of clearance. The original gels are presented in Supplementary Figs. 1 – 5 .
Characterization of xylanase
Ph optimum and stability.
The enzyme activity is greatly affected by pH because substrate binding and catalysis are dependent on the charge distribution of both the substrate and the enzyme molecules. The reaction pH was adjusted to 4.0–10.0 with various buffers as described above. The optimum pH of T. harzianum xylanase is pH 6.0 with an activity of 40 U/ml (Fig. 8 a). The enzyme is fairly stable at pH 6.0 and remains active (Fig. 9 ) retaining > 70% of its activity over 4 h. Souza et al. 64 reported that the xylanase from Thermoascus aurantiacus expressed in E. coli showed optimum activity and stability at a similar pH. Yadav et al. 29 reported that xylanase from Anoxybacillus kamchatkensis NASTPD13 showed high activity between pH 6.0 to 9.0 and at pH 6.0, the enzyme retained 71% of its activity over 24 h. The purified 60–79% ammonium sulphate fraction was further confirmed to contain the same protein as that purified in the 50% fraction as it displayed the same pH optimum and size (pH 6.0) (Fig. 8 a). Thus, the purified fractions of the 50–79% ammonium sulphate fractions can be combined to increase the yield (%).

Effect of pH ( a ) and temperature ( b ) on the activity of purified xylanases (50%, 60%, 70%, and 80% ammonium sulphate fractions). Data points represent the means ± SD (n = 4).

pH and temperature stability of purified xylanases (50% ammonium sulphate fraction) produced by the Trichoderma harzianum isolate. Data points represent the means ± SD (n = 4).
Optimum temperature and thermal stability
The experiment was carried out at different reaction temperatures ranging from 40 to 79 °C to find the optimal temperature of the xylanase. The highest activity of xylanase was observed at 65 °C (Fig. 8 b). Thermal stability data illustrated in Fig. 9 shows that the enzyme retained > 70% activity at 65 °C for 4 h. A similar result was reported by de Oliveira Simões et al. 65 . However, in that study, the enzyme was subjected to treatment for 24 h and was stable for 1 h. The purified 60–79% ammonium sulphate fraction contained the same purified protein as the 50% fraction, with the same molecular weight, pH and temperature optima obtained (65 °C) (Fig. 8 b). This confirms that these fractions are not isoforms of the xylanase produced. However, the shape of the curve for the 50% ammonium sulphate fraction is different from the other fractions, which seem to show an optimum rather than a broad bell shape.
The advantages of enzymes that prefer high temperatures are well known because the solubility of the reagents and products is increased, the viscosity is reduced, and the mass transfer rate is higher 66 . When looking for enzymes for industrial uses, stability, and activity at high temperatures are highly desirable.
Effect of metal ions and inhibitors
The effects of 8 metal ions (Ca 2+ , Co 2+ , Fe 2+ , Mg 2+ , Mn 2+ , Zn 2+ , K + , and Na + ) at a final concentration of 2 mM and 10 mM on xylanase activity were determined (Table 9 ) at the optimal pH and temperature (6.0 and 65 °C). Enzyme activity was slightly increased by 2 mM Mn2 + , K + , and Na + (101.11–101.77 U/ml) whereas the enzyme activity was slightly but significantly higher with 10 mM Ca 2+ , Co 2+ , Fe 2+ , Mg 2+ , Zn 2+ (104.27–110.89 U/ml) (p ≥ 0.05) and thus, these ions act as cofactors for the enzyme. Maximum enhancement was observed for Fe 2+ (10.88%) followed by Mg 2+ (9.43%) and Zn 2+ (8.43%) at 10 mM. Fu et al. 43 reported similar findings for xylanase from Trichoderma sp. TPS-36.
Inhibitory effects were observed for Fe 2+ (15.29%), Mg 2+ (3.44%), Zn 2+ (5.95%) at 2 mM and Na + (1.24%) at 10 mM, however, this inhibition of xylanase was weak (< 50%). Co 2+ and Ca 2+ had no effect on xylanase activity (100%) at either concentrations.
Fu et al . 43 also reported weak (< 50%) inhibition of xylanase with the same ions and that Co 2+ and Ca 2+ had no effect on xylanase activity (100%) at either concentrations.
Substrate specificity of purified xylanase
To determine the substrate specificity of the xylanase for polysaccharide degradation, potential substrates, including birchwood xylan, beechwood xylan, wheat arabinoxylan (soluble and insoluble), xylan from Larchwood, CMC and Avicel were tested under optimal conditions (pH 6.0 and temperature 65 °C) using the purified xylanase. Higher hydrolytic activity was observed for the xylans from beechwood, birchwood, and Larchwood compared to wheat arabinoxylan (Table 10 ). The xylanase most actively degraded birchwood xylan (174.07%), followed by Larchwood xylan (131.03%), and presented the lowest activity towards wheat arabinoxylan (soluble 70.54% and insoluble 46.62%). The purified xylanase exclusively hydrolyzed xylans, with no activity on CMC and Avicel. This suggested that xylanase's substrate-binding domain has a high affinity for xylans from softwood (birchwood and beechwood) 67 . This might be due to differences in xylan polymer structures and the presence of reactive groups on the surface that are more readily bound. The purified xylanase exhibited significant hydrolytic activity on the diverse xylan substrates, indicating that it might be classified as an endo-1,4-xylanase 68 .
Kinetic analysis
The Michaelis constant, K m , may be determined by measuring the substrate concentration at half the maximum velocity. K m is a constant that remains fixed for every given enzyme and substrate combination. As a result, a low K m improves the enzyme's affinity for the substrate 69 The concentration range of the substrate under investigation was 1–20 mg/ml, the study revealed K m and V max were 5.56 mg/ml and 1052.63 µmol/min/mg (Fig. 10 ). The value of K m is within the range of fungal xylanases reported in literature (0.14–14 mg/ml). Raj et al. 70 obtained similar values (4.96 mg/ml and lower V max 402 µmol/mg/ml) for xylanase from alkaliphilic Bacillus licheniformis . Fu et al. 43 reported high V max (1250 µmol/min/mg) similar to this study. Because xylanase has a high V max value and a low K m value, it has a high affinity for the substrate, beechwood xylan, and can catalyze it more efficiently and quickly than other substrates. Xylanases from Caldicoprobacter algeriensis sp. nov. strain TH7C1T were shown to have high selectivity for beechwood xylan 42 .

Double reciprocal plot of the purified (50% ammonium sulphate fraction) xylanase from Trichoderma harzianum on beechwood xylan. Data points represent the means ± SD (n = 3).
The current study describes the successful optimization of xylanase production via statistical modelling using PBD and BBD by a T. harzianum strain in submerged fermentation. The most influential independent variables were identified and optimized—resulting in a 4.16-fold and 2.24-fold increase in enzyme activity with BBD compared to the OFAT and PBD, respectively. PBD allowed for the consideration of various variables and avoided loss of information, which might be essential in the optimization of the fermentation process. The predictions of the mathematical models were validated by experimental results. Quadratic models with three independent variables were shown to accurately define xylanase production, with high R 2 values for correlations between the actual and predicted values of the response variables. Results showed high enzyme activities obtained within a high pH range which indicates the potential of the xylanase for use over a wide range of applications. Acidic-thermostable xylanase was purified with a 10.42% recovery and 2.52-fold purity. The specific activity of purified xylanase was 254.63 U/mg. The acidic-thermostability of T. harzianum xylanase is advantageous for animal feed manufacturing. Future studies will include scaling up the production of xylanase from T. harzianum under optimized conditions, which include the factors and their variables that resulted in the highest xylanase activity (RSM, run 8). Studies will also include sequencing the xylanase protein to understand the structure-guided function of this enzyme.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request. Other data generated or analysed during this study are included in this article [and its supplementary information file] .
Broeker, J. et al. The hemicellulose-degrading enzyme system of the thermophilic bacterium Clostridium stercorarium : Comparative characterization and addition of new hemicellulolytic glycoside hydrolases. Biotechnol. Biofuels. 11 , 229 (2018).
Article PubMed PubMed Central Google Scholar
Bagewadi, Z. K., Mulla, S. I. & Ninnekar, H. Z. Purification, characterization, gene cloning and expression of GH-10 xylanase ( Penicillium citrinum isolate HZN13). 3 Biotech. 6 , 169 (2016).
Tenkanen, M. et al. Xylanase XYN IV from Trichoderma reesei showing exo- and endo-xylanase activity. FEBS J. 280 (1), 285–301 (2013).
Article CAS PubMed Google Scholar
Katsimpouras, C. et al. A novel fungal GH30 xylanase with xylobiohydrolase auxiliary activity. Biotechnol. Biofuels. 12 , 120 (2019).
Polizeli, M. D. L. T. D. M. et al. Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotecnol. 67 (5), 577–591 (2005).
Article CAS Google Scholar
Li, X., Dilokpimol, A., Kabel, M. A. & de Vries, R. P. Fungal xylanolytic enzymes: Diversity and applications. Bioresour. Technol. 344 , 126290 (2022).
Dar, F. M., & Dar, P. M. Fungal xylanases for different industrial applications. In Industrially Important Fungi for Sustainable Development . 515–539 (Springer, 2021).
Bhardwaj, N., Agrawal, K., & Verma, P. Xylanases: An overview of its diverse function in the field of biorefinery. Bioenergy Res. Commer. Oppor. Chall. 295–317 (2021).
Yi, Z. et al. Xylan deconstruction by thermophilic Thermoanaerobacterium bryantii , hemicellulases is stimulated by two oxidoreductases. Catalysts 12 , 182 (2022).
Golgeri, M. D. B. et al. A systematic review on potential microbial carbohydrases: Current and future perspectives. Crit. Rev. Food Sci. Nutr. (2022).
Chadha, B. S., Kaur, B., Basotra, N., Tsang, A. & Pandey, A. Thermostable xylanases from thermophilic fungi and bacteria: Current perspective. Bioresour. Technol. 277 , 195–203 (2019).
Intasit, R., Cheirsilp, B., Suyotha, W. & Boonsawang, P. Synergistic production of highly active enzymatic cocktails from lignocellulosic palm wastes by sequential solid state submerged fermentation and co-cultivation of different filamentous fungi. Biochem. Eng. J. 173 , 108086 (2021).
Walia, A., Guleria, S., Mehta, P., Chauhan, A. & Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech. 7 , 11 (2017).
Kereh, H., Mubarik, N. R., Palar, R., Santoso, P. & Yopi.,. Optimization of process parameters and scale-up of xylanase production using corn cob raw biomass by marine bacteria Bacillus subtilis LBF M8 in stirred tank bioreactor. PJBT. 15 (3), 707–714 (2018).
Google Scholar
Jain, P. & Pundir, R. K. Effect of fermentation medium, pH and temperature variations on antibacterial soil fungal metabolite production. J. Agric. Technol. 7 (2), 247–269 (2011).
Kurrataa, Y. & Meryandini, A. Characterization of xylanase activity produced by Paenibacillus sp. XJ18 from TNBD Jambi, Indonesia. J. Biosci. 22 , 20–26 (2015).
Wang, X. et al. Growth strategy of microbes on mixed carbon sources. Nat. Commun. 10 , 1279 (2019).
Article ADS CAS PubMed PubMed Central Google Scholar
Nasr, S., Soudi, M. R., Hatef Salmanian, A. & Ghadam, P. Partial optimization of endo-1, 4-Β-xylanase production by Aureobasidium pullulans using agro-industrial residues. Iran. J. Basic Med. Sci. 16 (12), 1245–1253 (2013).
PubMed PubMed Central Google Scholar
Uhoraningoga, A., Kinsella, G. K., Henehan, G. T. & Ryan, B. J. The goldilocks approach: A review of employing design of experiments in prokaryotic recombinant protein production. Bioeng. 5 (4), 89 (2018).
CAS Google Scholar
Khusro, A. & Aarti, C. Molecular identification of newly isolated Bacillus strains from poultry farm and optimization of process parameters for enhanced production of extracellular amylase using OFAT method. Res. J. Microbiol. 10 (9), 393–420 (2015).
Long, C., Liu, J., Gan, L., Zeng, B. & Long, M. Optimization of xylanase production by Trichoderma orientalis using corn cobs and wheat bran via statistical strategy. Waste Biomass Valoriz. 10 (1), 1277–1284 (2019).
Zhang, H. & Wu, J. Statistical optimization of aqueous ammonia pre-treatment and enzymatic hydrolysis of corn cob powder for enhancing sugar production. Biochem. Eng. J. 174 , 108106 (2021).
Irfan, M. et al. Statistical optimization of saccharification of alkali pre-treated wheat straw for bioethanol production. Waste Biomass Valoriz. 7 (6), 1289–1296 (2016).
Article Google Scholar
Ekpenyong, M. G., Antai, S. P., Asitok, A. D. & Ekpo, B. O. Plackett–Burman Design and response surface optimization of medium trace nutrients for glycolipopeptide biosurfactant production. Iran. Biomed. J. 21 (4), 249–260 (2017).
Sun, T. et al. The optimization of fermentation conditions for Pichia pastoris GS115 producing recombinant xylanase. Eng. Life Sci. 20 , 216–228 (2019).
Wu, W. J. & Ahn, B. Y. Statistical optimization of medium components by response surface methodology to enhance menaquinone-7 (vitamin k2) production by Bacillus subtilis . J. Microbiol. Biotechnol. 28 (6), 902–908 (2018).
Momeni, M. M., Kahforoushan, D., Abbasi, F. & Ghanbarian, S. Using chitosan/chpatc as a coagulant to remove colour and turbidity of industrial wastewater: Optimization through RSM design. J. Environ. Manag. 211 , 347–355 (2018).
Kiran, E. U., Akpinar, O. & Bakir, U. Improvement of enzymatic xylooligosaccharides production by the co-utilization of xylans from different origins. Food Bioprod. Process. 91 , 565–574 (2013).
Yadav, P. et al. Production, purification, and characterization of thermostable alkaline xylanase from Anoxybacillus kamchatkensis NASTPD13. Front. Bioeng. Biotechnol. 6 , 65 (2018).
Article MathSciNet PubMed PubMed Central Google Scholar
Periyasamy, K., Santhalembi, L. & Mortha, G. Production, partial purification and characterization of enzyme cocktail from Trichoderma citrinoviride AUKAR04 through solid-state fermentation. Arab. J. Sci. Eng. 42 , 53–63 (2017).
Kar, S. S. et al. Process optimization of xylanase production using cheap solid substrate by Trichoderma reesei SAF3 and study on the alteration of behavioural properties of enzyme obtained from SSF and SmF. Bioprocess Biosyst. Eng. 36 , 57–68 (2012).
Article PubMed Google Scholar
Carvalho, E. A. et al. Optimization of Xylanase production by Trichoderma stromaticum in solid state fermentation. Proceedings. 3, (2017).
Dhaver, P., Pletschke, B., Sithole, B. & Govinden, R. Isolation, screening and partial optimization of thermostable xylanase production under submerged fermentation by fungi in Durban, South Africa. Mycology. (2022).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31 , 426–428 (1959).
Ghosh, P. & Ghosh, U. Statistical optimization of laccase production by isolated strain Aspergillus flavus PUF5 utilizing ribbed gourd peels as the substrate and enzyme application on apple juice clarification. Indian J. Chem. Eng. 61 , 1–12 (2019).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2020). http://www.R-project.org/ .
Coman, G. & Bahrim, G. Optimization of xylanase production by Streptomyces sp. P12–137 using response surface methodology and central composite design. Ann. Microbiol. 61 (4), 773–779 (2011).
Article CAS PubMed PubMed Central Google Scholar
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis . 2nd ed. (Springer, 2009). https://doi.org/10.1007/978-0-387-98141-3 .
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 (12), 248–254 (1976).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 , 680–685 (1970).
Article ADS CAS PubMed Google Scholar
Franco, P. F., Ferreira, H. M. & Filho, E. X. Production and characterization of hemicellulase activities from Trichoderma harzianum strain T4. Biotechnol. Appl. Biochem. 40 (3), 255–259 (2004).
Amel, B. D. et al. Characterization of a purified thermostable xylanase from Caldicoprobacter algeriensis sp. Nov. strain TH7C1. Carbohydr. Res. 419 , 60–68 (2016).
Fu, L. H. et al. Purification and characterization of an endo-xylanase from Trichoderma sp., with xylobiose as the main product from xylan hydrolysis. World J. Microbiol. Biotechnol. 35 , 171 (2019).
Said, K. & Afizal, M. Overview on the response surface methodology (RSM) in extraction processes. J. Appl. Sci. Process Eng. 2 , 1 (2016).
Xie, Y. et al. Collaborative optimization of ground source heat pump-radiant ceiling air conditioning system based on response surface method and NSGA-II. Renew. Energy. 147 (1), 249–264 (2019).
Azzouz, Z., Bettache, A., Boucherba, N., Amghar, Z. & Benallaoua, S. Optimization of xylanase production by newly isolated strain Trichoderma afroharzianum isolate AZ12 in solid-state fermentation using Response Surface Methodology. Cellulose 54 , 451–462 (2020).
Chicco, D., Warrens, M. J. & Jurman, G. The coefficient of determination R squared is more informative than SMAPE, MAE, MAPE, MSE, and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 7 , 623 (2021).
Roy, S., Dutta, T., Sarkar, T. S. & Ghosh, S. Novel xylanases from Simplicillium obclavatum MTCC 9604: Comparative analysis of production, purification, and characterization of enzyme from submerged and solid state fermentation. Springerplus 2 , 382 (2013).
Zhang, S. et al. Synergistic mechanism of GH11 xylanases with different action modes from Aspergillus niger An76. Biotechnol. Biofuels. 14 , 118 (2013).
Liao, H. et al. Functional diversity and properties of multiple xylanases from Penicillium oxalicum GZ-2. Sci. Rep. 5 , 12631 (2015).
Okafor, U. A., Okochi, V. I., Onyegeme-okerenta, B. M. & Nwodo-Chinedu, S. Xylanase production by Aspergillus niger ANL 301 using agro-wastes. AJB. 6 , 1710–1714 (2007).
Abdel-Sater, M. A. & El-Said, A. H. M. Xylan-decomposing fungi and xylanolytic activity in agricultural and industrial wastes. Int. Biodeterior. Biodegrad. 47 , 15–21 (2001).
Thomas, L., Parameswaran, B. & Pandey, A. Hydrolysis of pre-treated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew. Energy. 98 , 9–15 (2016).
Maity, C. et al. Xylanase isozymes from the newly isolated Bacillus sp. CKBx1D and optimization of its deinking potentiality. Appl. Biochem. Biotechnol. 167 (5), 1208–1219 (2012).
Choudhury, B. et al. Biobleaching of nonwoody pulps using xylanase of Bacillus brevis BISR-062. Appl. Biochem. Biotechnol. 128 , 159–169 (2006).
Ribeiro Sales, M. et al. Cellulase and xylanase production by Aspergillus species. Ann. Microbiol. 61 , 917–924 (2011).
Abu, M. L., Nooh, H. M., Oslan, S. N. & Salleh, A. B. Optimization of physical conditions for the production of thermostable T1 lipase in Pichia guilliermondii strain SO using response surface methodology. BMC Biotechnol. 17 , 78 (2017).
Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S. & Escaleira, L. A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76 (5), 965–977 (2018).
He, X. et al. Efficient degradation of azo dyes by a newly isolated fungus Trichoderma tomentosum under non-sterile conditions. Ecotoxicol. Environ. Saf. 150 , 232–239 (2018).
Beg, Q. K., Bhushan, B., Kapoor, M. & Hoondal, G. S. Production and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11-3. J. Ind. Microbiol. Biotechnol. 23 , 396–402 (2000).
Liu, W., Lu, Y. L. & Ma, G. R. Induction and glucose repression of endo-beta-xylanase in the yeast Trichosporon cutaneum SL409. Process Biochem. 34 , 67–72 (1999).
Cui, F. & Zhao, L. Optimization of xylanase production from Penicillium sp. WX-Z1 by a two-step statistical strategy: Plackett–Burman and Box–Behnken experimental design. Int. J. Mol. Sci. 13 (8), 10630–10646 (2012).
Bhardwaj, N., Kumar, B. & Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 6 , 40 (2019).
Souza, A. R. et al. Engineering increased thermostability in the GH-10 endo-1, 4-β-xylanase from Thermoascus aurantiacus CBMAI 756. Int. J. Biol. Macromol. 93 , 20–26 (2016).
de Oliveira Simões, L. C. et al. Purification and physicochemical characterization of a novel thermostable xylanase secreted by the fungus Myceliophthora heterothallica F.2.1.4. Appl. Biochem. Biotechnol. 188 (4), 991–1008 (2019).
Vieille, C. & Zeikus, G. J. Hyper thermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability. MMBR. 65 (1), 1–43 (2001).
Yin, L., Lin, H., Chiang, Y. & Jiang, S. T. Bio properties and purification of xylanase from Bacillus sp. YJ6. J. Agric. Food Chem. 58 (1), 557–562 (2010).
Fang, Z., Smith, J., Richard, L. & Tian, X. Isolation, purification, and potential applications of xylan. Sustain. Biomass Resour. 9 (1), 3–35 (2019).
Deshmukh, R. A. et al. Purification, biochemical characterization and structural modelling of alkali-stable β-1,4-xylan xylanohydrolase from Aspergillus fumigatus R1 isolated from soil. BMC Biotechnol. 16 , 11 (2016).
Raj, A. S., Kumar, S., Singh, S. K. & Prakash, J. Production and purification of xylanase from alkaliphilic Bacillus licheniformis and its pre-treatment of eucalyptus kraft pulp. Biocatal. Agric. Biotechnol. 15 , 199–209 (2018).
Download references
Acknowledgements
This work was supported by the Technology Innovation Agency (TIA) managed DST/CON/0177/2018: SIIP: ENZYME AND MICROBIAL TECHNOLOGIES (EMT) grant and the Biorefinery Industry Development Facility (BIDF) at the Council for Scientific and Industrial Research (CSIR), Durban, South Africa.
Author information
Authors and affiliations.
Discipline of Microbiology, School of Life Sciences, Westville Campus, University of KwaZulu-Natal, Durban, 4000, South Africa
Priyashini Dhaver & Roshini Govinden
Enzyme Science Programme (ESP), Department of Biochemistry and Microbiology, Rhodes University, Makhanda (Grahamstown), Eastern Cape, South Africa, 6139
Brett Pletschke
Biorefinery Industry Development Facility, Council for Scientific and Industrial Research, Durban, 4000, South Africa
- Bruce Sithole
Discipline of Chemical Engineering, University of KwaZulu-Natal, Durban, 4000, South Africa
You can also search for this author in PubMed Google Scholar
Contributions
This study was carried out by P.D. and supervision was undertaken by R.G., B.S., and B.P. The manuscript was written by P.D. and all authors reviewed, edited and proof checked the manuscript. The study was supported by B.S. and T.I.A. and C.S.I.R.
Corresponding author
Correspondence to Priyashini Dhaver .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary figures., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Dhaver, P., Pletschke, B., Sithole, B. et al. Optimization, purification, and characterization of xylanase production by a newly isolated Trichoderma harzianum strain by a two-step statistical experimental design strategy. Sci Rep 12 , 17791 (2022). https://doi.org/10.1038/s41598-022-22723-x
Download citation
Received : 06 September 2022
Accepted : 18 October 2022
Published : 22 October 2022
DOI : https://doi.org/10.1038/s41598-022-22723-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Hyper β-glucosidase producer beauveria bassiana san01—optimization of fermentation conditions and evaluation of saccharification potential.
- Buka Magwaza
- Ayodeji Amobonye
- Santhosh Pillai
Biomass Conversion and Biorefinery (2024)
In silico studies of fungal xylanase enzymes: structural and functional insight towards efficient biodegradation of lignocellulosic biomass
- Barsha Samantaray
- Rashmi Ranjan Behera
- Hrudayanath Thatoi
Systems Microbiology and Biomanufacturing (2024)
Statistically assisted optimisation for the simultaneous production of Trichoderma harzianum and Aspergillus fumigatus cellulolytic enzymes
- Hwee Li Teo
- Roswanira Abdul Wahab
- Aida Rasyidah Azman
A glucose tolerant β-glucosidase from a newly isolated Neofusicoccum parvum strain F7: production, purification, and characterization
- Nivisti Singh
- Roshini Govinden
Scientific Reports (2023)
Purification and characterization of the low molecular weight xylanase from Bacillus cereus L-1
Brazilian Journal of Microbiology (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Microbial xylanases and their industrial applications: a review
Affiliation.
- 1 Department of Microbiology, University of Delhi, New Delhi, India. [email protected]
- PMID: 11548999
- DOI: 10.1007/s002530100704
Despite an increased knowledge of microbial xylanolytic systems in the past few years, further studies are required to achieve a complete understanding of the mechanism of xylan degradation by microorganisms and their enzymes. The enzyme system used by microbes for the metabolism of xylan is the most important tool for investigating the use of the second most abundant polysaccharide (xylan) in nature. Recent studies on microbial xylanolytic systems have generally focussed on induction of enzyme production under different conditions, purification, characterization, molecular cloning and expression, and use of enzyme predominantly for pulp bleaching. Rationale approaches to achieve these goals require a detailed knowledge of the regulatory mechanism governing enzyme production. This review will focus on complex xylan structure and the microbial enzyme complex involved in its complete breakdown, studies on xylanase regulation and production and their potential industrial applications, with special reference to biobleaching.
PubMed Disclaimer
Similar articles
- Xylanolytic enzymes from fungi and bacteria. Sunna A, Antranikian G. Sunna A, et al. Crit Rev Biotechnol. 1997;17(1):39-67. doi: 10.3109/07388559709146606. Crit Rev Biotechnol. 1997. PMID: 9118232 Review.
- Xylanases from fungi: properties and industrial applications. Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Polizeli ML, et al. Appl Microbiol Biotechnol. 2005 Jun;67(5):577-91. doi: 10.1007/s00253-005-1904-7. Epub 2005 Jan 27. Appl Microbiol Biotechnol. 2005. PMID: 15944805 Review.
- Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Subramaniyan S, Prema P. Subramaniyan S, et al. Crit Rev Biotechnol. 2002;22(1):33-64. doi: 10.1080/07388550290789450. Crit Rev Biotechnol. 2002. PMID: 11958335 Review.
- Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. Walia A, Guleria S, Mehta P, Chauhan A, Parkash J. Walia A, et al. 3 Biotech. 2017 May;7(1):11. doi: 10.1007/s13205-016-0584-6. Epub 2017 Apr 8. 3 Biotech. 2017. PMID: 28391477 Free PMC article.
- Screening and production study of microbial xylanase producers from Brazilian Cerrado. Alves-Prado HF, Pavezzi FC, Leite RS, de Oliveira VM, Sette LD, Dasilva R. Alves-Prado HF, et al. Appl Biochem Biotechnol. 2010 May;161(1-8):333-46. doi: 10.1007/s12010-009-8823-5. Epub 2009 Nov 8. Appl Biochem Biotechnol. 2010. PMID: 19898784
- Valorization of Wheat Bran by Co-Cultivation of Fungi with Integrated Hydrolysis to Provide Sugars and Animal Feed. Mittermeier F, Fischer F, Hauke S, Hirschmann P, Weuster-Botz D. Mittermeier F, et al. BioTech (Basel). 2024 May 18;13(2):15. doi: 10.3390/biotech13020015. BioTech (Basel). 2024. PMID: 38804297 Free PMC article.
- Cloning and characterization of a novel mesophilic xylanase gene Fgxyn3 from Fusarium graminearum Z-1. Zhang D, Zhu Z, Su X, Gao T, Li N, Huang W, Wu M. Zhang D, et al. 3 Biotech. 2024 Jun;14(6):162. doi: 10.1007/s13205-024-03973-0. Epub 2024 May 25. 3 Biotech. 2024. PMID: 38803445
- Activity-stability trade-off observed in variants at position 315 of the GH10 xylanase XynR. Nakamura T, Takita T, Kuwata K, Mizutani K, Mikami B, Nakamura S, Yasukawa K. Nakamura T, et al. Sci Rep. 2024 Apr 2;14(1):7767. doi: 10.1038/s41598-024-57819-z. Sci Rep. 2024. PMID: 38565938 Free PMC article.
- High-Molecular-Weight Xylanase from B. pumilus US570 Strain: Purification, Characterization and Application in Banana and Orange Peels Hydrolysis and Breadmaking. Elgharbi F, Salem K, Elbedoui M, Hmida-Sayari A. Elgharbi F, et al. Appl Biochem Biotechnol. 2024 Feb 23. doi: 10.1007/s12010-024-04866-x. Online ahead of print. Appl Biochem Biotechnol. 2024. PMID: 38393579
- Influence of Salinity on the Extracellular Enzymatic Activities of Marine Pelagic Fungi. Salazar-Alekseyeva K, Herndl GJ, Baltar F. Salazar-Alekseyeva K, et al. J Fungi (Basel). 2024 Feb 13;10(2):152. doi: 10.3390/jof10020152. J Fungi (Basel). 2024. PMID: 38392824 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources, other literature sources.
- The Lens - Patent Citations
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- DOI: 10.33451/florafauna.v28i2pp231-240
- Corpus ID: 254671708
Microbial Xylanases: A Review
- Jyoti Richhariya , T. Sharma , S. Dassani
- Published in FLORA AND FAUNA 2022
- Biology, Environmental Science, Chemistry
78 References
Production and optimization of xylanase enzyme from bacillus subtilis using agricultural wastes by solid state fermentation, xylanases from fungi: properties and industrial applications, production of fungal xylanases, engineering thermostable microbial xylanases toward its industrial applications, microbial xylanases and their industrial application in pulp and paper biobleaching: a review, biotechnology of microbial xylanases: enzymology, molecular biology, and application, production and industrial applications of xylanase: a review, xylanases and their applications in baking industry, production and characterization of xylanase from aspergillus niger using wheat bran, corn cobs and sugar cane bagasse as carbon sources with different concentrations, a review of xylanase production by the fermentation of xylan: classification, characterization and applications, related papers.
Showing 1 through 3 of 0 Related Papers
Xylanases: An Overview of its Diverse Function in the Field of Biorefinery
- First Online: 11 August 2021
Cite this chapter

- Nisha Bhardwaj 6 na1 ,
- Komal Agrawal 6 na1 &
- Pradeep Verma 6 na1
Part of the book series: Clean Energy Production Technologies ((CEPT))
494 Accesses
4 Citations
Hemicellulases are enzymatic complex that has its application in biorefinery and can effectively catalyse the hydrolysis process of xylan (a principal hemicellulose), comprising of a linear polymeric chain made up of β-D-xylopyranosyl units linked with β-1,4-glycosidic linkages. Hemicellulases are produced by various microorganisms such as bacteria, fungi, algae, insects, etc. However, xylanase from filamentous fungi such as Aspergillus sp. and Trichoderma sp. is more preferred over other sources. Endo-1,4-β-xylanase and β-xylosidase are found to be most important hemicellulases and have tremendous application in biorefinery as well as other industries. The growing interest of industries such as biorefinery, animal feed, pharmaceuticals, paper and pulp industries, etc., has gained interest of the researchers and focused the research to find new sources of xylanases that utilise economically feasible production methods and be sustainable as well. Thus, the present chapter will describe the application of xylanases in biorefinery as well as different industries along with the general production strategy applied by the researchers in current time.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others
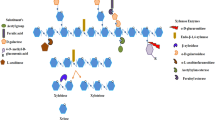
A detailed overview of xylanases: an emerging biomolecule for current and future prospective

Xylanases: For Sustainable Bioproduct Production

Recent Advances and Industrial Applications of Microbial Xylanases: A Review
Adigüzel AO, Tunçer M (2016) Production, characterization and application of a xylanase from Streptomyces sp. AOA40 in fruit juice and bakery industries. Food Biotechnol 30(3):189–218
Article CAS Google Scholar
Adiguzel G, Faiz O, Sisecioglu M, Sari B, Baltaci O, Akbulut S, Genc B, Adiguzel A (2019) A novel endo-β-1, 4-xylanase from Pediococcus acidilactici GC25; purification, characterization and application in clarification of fruit juices. Int J Biol Macromol 129:571–578
Article CAS PubMed Google Scholar
Adsul MG, Bastawde KB, Gokhale DV (2009) Biochemical characterization of two xylanases from yeast Pseudozyma hubeiensis producing only xylooligosaccharides. Bioresour Technol 100(24):6488–6495
Agrawal K, Alam A, Verma P (2021) Bioprospecting and applications of fungi: a game changer in present scenario. Recent trends in mycological research: Volume 2: Environmental and Industrial Perspective. Springer, Cham, p 1
Google Scholar
Agrawal K, Bhardwaj N, Kumar B, Chaturvedi V, Verma P (2019) Process optimization, purification and characterization of alkaline stable white laccase from Myrothecium verrucaria ITCC-8447 and its application in delignification of agroresidues. Int J Biol Macromol 125:1042–1055
Agrawal K, Verma P (2020a) Production optimization of yellow laccase from Stropharia sp. ITCC 8422 and enzyme-mediated depolymerization and hydrolysis of lignocellulosic biomass for biorefinery application. In: Biomass conversion and biorefinery. Springer, pp 1–20
Agrawal K, Verma P (2020b) Laccase-mediated synthesis of bio-material using agro-residues. In: Biotechnological applications in human health. Springer, Singapore, pp 87–93
Agrawal K, Verma P (2021a) Applications of biomolecules of endophytic fungal origin and its future prospect. In fungi bio-prospects in sustainable agriculture, environment and nano-technology, 207-230). Academic Press.
Agrawal K, Verma P (2021b) Fungal metabolites: a recent trend and its potential biotechnological applications. In: New and future developments in microbial biotechnology and bioengineering, Elsevier
Agrawal K, Verma P (2021c) “Omics”—A step toward understanding of complex diversity of the microbial community. In: Wasewater treatment. Elsevier, pp 471–487
Agrawal K, Chaturvedi V, Verma P (2018) Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess 5(1)
Ahmad Z, Butt MS, Ahmed A, Riaz M, Sabir SM, Farooq U, Rehman FU (2014) Effect of Aspergillus Niger xylanase on dough characteristics and bread quality attributes. J Food Sci Technol 51(10):2445–2453
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanases: an overview. Appl Microbiol Biotechnol 84(1):19–35
Akpinar O, Ak O, Kavas A, Bakir U, Yilmaz L (2007) Enzymatic production of xylooligosaccharides from cotton stalks. J Agric Food Chem 55(14):5544–5551
Altınel B, Ünal SS (2017) The effects of amyloglucosidase, glucose oxidase and hemicellulase utilization on the rheological behaviour of dough and quality characteristics of bread. Int J Food Eng 13(2):66
Aragon CC, Santos AF, Ruiz-Matute AI, Corzo N, Guisan JM, Monti R, Mateo C (2013) Continuous production of xylooligosaccharides in a packed bed reactor with immobilized–stabilized biocatalysts of xylanase from Aspergillus versicolor . J Mol Catal B Enzym 98:8–14
Bai Y, Wang J, Zhang Z, Shi P, Luo H, Huang H, Luo C, Yao B (2010) A novel family 9 β-1, 3 (4)-glucanase from thermoacidophilic Alicyclobacillus sp. A4 with potential applications in the brewing industry. Appl Microbiol Biotechnol 87(1):251–259
Bajaj BK, Manhas K (2012) Production and characterization of xylanase from Bacillus licheniformis P11 (C) with potential for fruit juice and bakery industry. Biocatal Agric Biotechnol 1(4):330–337
Bamforth C (2009) Current perspectives on the role of enzymes in brewing. J Cereal Sci 50(3):353–357
Battan B, Sharma J, Dhiman SS, Kuhad RC (2007) Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzym Microb Technol 41(6-7):733–739
Bhardwaj N, Agrawal K, Kumar B, Verma P (2021) Role of enzymes in deconstruction of waste biomass for sustainable generation of value-added products. In Bioprospecting of enzymes in industry, healthcare and sustainable environment, 219-250). Springer, Singapore.
Bhardwaj N, Chanda K, Kumar B, Prasad HK, Sharma GD, Verma P (2017) Statistical optimization of nutritional and physical parameters for xylanase production from newly isolated Aspergillus oryzae LC1 and its application in the hydrolysis of lignocellulosic agro-residues. Bioresources 12(4):8519–8538
CAS Google Scholar
Bhardwaj N, Kumar B, Agarwal K, Chaturvedi V, Verma P (2019a) Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in Xylo-oligosaccharides production from lignocellulosic agricultural wastes. Int J Biol Macromol 122:1191–1202
Bhardwaj N, Kumar B, Agrawal K, Verma P (2019b) Bioconversion of rice straw by synergistic effect of in-house produced ligno-hemicellulolytic enzymes for enhanced bioethanol production. Bioresour Technol Rep 10:100352
Article Google Scholar
Bhardwaj B, Kumar B, Verma P (2019c) A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess 6(1)
Bhardwaj N, Verma P (2020) Extraction of fungal xylanase using ATPS-PEG/sulphate and its application in hydrolysis of agricultural residues. In: biotechnological applications in human health. Springer, Singapore, pp 95–105
Bhardwaj N, Verma P (2021) Microbial xylanases: A helping module for the enzyme biorefinery platform. Bioenergy Research: Evaluating strategies for commercialization and sustainability, 129-152, Wiley.
Bhardwaj N, Verma VK, Chaturvedi V, Verma P (2018) GH10 XynF1 and Xyn11A: the predominant xylanase identified in the profiling of extracellular proteome of Aspergillus oryzae LC1. Ann Microbiol 68(11):731–742
Boonchuay P, Takenaka S, Kuntiya A, Techapun C, Leksawasdi N, Seesuriyachan P, Chaiyaso T (2016) Purification, characterization, and molecular cloning of the xylanase from Streptomyces thermovulgaris TISTR1948 and its application to xylooligosaccharide production. J Mol Catal B Enzym 129:61–68
Boonyapakron K, Jaruwat A, Liwnaree B, Nimchua T, Champreda V, Chitnumsub P (2017) Structure-based protein engineering for thermostable and alkaliphilic enhancement of endo-β-1, 4-xylanase for applications in pulp bleaching. J Biotechnol 259:95–102
Boruah P, Dowarah P, Hazarika R, Yadav A, Barkakati P, Goswami T (2016) Xylanase from Penicillium meleagrinum var. viridiflavum –a potential source for bamboo pulp bleaching. J Clean Prod 116:259–267
Buchert J, Pere J, Puolakka A, Nousiainen P (1998) Enzymatic scouring of cotton. Book of papers, AATCC international conference and exhibition. pp 493–499
Buchert J, Ranua M, Kantelinen A, Viikari L (1992) The role of two Trichoderma reesei xylanases in the bleaching of pine Kraft pulp. Appl Microbiol Biotechnol 37(6):825–829
Cakmak U, Ertunga NS (2016) Gene cloning, expression, immobilization and characterization of endo-xylanase from Geobacillus sp. TF16 and investigation of its industrial applications. J Mol Catal B Enzym 133:S288–S298
Campioni TS, de Jesus Moreira L, Moretto E, Nunes NSS, de Oliva Neto P (2019) Biobleaching of Kraft pulp using fungal xylanases produced from sugarcane straw and the subsequent decrease of chlorine consumption. Biomass Bioenergy 121:22–27
Carvalho EA, dos Santos Góes LM, Uetanabaro APT, da Silva EGP, Rodrigues LB, Pirovani CP, da Costa AM (2017) Thermoresistant xylanases from Trichoderma stromaticum : application in bread making and manufacturing xylo-oligosaccharides. Food Chem 221:1499–1506
Chang S, Guo Y, Wu B, He B (2017) Extracellular expression of alkali tolerant xylanase from Bacillus subtilis Lucky9 in E. coli and application for xylooligosaccharides production from agro-industrial waste. Int J Biol Macromol 96:249–256
Chapla D, Pandit P, Shah A (2012) Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour Technol 115:215–221
Chaturvedi V, Verma P (2013) An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 3:415–431
Chng LM, Lee KT, Chan DJC (2017) Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers Manag 141:410–419
Courtin C, Gelders G, Delcour J (2001) Use of two endoxylanases with different substrate selectivity for understanding arabinoxylan functionality in wheat flour breadmaking. Cereal Chem 78(5):564–571
Csiszár El, Losonczi A, Szakács G, Rusznák I, Bezúr L, Reicher J (2001) Enzymes and chelating agent in cotton pretreatment. J Biotechnol 89(2-3):271–279
Article PubMed Google Scholar
Cunha FM, Badino AC, Farinas CS (2017) Effect of a novel method for in-house cellulase production on 2G ethanol yields. Biocatal Agric Biotechnol 9:224–229
da Costa AC, Cavalheiro GF, de Queiroz Vieira ER, Gandra JR, e Buschinelli, R.H.d.T., da Paz, M.F., Fonseca, G.G., Leite, R.S.R. (2019) Catalytic properties of xylanases produced by Trichoderma piluliferum and Trichoderma viride and their application as additives in bovine feeding. Biocatal Agric Biotechnol 19:101161
da Silva PO, de Alencar Guimarães NC, Serpa JDM, Masui DC, Marchetti CR, Verbisck NV, Zanoelo FF, Ruller R, Giannesi GC (2019) Application of an endo-xylanase from Aspergillus japonicus in the fruit juice clarification and fruit peel waste hydrolysis. Biocatal Agric Biotechnol 21:101312
Damen B, Pollet A, Dornez E, Broekaert WF, Van Haesendonck I, Trogh I, Arnaut F, Delcour JA, Courtin CM (2012) Xylanase-mediated in situ production of arabinoxylan oligosaccharides with prebiotic potential in whole meal breads and breads enriched with arabinoxylan rich materials. Food Chem 131(1):111–118
de Castro AM, de Andréa TV, Carvalho DF, Teixeira MMP, dos Reis Castilho L, Freire DMG (2011) Valorization of residual agroindustrial cakes by fungal production of multienzyme complexes and their use in cold hydrolysis of raw starch. Waste Biomass Valorization 2(3):291–302
Dhiman SS, Garg G, Sharma J, Mahajan R (2011) Characterization of statistically produced xylanase for enrichment of fruit juice clarification process. New Biotechnol 28(6):746–755
Ding C, Li M, Hu Y (2018) High-activity production of xylanase by Pichia stipitis : purification, characterization, kinetic evaluation and xylooligosaccharides production. Int J Biol Macromol 117:72–77
Dogaris I, Vakontios G, Kalogeris E, Mamma D, Kekos D (2009) Induction of cellulases and hemicellulases from Neurospora crassa under solid-state cultivation for bioconversion of sorghum bagasse into ethanol. Ind Crop Prod 29(2-3):404–411
Driss D, Zouari-Ellouzi S, Chaari F, Kallel F, Ghazala I, Bouaziz F, Chaabouni SE (2014) Production and in vitro evaluation of xylooligosaccharides generated from corncobs using immobilized Penicillium occitanis xylanase . J Mol Catal B Enzym 102:146–153
Du Y, Shi P, Huang H, Zhang X, Luo H, Wang Y, Yao B (2013) Characterization of three novel thermophilic xylanases from Humicola insolens Y1 with application potentials in the brewing industry. Bioresour Technol 130:161–167
Dutta PD, Neog B, Goswami T (2019) Xylanase enzyme production from bacillus australimaris P5 for prebleaching of bamboo ( Bambusa tulda ) pulp. Mater Chem Phys 243:122227
El Aty AAA, Saleh SA, Eid BM, Ibrahim NA, Mostafa FA (2018) Thermodynamics characterization and potential textile applications of Trichoderma longibrachiatum KT693225 xylanase. Biocatal Agric Biotechnol 14:129–137
Filipčev B, Šimurina O, Bodroža-Solarov M (2014) Combined effect of xylanase, ascorbic and citric acid in regulating the quality of bread made from organically grown spelt cultivars. J Food Qual 37(3):185–195
Garg G, Dhiman SS, Mahajan R, Kaur A, Sharma J (2011) Bleach-boosting effect of crude xylanase from Bacillus stearothermophilus SDX on wheat straw pulp. New Biotechnol 28(1):58–64
Ghoshal G, Shivhare U, Banerjee U (2017) Rheological properties and microstructure of xylanase containing whole wheat bread dough. J Food Sci Technol 54(7):1928–1937
Article CAS PubMed PubMed Central Google Scholar
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Gomes HAR, da Silva AJ, Gómez-Mendoza DP, dos Santos Júnior ACM, di Cologna, N.d.M., Almeida, R.M., Miller, R.N.G., Fontes, W., de Sousa, M.V., Ricart, C.A.O. (2017) Identification of multienzymatic complexes in the Clonostachys byssicola secretomes produced in response to different lignocellulosic carbon sources. J Biotechnol 254:51–58
Gonçalves FA, Ruiz HA, da Costa Nogueira C, dos Santos ES, Teixeira JA, de Macedo GR (2014) Comparison of delignified coconuts waste and cactus for fuel-ethanol production by the simultaneous and semi-simultaneous saccharification and fermentation strategies. Fuel 131:66–76
Gowdhaman D, Ponnusami V (2015) Production and optimization of xylooligosaccharides from corncob by Bacillus aerophilus KGJ2 xylanase and its antioxidant potential. Int J Biol Macromol 79:595–600
Guan G-Q, Zhao P-X, Zhao J, Wang M-J, Huo S-H, Cui F-J, Jiang J-X (2016) Production and partial characterization of an alkaline xylanase from a novel fungus Cladosporium oxysporum . BioMed Res Int 2016:4575024
PubMed PubMed Central Google Scholar
Guo Y, Gao Z, Xu J, Chang S, Wu B, He B (2018) A family 30 glucurono-xylanase from Bacillus subtilis LC9: expression, characterization and its application in Chinese bread making. Int J Biol Macromol 117:377–384
Hardt N, Boom R, Van Der Goot A (2014) Wheat dough rheology at low water contents and the influence of xylanases. Food Res Int 66:478–484
Hartzell MM, Hsieh Y-L (1998) Enzymatic scouring to improve cotton fabric wettability. Text Res J 68(4):233–241
Haven MØ, Jørgensen H (2013) Adsorption of β-glucosidases in two commercial preparations onto pretreated biomass and lignin. Biotechnol Biofuels 6(1):165
He X, Yu B, He J, Huang Z, Mao X, Zheng P, Luo Y, Luo J, Wang Q, Wang H (2020) Effects of xylanase on growth performance, nutrients digestibility and intestinal health in weaned piglets. Livest Sci 233:103940
Hemalatha M, Leelavathi K, Salimath P, Rao UP (2014) Control of chapati staling upon treatment of dough with amylases and xylanase. Food Biosci 5:73–84
Hernandez A, Kholif A, Lugo-Coyote R, Elghandour M, Cipriano M, Rodríguez G, Odongo N, Salem A (2017) The effect of garlic oil, xylanase enzyme and yeast on biomethane and carbon dioxide production from 60-d old Holstein dairy calves fed a high concentrate diet. J Clean Prod 142:2384–2392
Jia C, Huang W, Abdel-Samie MA-S, Huang G, Huang G (2011) Dough rheological, Mixolab mixing, and nutritional characteristics of almond cookies with and without xylanase. J Food Eng 105(2):227–232
Jiang Z, Li X, Yang S, Li L, Tan S (2005) Improvement of the breadmaking quality of wheat flour by the hyperthermophilic xylanase B from Thermotoga maritima . Food Res Int 38(1):37–43
Juodeikiene G, Basinskiene L, Vidmantiene D, Makaravicius T, Bartkiene E (2012) Benefits of β-xylanase for wheat biomass conversion to bioethanol. J Sci Food Agric 92(1):84–91
Kamei I, Hirota Y, Meguro S (2012) Integrated delignification and simultaneous saccharification and fermentation of hard wood by a white-rot fungus, Phlebia sp. MG-60. Bioresour Technol 126:137–141
Karmakar SR (1999) Chemical technology in the pre-treatment processes of textiles. Elsevier, Amsterdam
Khambhaty Y, Akshaya R, Suganya CR, Sreeram KJ, Rao JR (2018) A logical and sustainable approach towards bamboo pulp bleaching using xylanase from Aspergillus nidulans. Int J Biol Macromol 118:452–459
Kiran EU, Liu Y (2015) Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 159:463–469
Knob A, Fortkamp D, Prolo T, Izidoro SC, Almeida JM (2014) Agro-residues as alternative for xylanase production by filamentous fungi. Bioresources 9(3):5738–5773
Knob A, Terrasan CF, Carmona E (2010) β-Xylosidases from filamentous fungi: an overview. World J Microbiol Biotechnol 26(3):389–407
Ko C-H, Lin Z-P, Tu J, Tsai C-H, Liu C-C, Chen H-T, Wang T-P (2010) Xylanase production by Paenibacillus campinasensis BL11 and its pretreatment of hardwood Kraft pulp bleaching. Int Biodeterior Biodegradation 64(1):13–19
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23(4):411–456
Kumar B, Bhardwaj N, Alam A, Agrawal K, Prasad H, Verma P (2018) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Exp 8(1):173
Kumar B, Bhardwaj N, Verma P (2019) Pretreatment of rice straw using microwave assisted FeCl 3 -H 3 PO 4 system for ethanol and oligosaccharides generation. Bioresour Technol Rep 7:100295
Kumar B, Verma P (2020) Application of hydrolytic enzymes in biorefinery and its future prospects. In: Microbial strategies for techno-economic biofuel production. Springer, Singapore, pp 59–83
Chapter Google Scholar
Kumar B, Verma P (2021) Biomass-based biorefineries: an important architype towards a circular economy. Fuel 288:119622
Kumar V, Satyanarayana T (2011) Applicability of thermo-alkali-stable and cellulase-free xylanase from a novel thermo-halo-alkaliphilic Bacillus halodurans in producing xylooligosaccharides. Biotechnol Lett 33(11):2279
Lee KY, Balasubramanian B, Kim JK, Kim IH (2018) Dietary inclusion of xylanase improves growth performance, apparent total tract nutrient digestibility, apparent ileal digestibility of nutrients and amino acids and alters gut microbiota in growing pigs. Anim Feed Sci Technol 235:105–109
Lee S, Apajalahti J, Vienola K, González-Ortiz G, Fontes C, Bedford M (2017) Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim Feed Sci Technol 234:29–42
Li C, Kumar A, Luo X, Shi H, Liu Z, Wu G (2020) Highly alkali-stable and cellulase-free xylanases from Fusarium sp. 21 and their application in clarification of orange juice. Int J Biol Macromol 155:572–580
Li L, Tian H, Cheng Y, Jiang Z, Yang S (2006) Purification and characterization of a thermostable cellulase-free xylanase from the newly isolated Paecilomyces themophila. Enzym Microb Technol 38(6):780–787
Li X, Li E, Zhu Y, Teng C, Sun B, Song H, Yang R (2012) A typical endo-xylanase from Streptomyces rameus L2001 and its unique characteristics in xylooligosaccharide production. Carbohydr Res 359:30–36
Li Y, Hardin IR (1998) Enzymatic scouring of cotton-surfactants, agitation, and selection of enzymes. Text Chem Color 30(9):23–29
Lin XQ, Han SY, Zhang N, Hu H, Zheng SP, Ye YR, Lin Y (2013) Bleach boosting effect of xylanase A from Bacillus halodurans C-125 in ECF bleaching of wheat straw pulp. Enzym Microb Technol 52(2):91–98
Lipp-Symonowicz B, Tańska B, Wołukanis A, Wrzosek H (2004) Influence of enzymatic treatment on the flax fibre morphological structure, physico-chemical properties and metrological parameters of yarn. Fibres Text East Eur 1(45):61–65
Liu D, Li J, Zhao S, Zhang R, Wang M, Miao Y, Shen Y, Shen Q (2013a) Secretome diversity and quantitative analysis of cellulolytic Aspergillus fumigatus Z5 in the presence of different carbon sources. Biotechnol Biofuels 6(1):149
Liu MQ, Huo WK, Xu X, Weng XY (2017a) Recombinant bacillus amyloliquefaciens xylanase A expressed in Pichia pastoris and generation of xylooligosaccharides from xylans and wheat bran. Int J Biol Macromol 105:656–663
Liu W, Brennan MA, Serventi L, Brennan CS (2017b) Effect of cellulase, xylanase and α-amylase combinations on the rheological properties of Chinese steamed bread dough enriched in wheat bran. Food Chem 234:93–102
Liu X, Liu Y, Jiang Z, Liu H, Yang S, Yan Q (2018) Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chem 264:310–318
Liu Z, Zhao X, Bai F (2013b) Production of xylanase by an alkaline-tolerant marine-derived Streptomyces viridochromogenes strain and improvement by ribosome engineering. Appl Microbiol Biotechnol 97(10):4361–4368
Losonczi A, Csiszár E, Szakács G, Bezur L (2005) Role of the EDTA chelating agent in bioscouring of cotton. Text Res J 75(5):411–417
Lu PY, Wang J, Wu SG, Gao J, Dong Y, Zhang HJ, Qi GH (2020) Standardized ileal digestible amino acid and metabolizable energy content of wheat from different origins and the effect of exogenous xylanase on their determination in broilers. Poult Sci 99(2):992–1000
Maalej-Achouri I, Guerfali M, Romdhane IB-B, Gargouri A, Belghith H (2012) The effect of Talaromyces thermophilus cellulase-free xylanase and commercial laccase on lignocellulosic components during the bleaching of Kraft pulp. Int Biodeterior Biodegradation 75:43–48
Martins MD, Guimarães MW, de Lima VA, Gaglioti AL, Da-Silva PR, Kadowaki MK, Knob A (2018) Valorization of passion fruit peel by-product: xylanase production and its potential as bleaching agent for Kraft pulp. Biocatal Agric Biotechnol 16:172–180
Mathlouthi N, Saulnier L, Quemener B, Larbier M (2002) Xylanase, β-glucanase, and other side enzymatic activities have greater effects on the viscosity of several feedstuffs than xylanase and β-glucanase used alone or in combination. J Agric Food Chem 50(18):5121–5127
Milagres AM, Prade RA (1994) Production of xylanases from Penicillium janthinellum and its use in the recovery of cellulosic textile fibers. Enzym Microb Technol 16(7):627–632
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Moss AF, Chrystal PV, Dersjant-Li Y, Selle PH, Liu SY (2018) Responses in digestibilities of macro-minerals, trace minerals and amino acids generated by exogenous phytase and xylanase in canola meal diets offered to broiler chickens. Anim Feed Sci Technol 240:22–30
Moure A, Gullón P, Domínguez H, Parajó JC (2006) Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem 41(9):1913–1923
Mussatto SI, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohydr Polym 68(3):587–597
Nagar S, Mittal A, Gupta VK (2012) Enzymatic clarification of fruit juices (apple, pineapple, and tomato) using purified Bacillus pumilus SV-85S xylanase. Biotechnol Bioprocess Eng 17(6):1165–1175
Nie S, Wang S, Qin C, Yao S, Ebonka JF, Song X, Li K (2015) Removal of hexenuronic acid by xylanase to reduce adsorbable organic halides formation in chlorine dioxide bleaching of bagasse pulp. Bioresour Technol 196:413–417
Nieto-Domínguez M, de Eugenio LI, York-Durán MJ, Rodríguez-Colinas B, Plou FJ, Chenoll E, Pardo E, Codoñer F, Martínez MJ (2017) Prebiotic effect of xylooligosaccharides produced from birchwood xylan by a novel fungal GH11 xylanase. Food Chem 232:105–113
Article PubMed CAS Google Scholar
Nikhil B, Adhyaru D, Thakor P (2012) Production of xylanase by Aspergillus flavus FPDN1 on pearl millet bran: optimization of culture conditions and application in bioethanol production. Int J Res Chem Environ 2(3):204–210
Ninawe S, Kuhad RC (2006) Bleaching of wheat straw-rich soda pulp with xylanase from a thermoalkalophilic Streptomyces cyaneus SN32. Bioresour Technol 97(18):2291–2295
Ognean M, Ognean CF, Bucur A (2011) Rheological effects of some xylanase on doughs from high and low extraction flours. Procedia Food Sci 1:308–314
Ohbuchi T, Sakaino M, Takahashi T, Azumi N, Ishikawa K, Kawazoe S, Kobayashi Y, Kido Y (2010) Oral administration of acidic xylooligosaccharides prevents the development of atopic dermatitis-like skin lesions in NC/Nga mice. J Nutr Sci Vitaminol 56(1):54–59
Olfa E, Mondher M, Issam S, Ferid L, Nejib MM (2007) Induction, properties and application of xylanase activity from Sclerotinia sclerotiorum S2 fungus. J Food Biochem 31(1):96–107
Paice M, Gurnagul N, Page D, Jurasek L (1992) Mechanism of hemicellulose-directed prebleaching of Kraft pulps. Enzym Microb Technol 14(4):272–276
Paloheimo M, Piironen J, Vehmaanpera J (2010) Xylanases and cellulases as feed additives. Enzymes Farm Anim Nutr 12–53
Pandey AK, Edgard G, Negi S (2016) Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew Energy 98:51–56
Pereira GF, de Bastiani D, Gabardo S, Squina F, Ayub MAZ (2018) Solid-state cultivation of recombinant Aspergillus nidulans to co-produce xylanase, arabinofuranosidase, and xylooligosaccharides from soybean fibre. Biocatal Agric Biotechnol 15:78–85
Prasoulas G, Gentikis A, Konti A, Kalantzi S, Kekos D, Mamma D (2020) Bioethanol production from food waste applying the multienzyme system produced on-site by Fusarium oxysporum F3 and mixed microbial cultures. Fermentation 6(2):39
Qiu Z, Shi P, Luo H, Bai Y, Yuan T, Yang P, Liu S, Yao B (2010) A xylanase with broad pH and temperature adaptability from Streptomyces megasporus DSM 41476, and its potential application in brewing industry. Enzym Microb Technol 46(6):506–512
Rahmani N, Kahar P, Lisdiyanti P, Lee J, Prasetya B, Ogino C, Kondo A (2019) GH-10 and GH-11 Endo-1, 4-β-xylanase enzymes from Kitasatospora sp. produce xylose and xylooligosaccharides from sugarcane bagasse with no xylose inhibition. Bioresour Technol 272:315–325
Rajagopalan G, Shanmugavelu K, Yang K-L (2017) Production of prebiotic-xylooligosaccharides from alkali pretreated mahogany and mango wood sawdust by using purified xylanase of Clostridium strain BOH3. Carbohydr Polym 167:158–166
Rattanachomsri U, Tanapongpipat S, Eurwilaichitr L, Champreda V (2009) Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis . J Biosci Bioeng 107(5):488–493
Reddy SS, Krishnan C (2016) Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT-Food Sci Technol 65:237–245
Rodrigues IDSV, Barreto JT, Moutinho BL, MMG O, da Silva RS, Fernandes MF, RPM F (2020) Production of xylanases by Bacillus sp. TC-DT13 in solid state fermentation using bran wheat. Prep Biochem Biotechnol 50(1):91–97
Rosell CM, Rojas JA, De Barber CB (2001) Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll 15(1):75–81
Rosmine E, Sainjan NC, Silvester R, Alikkunju A, Varghese SA (2017) Statistical optimisation of xylanase production by estuarine Streptomyces sp. and its application in clarification of fruit juice. J Gen Eng Biotechnol 15(2):393–401
Rouette H-K, Schwager B (2001) Encyclopedia of textile finishing. Springer, Berlin
Book Google Scholar
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40(12):3693–3700
Sandrim V, Rizzatti A, Terenzi H, Jorge J, Milagres A, Polizeli M (2005) Purification and biochemical characterization of two xylanases produced by Aspergillus caespitosus and their potential for Kraft pulp bleaching. Process Biochem 40(5):1823–1828
Saputra F, Shiu Y-L, Chen Y-C, Puspitasari AW, Danata RH, Liu C-H, Hu S-Y (2016) Dietary supplementation with xylanase-expressing B. amyloliquefaciens R8 improves growth performance and enhances immunity against Aeromonas hydrophila in Nile tilapia ( Oreochromis niloticus ). Fish Shellfish Immunol 58:397–405
Schoenlechner R, Szatmari M, Bagdi A, Tömösközi S (2013) Optimisation of bread quality produced from wheat and proso millet ( Panicum miliaceum L. ) by adding emulsifiers, transglutaminase and xylanase. LWT-Food Sci Technol 51(1):361–366
Shah AR, Shah R, Madamwar D (2006) Improvement of the quality of whole wheat bread by supplementation of xylanase from Aspergillus foetidus . Bioresour Technol 97(16):2047–2053
Sharma A, Thakur VV, Shrivastava A, Jain RK, Mathur RM, Gupta R, Kuhad RC (2014) Xylanase and laccase based enzymatic Kraft pulp bleaching reduces adsorbable organic halogen (AOX) in bleach effluents: A pilot scale study. Bioresour Technol 169:96–102
Sharma HP, Patel H, Sugandha (2017) Enzymatic added extraction and clarification of fruit juices–A review. Crit Rev Food Sci Nutr 57(6):1215–1227
Shin SK, Hyeon JE, Kim YI, Kang DH, Kim SW, Park C, Han SO (2015) Enhanced hydrolysis of lignocellulosic biomass: bi-functional enzyme complexes expressed in Pichia pastoris improve bioethanol production from Miscanthus sinensis . Biotechnol J 10(12):1912–1919
Singh A, Patel AK, Adsul M, Mathur A, Singhania RR (2017) Genetic modification: a tool for enhancing cellulase secretion. Biofuel Res J 4(2):600–610
Singh A, Sharma D, Varghese LM, Mahajan R (2020) Fast flow rate processes for purification of alkaline xylanase isoforms from Bacillus pumilus AJK and their biochemical characterization for industrial application purposes. Biotechnol Prog 36(1):e2898
Singh S, Tyagi C, Dutt D, Upadhyaya J (2009) Production of high level of cellulase-poor xylanases by wild strains of white-rot fungus Coprinellus disseminatus in solid-state fermentation. New Biotechnol 26(3-4):165–170
Sonia K, Chadha B, Saini H (2005) Sorghum straw for xylanase hyper-production by Thermomyces lanuginosus (D2W3) under solid-state fermentation. Bioresour Technol 96(14):1561–1569
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol 22(1):33–64
Suryawanshi RK, Jana UK, Prajapati BP, Kango N (2019) Immobilization of Aspergillus quadrilineatus RSNK-1 multi-enzymatic system for fruit juice treatment and mannooligosaccharide generation. Food Chem 289:95–102
Tabka MG, Herpoël-Gimbert I, Monod F, Asther M, Sigoillot J-C (2006) Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzym Microb Technol 39(4):897–902
Thomas L, Parameswaran B, Pandey A (2016) Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renew Energy 98:9–15
Twomey L, Pluske J, Rowe JB, Choct M, Brown W, McConnell M, Pethick D (2003) The effects of increasing levels of soluble non-starch polysaccharides and inclusion of feed enzymes in dog diets on faecal quality and digestibility. Anim Feed Sci Technol 108(1-4):71–82
Valeri D, Lopes AM, Pessoa-Júnior A (2011) Evaluation of xylanases from Aspergillus Niger and Trichoderma sp. on dough rheological properties. Afr J Biotechnol 10(45):9132–9136
Van Dorn R, Shanahan D, Ciofalo V (2018) Safety evaluation of xylanase 50316 enzyme preparation (also known as VR007), expressed in Pseudomonas fluorescens , intended for use in animal feed. Regul Toxicol Pharmacol 97:48–56
Verschraege L (1989) Cotton fibre impurities: neps, motes, and seed coat fragments: ICAC review articles on cotton production research, vol 1. CAB International, Wallingford, UK
Verma P, Mai C (2010) Hydrolysis of cellulose and wood powder treated with DMDHEU by a hydrolase enzyme complex, Fenton’s reagent, and in a liquid culture of Trametes versicolor . Holzforschung, Walter de Gruyter 64:69–75
Verma P, Watanabe T, Honda Y, Watanabe T (2011) Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated by H 2 O 2 . Bioresour Technol 102:3941–3945
Viëtor R, Voragen A, Angelino S, Pilnik W (1991) Non-starch polysaccharides in barley and malt: a mass balance of flour fractionation. J Cereal Sci 14(1):73–83
Viikari L, Kantelinen A, Sundquist J, Linko M (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev 13(2-3):335–350
Wang M, Hamer R, Van Vliet T, Gruppen H, Marseille H, Weegels P (2003) Effect of water unextractable solids on gluten formation and properties: mechanistic considerations. J Cereal Sci 37(1):55–64
Whiting I, Rose S, Mackenzie A, Amerah A, Pirgozliev V (2019) Effect of wheat distillers dried grains with solubles and exogenous xylanase on laying hen performance and egg quality. Poult Sci 98(9):3756–3762
Xiao W, Li H, Xia W, Yang Y, Hu P, Zhou S, Hu Y, Liu X, Dai Y, Jiang Z (2019) Co-expression of cellulase and xylanase genes in Sacchromyces cerevisiae toward enhanced bioethanol production from corn Stover. Bioengineered 10(1):513–521
Yegin S, Altinel B, Tuluk K (2018) A novel extremophilic xylanase produced on wheat bran from Aureobasidium pullulans NRRL Y-2311-1: effects on dough rheology and bread quality. Food Hydrocoll 81:389–397
Zhang Z, Tun HM, Li R, Gonzalez BJ, Keenes HC, Nyachoti CM, Kiarie E, Khafipour E (2018) Impact of xylanases on gut microbiota of growing pigs fed corn-or wheat-based diets. Anim Nutr 4(4):339–350
Article PubMed PubMed Central Google Scholar
Zhao H, Hua X, Yang R, Zhao L, Zhao W, Zhang Z (2012) Diafiltration process on xylo-oligosaccharides syrup using nanofiltration and its modelling. Int J Food Sci Technol 47(1):32–39
Zhao L, Meng K, Shi P, Bai Y, Luo H, Huang H, Wang Y, Yang P, Yao B (2013) A novel thermophilic xylanase from Achaetomium sp. Xz-8 with high catalytic efficiency and application potentials in the brewing and other industries. Process Biochem 48(12):1879–1885
Download references
Author information
Nisha Bhardwaj and Komal Agrawal contributed equally with all other contributors.
Authors and Affiliations
Bioprocess and Bioenergy Laboratory, Department of Microbiology, Central University of Rajasthan, NH-8, Bandarsindri, Kishangarh, Ajmer, Rajasthan, India
Nisha Bhardwaj, Komal Agrawal & Pradeep Verma
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Pradeep Verma .
Editor information
Editors and affiliations.
Department of Chemical Engineering & Technology, Indian Institute of Technology BHU, Varanasi, Uttar Pradesh, India
Manish Srivastava
Department of Chemical Engineering & Technology, Indian Institute of Technology (BHU), Varanasi, Uttar Pradesh, India
Neha Srivastava
Department of Environment Studies, Satyawati College, University of Delhi, Delhi, India
Rajeev Singh

Rights and permissions
Reprints and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Bhardwaj, N., Agrawal, K., Verma, P. (2021). Xylanases: An Overview of its Diverse Function in the Field of Biorefinery. In: Srivastava, M., Srivastava, N., Singh, R. (eds) Bioenergy Research: Commercial Opportunities & Challenges . Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-16-1190-2_10
Download citation
DOI : https://doi.org/10.1007/978-981-16-1190-2_10
Published : 11 August 2021
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-1189-6
Online ISBN : 978-981-16-1190-2
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Sustainable Degradation of Lignocellulosic Biomass - Techniques, Applications and Commercialization
A Review of Xylanase Production by the Fermentation of Xylan: Classification, Characterization and Applications
Submitted: 12 April 2012 Published: 15 May 2013
DOI: 10.5772/53544
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Sustainable Degradation of Lignocellulosic Biomass - Techniques, Applications and Commercialization
Edited by Anuj K. Chandel and Silvio Silvério da Silva
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
10,370 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Overall attention for this chapters
Author Information
F. l. motta *.
- Development of Biotechnological Processes Laboratory, School of Chemical Engineering, University of Campinas, Campinas, Brazil
C. C. P. Andrade
- Bioprocess Engineering Laboratory, Food Engineering Department, University of Campinas, Campinas, Brazil
M. H. A. Santana
*Address all correspondence to: [email protected]
1. Introduction
The enzymatic hydrolysis of xylan, which is the second most abundant natural polysaccharide, is one of the most important industrial applications of this polysaccharide [ 1 , 2 ]. The primary chain of xylan is composed of β-xylopyranose residues, and its complete hydrolysis requires the action of several enzymes, including endo-1,4-β-D-xylanase (EC3.2.1.8), which is crucial for xylan depolymerization [ 2 ]. Due to the diversity in the chemical structures of xylans derived from the cell walls of wood, cereal or other plant materials, a large variety of xylanases with various hydrolytic activities, physicochemical properties and structures are known. Moreover, xylan derivatives are frequently used to induce the production of xylanases [ 3 ] by microorganisms [ 4 ], using either solid-state or submerged fermentation [ 5 ].
Xylanases and the microorganisms that produce them are currently used in the management of waste, to degrade xylan to renewable fuels and chemicals, in addition to their use in food, agro-fiber, and the paper and pulp industries, where the enzymes help to reduce their environmental impact [ 6 ]. Oligosaccharides produced by the action of xylanases are further used as functional food additives or alternative sweeteners with beneficial properties [ 7 ].
To meet the needs of industry, more attention has been focused on the enzyme stability under different processing conditions, such as pH, temperature and inhibitory irons, in addition to its ability to hydrolyze soluble or insoluble xylans. Although many wild-type xylanases contain certain desired characteristics, such as thermostability, pH stability or high activity, no individual xylanase is capable of meeting all of the requirements of the feed and food industries. Moreover, as industrial applications require cheaper enzymes, the elevation of expression levels and the efficient secretion of xylanases are crucial to ensure the viability of the process; therefore, genetic engineering and recombinant DNA technology have an important role in the large-scale expression of xylanases in homologous or heterologous protein-expression hosts.
Considering the future prospects of xylanases in biotechnological applications, the goal of this review chapter is to present an overview of xylanase production via fermentation and to describe some of the characteristics of these enzymes and their primary substrate, xylan. Moreover, this review will discuss the fermentation processes as well as the genetic techniques applied to improve xylanase yields.
The three main components that constitute lignocellulosic substrates are cellulose, hemicellulose and lignin [ 8 ]. Schulze [ 9 ] first introduced the term ‘hemicellulose’ to represent the fractions isolated or extracted from plant materials using a dilute alkali. Hemicelluloses are composed of complex mixtures of xylan, xyloglucan, glucomannan, galactoglucomannan, arabinogalactan or other heteropolymers [ 8 ].
The substrate of xylanase, xylan, is the second most-abundant polysaccharide in nature, accounting for approximately one-third of the renewable organic carbon on Earth [ 10 ], and it constitutes the major component of hemicellulose, a complex of polymeric carbohydrates, including xylan, xyloglucan (heteropolymer of D-xylose and D-glucose), glucomannan (heteropolymer of D-glucose and D-mannose), galactoglucomannan (heteropolymer of D-galactose, D-glucose and D-mannose) and arabinogalactan (heteropolymer of D-galactose and arabinose) [ 11 ]. Xylan is primarily present in the secondary cell wall and together with cellulose (1,4-β-glucan) and lignin (a complex polyphenolic compound) make up the major polymeric constituents of plant cell walls [ 12 ]. Within the cell wall structure, all three constituents interact via covalent and non-covalent linkages, with xylan being found at the interface between lignin and cellulose, where it is believed to be important for fiber cohesion and plant cell wall integrity [ 1 ].
2.1. Structure and distribution
A complex, highly branched heteropolysaccharide, xylan varies in structure between different plant species, and the homopolymeric backbone chain of 1,4-linked β-D-xylopyranosyl units can be substituted to varying degrees with glucuronopyranosyl, 4-O-methyl-D-glucuronopyranosyl, α-L-arabinofuranosyl, acetyl, feruloyl or p-coumaroyl side-chain groups [ 12 , 13 ] ( Figure 1) .
Xylan is distributed in several types of tissues and cells and is present in a variety of plant species [ 12 ], being found in large quantities in hardwoods from angiosperms (15–30% of the cell wall content) and softwoods from gymnosperms (7–10%), as well as in annual plants (<30%) [ 14 ]. Wood xylan exists as O-acetyl-4-O-methylglucuronoxylan in hardwoods and as arabino-4-O-methylglucuronoxylan in softwoods, while xylans in grasses and annual plants are typically arabinoxylans [ 12 ]. Linear unsubstituted xylan has also been reported in esparto grass [ 15 ], tobacco [ 16 ] and certain marine algae [ 17 , 18 ], with the latter containing xylopyranosyl residues linked by both 1,3-β and 1,4-β linkages [ 17 , 19 ].
Similar to other polysaccharides of plant origin, xylan has a large polydiversity and polymolecularity [ 20 ]. The degree of polymerization in xylans is also variable, with, for example, hardwood and softwood xylans generally consisting of 150-200 and 70-130 β-xylopyranose residues, respectively [ 12 ].

Structure of xylan and the xylanolytic enzymes involved in its degradation. Ac: Acetyl group; α-Araf: α-arabinofuranose; α-4-O-Me-GlcA: α-4-O-methylglucuronic acid. Source: Sunna and Antranikian [ 20 ].
Based on the common substituents found on the backbone, xylans are categorized as linear homoxylan, arabinoxylan, glucuronoxylan or glucuronoarabinoxylan. Homoxylans consisting exclusively of xylosyl residues are not widespread in nature; they have been isolated from limited sources, such as esparto grass, tobacco stalks and guar seed husks [ 20 ]. However, based on the nature of its substituents, a broad distinction may therefore be made among xylans, in which the complexity increases from linear to highly substituted xylans. Four main families of xylans can be considered [ 21 ]:
Arabinoxylans, having only side chains of single terminal units of α-L-arabinofuranosyl substituents. In the particular case of cereals, arabinoxylans vary in the degree of arabinosyl substitution, with either 2-O- and 3-O-mono-substituted or double (2-O-, 3-O-) substituted xylosyl residues.
Glucuronoxylans, in which α-D-glucuronic acid or its 4-O-methyl ether derivative represents the only substituent.
Glucuronoarabinoxylan, in which α-D-glucuronic (and 4-O-methyl-α-D-glucuronic) acid and α-L-arabinose are both present.
Galactoglucuronoarabinoxylans, which are characterized by the presence of terminal β-D-galactopyranosyl residues on complex oligosaccharide side chains of xylans and are typically found in perennial plants.
In each category there exists microheterogeneity with respect to the degree and nature of branching. The side chains determine the solubility, physical conformation and reactivity of the xylan molecule with the other hemicellulosic components and hence greatly influence the mode and extent of enzymatic cleavage [ 12 ]. Endospermic arabinoxylans of annual plants, also called pentosans, are more soluble in water and alkaline solutions than xylans of lignocellulosic materials because of their branched structures [ 22 ].
2.2. Enzymatic hydrolysis of xylan
Due to the heterogeneity and complex chemical nature of plant xylan, its complete breakdown requires the action of a complex of several hydrolytic enzymes with diverse specificities and modes of action. Thus, it is not surprising for xylan-degrading cells to produce an arsenal of polymer-degrading proteins [ 1 ]. The xylanolytic enzyme system that carries out the xylan hydrolysis is normally composed of a repertoire of hydrolytic enzymes, including endoxylanase (endo-1,4-β-xylanase, E.C.3.2.1.8), β-xylosidase (xylan-1,4-β-xylosidase, E.C.3.2.1.37), α-glucuronidase (α-glucosiduronase, E.C.3.2.1.139), α-arabinofuranosidase (α-L-arabinofuranosidase, E.C.3.2.1.55) and acetylxylan esterase (E.C.3.1.1.72) [ 23 ]. All of these enzymes act cooperatively to convert xylan into its constituent sugars [ 24 ]. Among all xylanases, endoxylanases are the most important due to their direct involvement in cleaving the glycosidic bonds and in liberating short xylooligosaccharides [ 8 ].
Xylan, being a high molecular mass polymer, cannot penetrate the cell wall. The low molecular mass fragments of xylan play a key role in the regulation of xylanase biosynthesis. These fragments include xylose, xylobiose, xylooligosaccharides, heterodisaccharides of xylose and glucose and their positional isomers. These molecules are liberated from xylan through the action of small amounts of constitutively produced enzymes [ 12 ]. Xylanase catalyzes the random hydrolysis of xylan to xylooligosaccharides, while β-xylosidase releases xylose residues from the nonreducing ends of xylooligosaccharides. However, a complete degradation requires the synergistic action of acetyl esterase to remove the acetyl substituents from the β-1,4-linked D-xylose backbone of xylan [ 25 , 26 ].
3. Xylanases
3.1. classification and mode of action.
Xylanases, as glycoside hydrolase members, are able to catalyze the hydrolysis of the glycosidic linkage (β-1,4) of xylosides, leading to the formation of a sugar hemiacetal and the corresponding free aglycone (nonsugar compound remaining after replacement of the glycoside by a hydrogen atom [ 27 ]). Xylanases have been classified in at least three ways: based on the molecular weight and isoelectric point (pI) [ 28 ], the crystal structure [ 29 ] and kinetic properties, or the substrate specificity and product profile. As the first classification is not sufficient to describe all xylanases, several exceptions have been identified [ 10 ] because not all xylanases have a high molecular mass (above 30 kDa) and low pI or a low molecular mass (less than 30 kDa) and high pI [ 6 ]. Therefore, a more complete system, based on the primary structure and comparison of the catalytic domains, was introduced [ 10 , 30 ], analyzing both the structural and mechanistic features [ 10 ].
Updated information on the characteristics and classification of enzymes may be found in the Carbohydrate-Active Enzyme (CAZy) database. This is a knowledge-based resource specializing on enzymes that build and breakdown complex carbohydrates and glycoconjugates. This database contains information from sequence annotations found in publicly available sources (such as the National Center for Biotechnology Information, NCBI), family classifications and known functional information [ 31 ]. According to the CAZy database (http://www.cazy.org), xylanases (EC3.2.1.8) are related to glycoside hydrolase (GH) families 5, 7, 8, 9, 10, 11, 12, 16, 26, 30, 43, 44, 51 and 62. However, the sequences classified in families 16, 51 and 62 appear to be bifunctional enzymes containing two catalytic domains, unlike families 5, 7, 8, 10, 11 and 43, which have a truly distinct catalytic domain with endo-1,4-β-xylanase activity [ 10 ]. Using the same analysis, families 9, 12, 26, 30 and 44 may have residual or secondary xylanase activity.
Xylanases have been primarily classified as GH 10 and 11 based on the hydrophobic cluster analysis of the catalytic domains and similarities in the amino acid sequences [ 8 ]. Although members of these two families have been thoroughly studied, the catalytic properties of the members of the remaining families (5, 7, 8 and 43) are recent and remain very limited [ 32 ].
Members of GH families 5, 7, 8, 10, 11 and 43 differ in their physicochemical properties, structure, mode of action and substrate specificities [ 10 ]. Several models have been proposed to explain the mechanism of xylanase action. Xylanase activity leads to the hydrolysis of xylan. Generally, this hydrolysis may result either in the retention (GH families 5, 7, 10 and 11) or the inversion (GH families 8 and 43) of the anomeric center of the reducing sugar monomer of the carbohydrate [ 33 , 34 ].
Families 5, 7, 10 and 11 contain enzymes that catalyze the hydrolysis with the retention of the anomeric configuration, with two glutamate residues being implicated in the catalytic mechanism. This indicates a double-displacement mechanism, in which a covalent glycosyl-enzyme intermediate is formed and subsequently hydrolyzed, and two carboxylic acid residues, suitably located in the active site, are involved in the formation of the intermediate; one acts as a general acid catalyst by protonating the substrate, while the second performs a nucleophilic attack, which results in the departure of the leaving group and the formation of the α-glycosyl enzyme intermediate (β to α inversion). In the second step, the first carboxylate group instead functions as a general base, abstracting a proton from a nucleophilic water molecule, which attacks the anomeric carbon. This leads to a second substitution, in which the anomeric carbon again passes via a transition state to give rise to a product with the β configuration (α to β inversion) [ 10 , 34 ].
In contrast to the mechanism mentioned above, the enzymes in families 8 and 43 generally act via an inversion of the anomeric center, and glutamate and aspartate may be the catalytic residues. Inverting enzymes work via a single displacement reaction, in which one carboxylate provides for a general acid-catalyzed leaving group departure. The second function of these enzymes, acting as general base, activates a nucleophilic water molecule to attack the anomeric carbon, thereby cleaving the glycosidic bond and leading to an inversion of the configuration at the anomeric carbon. Generally, the distance between the two residues allows for the accommodation of the water molecule between the anomeric carbon and the general base [ 10 , 34 ].
3.1.1. GH families 10 and 11
Xylanase from the GH10 family (or family G) have a low molecular mass with a pI between 8–9.5, while those from the GH11 family (or family F) have a high molecular mass and lower pI values [ 35 , 36 ].
Glycoside hydrolase family 10 is composed of endo-1,4-β-xylanases and endo-1,3-β-xylanases (EC 3.2.1.32) [ 34 ]. Members of this family are also capable of hydrolyzing the aryl β-glycosides of xylobiose and xylotriose at the aglyconic bond. Furthermore, these enzymes are highly active on short xylooligosaccharides, thereby indicating small substrate-binding sites. Crystal structure analyses, kinetic analyses of the activity on xylooligosaccharides of various sizes and end product analyses have indicated that family 10 xylanases typically have four to five substrate-binding sites [ 37 ]. Members of this family also typically have a high molecular mass, a low pI and display an (α/β)8-barrel fold [ 10 , 34 , 38 ].
Compared to other xylanases, GH11 members display several interesting properties, such as high substrate selectivity and high catalytic efficiency, a small size, and a variety of optimum pH and temperature values, making them suitable in various conditions and in many applications [ 39 ]. Family 11 is composed only of xylanases (EC3.2.1.8), leading to their consideration as “true xylanases,” as they are exclusively active on D-xylose-containing substrates. GH11 enzymes are generally characterized by a high pI, a low molecular weight, a double-displacement catalytic mechanism, two glutamates that act as the catalytic residues and a β-jelly roll fold structure. Additionally, the products of their action can be further hydrolyzed by the family 10 enzymes [ 37 ]. Similar to family 10 xylanases, these enzymes can hydrolyze the aryl β-glycosides of xylobiose and xylotriose at the aglyconic bond, but they are inactive on aryl cellobiosides. Furthermore, in contrast to the family 10 xylanases, but similar to the family 8 cold-adapted xylanases, these enzymes are most active on long-chain xylooligosaccharides, and it has been found that they have larger substrate-binding clefts, containing at least seven subsites [ 10 ].
Xylanases belonging to GH10 exhibit greater catalytic versatility and lower substrate specificity than those belonging to GH11 [ 37 , 40 ]. According to Davies et al . [ 41 ], the binding sites for xylose residues in xylanases are termed subsites, with bond cleavage occurring between the sugar residues at the -1 (non-reducing) and the +1 (reducing) ends of the polysaccharide substrate. As observed in assays using arabinoxylan as the substrate, GH10 products have arabinose residues substituted on xylose at the +1 subsite, whereas GH 11 products have arabinose residues substituted at the +2 subsite [ 42 ]. These results suggest that GH 10 enzymes are able to hydrolyze xylose linkages closer to the side-chain residues [ 43 ]. Therefore, xylanases from family 11 preferentially cleave the unsubstituted regions of the arabinoxylan backbone, whereas GH10 enzymes cleave the decorated regions, being less hampered by the presence of substituents along the xylan backbone [ 37 ]. The xylan side-chain decorations are recognized by xylanases, and the degree of substitution in xylan will influence the hydrolytic products; this difference in substrate specificity has important implications in the deconstruction of xylan [ 43 ].
3.1.2. GH families 5, 7, 8 and 43
GH family 5 (or family A) is the largest glycoside hydrolase family, and only seven amino acid residues, including the nucleophile and the general acid/base residue, are strictly conserved among all members [ 10 ]. Structural alignment among the members of family 5 and 10 showed that these enzymes are as structurally different within family 5 as they are to the family 10 enzymes, therefore both families are classified into clan GH-A. The concept of clan or superfamily demonstrates a broader relationship between GH families, suggesting a more distant common evolutionary ancestor [ 44 , 45 ]. Furthermore, the activity of these enzymes is affected by substituents on the xylan main chain, and it is unable to cleave linkages adjacent to substituted residues. Hydrolysis studies have shown that the shortest substituted fragments formed from glucuronoxylan and arabinoxylan are substituted xylotrioses, with the substitution being found on the internal xylose residue. Therefore, the products produced by family 5 are shorter than those produced by family 7 [ 10 ].
GH family 8 (or family D) is composed of cellulases (EC 3.2.1.4), and also contains chitosanases (EC 3.2.1.132), lichenases (EC 3.2.1.73) and endo-1,4-β-xylanases (EC 3.2.1.8). This family of cold-adapted xylanases was found to hydrolyze xylan to xylotriose and xylotetraose and was most active on long-chain xylooligsaccharides. Similar to family 11 xylanases, a large substrate binding cleft containing at least six xylose-binding residues, with the catalytic site in the middle, was proposed [ 6 ]. However, unlike family 10 and 11 xylanases, enzymes from family 8 were found to catalyze hydrolysis with the inversion of the anomeric configuration and, under the conditions used, were found to be inactive on aryl β-glycosides of xylose, xylobiose and xylotriose [ 10 , 31 ].
GH families 7 and 43 contain only a few enzymes exhibiting xylanase activity that have been identified and studied. Family 7 has characteristics in common with both family 10 and 11 xylanases. Similar to the former family, those in family 7 have a high molecular weight and low pI, as well as a small substrate-binding site, containing approximately four subsites, with the catalytic site in the middle [ 10 ]. The members of family 43 have not been as thoroughly studied, and the structure of only one member has been determined, indicating that members of this family may display a five-blade β-propeller fold. Furthermore, a glutamate and aspartate in the center of a long V-shaped surface groove formed across the face of the propeller have been suggested as the catalytic residues. Family 43 is grouped with family 62 in clan GH-F, and, as also demonstrated in the family 8 enzymes, its members are believed to catalyze hydrolysis via a single displacement mechanism [ 10 , 31 ].
3.2. Properties and applications
The heterogeneity and complexity of xylan have resulted in a diverse range of xylanases, which differ in their physicochemical properties, structure, mode of action and substrate specificities [ 10 ]. As the xylosidic linkages in lignocellulose are neither equivalent nor equally accessible, the production of an enzymatic system with specialized functions is a strategy to achieve superior xylan hydrolysis [ 28 ]. Together with the heterogeneous nature of xylan, the multiplicity of xylanases in microorganisms may be caused by a redundancy in gene expression. Generally, a single xylanase gene encodes multiple xylanases, and xylanase multiplicity may arise from posttranslational modifications, such as differential glycosylation, proteolysis or both [ 23 ].
The potential applications of xylanases also include the bioconversion of lignocellulosic material and agro-wastes into fermentative products, the clarification of juices, the improvement of the consistency of beer and the digestibility of animal feedstocks [ 28 ]. One of the most important biotechnological applications of xylanase is its use in pulp bleaching [ 46 ]. Xylanases may also be applicable to the production of rayon, cellophane and several chemicals such as cellulose esters (acetates, nitrates, propionates and butyrates) and cellulose ethers (carboxymethyl cellulose and methyl and ethyl cellulose), which are all produced by dissolving pulp and purifying fibers from other carbohydrates [ 33 ].
3.2.1. The paper and pulp industries
During the past several years, the use of enzymes in paper and pulp bleaching has caught the attention of researchers and industries all over the world. Xylanase enzymes have proven to be a cost-effective means for mills to take advantage of a variety of bleaching benefits [ 47 ]. Xylanases and other side-cleaving enzymes have been used in pulp bleaching primarily to reduce lignin and increase the brightness of the pulp [ 20 , 46 ]. The importance of xylanase in the pulp and paper industries is related to the hydrolysis of xylan, which facilitates the release of lignin from paper pulp and, consequently, reduces the usage of chlorine as the bleaching agent [ 33 ].
Bleaching is the process of lignin removal from chemical pulps to produce bright or completely white finished pulp [ 1 ]. Thus, the bleaching of pulp using enzymes or ligninolytic microorganisms is called biobleaching [ 48 ]. This process is necessary due to the presence of residual lignin and its derivatives in the pulping process, which causes the resultant pulp to gain a characteristic brown color. The intensity of this pulp color is related to the amount and chemical state of the remaining lignin [ 33 ].
The bleaching of pulp involves the destruction, alteration or solubilization of the lignin, colored organic matter and other undesirable residues on the fibers [ 33 ]. Bleaching of kraft pulp usually requires large amounts of chlorine-based chemicals and sodium hydrosulfite, which cause several effluent-based problems in the pulp and paper industries. The use of these chemicals generates chlorinated organic substances, some of which are toxic, mutagenic, persistent, and highly resistant to biodegradation, in addition to causing numerous harmful disturbances in biological systems and forming one of the major sources of environmental pollution [ 1 , 33 , 49 ].
As hemicellulose is easier to depolymerize than lignin, biobleaching of pulp appears to be more effective with the use of xylanases than with lignin-degrading enzymes. This is due to the fact of the removal of even a small portion of the hemicellulose could be sufficient to open up the polymer, which facilitates removal of the residual lignin by mild oxidants [ 33 , 50 ].
The use of xylanase in bleaching pulp requires the use of enzymes with special characteristics. A key requirement is to be cellulose-free, to avoid damaging the pulp fibers [ 3 ], as cellulose is the primary product in the paper industry [ 33 ]. Other desirable characteristics are stability at high temperatures [ 51 ] and an alkaline optimal pH [ 48 ].
Madlala et al . [ 52 ] used different preparations of commercial Xylanase P and crude xylanase from Thermomyces lanuginosus to evaluate the bleaching process of paper pulp. It was demonstrated that the use of enzymes could increase the pulp brightness (over 5 brightness points over the control) and reduce the amount of bleaching chemicals used (up to 30% for chlorine dioxide). Chipeta et al . [ 53 ] evaluated crude xylanase preparations from Aspergillus oryzae NRRL 3485 and Aspergillus phoenicis ATCC 13157 and found that at a charge of 10 U per gram of pulp it was possible to reduce the usage of chlorine dioxide up to 30% without compromising the pulp brightness.
3.2.2. Bioconversion of lignocellulose in biofuels
Currently, second-generation biofuels are the primary products of the bioconversion of lignocellulosic materials. According to Taherzadech and Karimi [ 54 ], ethanol is the most important renewable fuel in terms of volume and market value, and following the fossil fuel crisis, it has been identified as an alternative fuel [ 48 ]. Despite the primarily first-generation production of ethanol, from sugar and starch, the second-generation production of ethanol has only begun to be tested in pilot plants [ 55 ]. And, unlike first-generation biofuels, second-generation biofuels do not compete with food production and can provide environmental, economic, and strategic benefits for the production of fuels [ 56 ].
Xylanase, together with other hydrolytic enzymes, can be used for the generation of biological fuels, such as ethanol, from lignocellulosic biomass [ 1 , 57 ]. However, enzymatic hydrolysis is still a major cost factor in the conversion of lignocellulosic raw materials to ethanol [ 56 ]. In bioethanol fuel production, the first step is the delignification of lignocellulose, to liberate cellulose and hemicellulose from their complex with lignin. The second step is a depolymerization of the carbohydrate polymers to produce free sugars, followed by the fermentation of mixed pentose and hexose sugars to produce ethanol [ 1 , 58 ]. Simultaneous saccharification and fermentation is an alternative process, in which both hydrolytic enzymes and fermentative microorganism are present in the reaction [ 48 , 59 ].
3.2.3. The pharmaceutical, food and feed industries
Xylanase, together with pectinase, carboxymethylcellulase and amylase, can be used for the clarification of juices because the turbidity observed is due to both pectic materials and other materials suspended in a stable colloidal system [ 60 ]. Xylanase may also improve the extraction of coffee, plant oils, and starch [ 25 ]. The xylose resulting from xylan depolymerization may also be converted to xylitol, a valuable sweetener that has applications in both the pharmaceutical and food industries [ 61 - 63 ].
In the bakery industry, xylanase may improve the quality of bread, by increasing the bread’s specific volume. In combination with amylases, this characteristic was enhanced, as observed upon the introduction of Aspergillus niger var. awamori [ 64 ]. According to Collins et al . [ 65 ], psychrophilic enzymes may be suitable for use in the baking industry as they are generally optimally active at the temperatures most frequently used for dough preparation (at or below 35 °C). These enzymes could also be used as more efficient baking additives than the currently used commercial mesophilic enzymes, which are optimally active at higher temperatures.
Xylanase may also improve the nutritional properties of agricultural silage and grain feed. The use of this enzyme in poultry diets showed that the decrease in weight gain and feed conversion efficiency in rye-fed broiler chicks has been associated with intestinal viscosity [ 66 ]. The incorporation of xylanase from Trichoderma longibrachiatum into the rye-based diet of broiler chickens reduced intestinal viscosity, thus, improving both the weight gain of the chicks and their feed conversion efficiency [ 67 ].
Xylanases can also be used in cereals as a pretreatment for arabinoxylan-containing substrates, as arabinoxylans are partly water soluble and result in a highly viscous aqueous solution. This high viscosity of cereal grain water extract may lead to brewing problems, by decreasing the rate of filtration or haze formation in beer. Additionally, it is unfavorable in the cereal grains used in animal feeding [ 68 , 69 ].
The enzymatic hydrolysis of xylan may also result in oligomers known as xylooligosaccharides (XOs), which may be used in pharmaceutical, agriculture and feed products. XOs have prebiotic effects, as they are neither hydrolyzed nor absorbed in the upper gastrointestinal tract, and they affect the host by selectively stimulating the growth or activity of one or a number of bacteria in the colon, thus improving health [ 70 - 72 ]. Among their key physiological advantages are the reduction of cholesterol, maintenance of gastrointestinal health, and improvement of the biological availability of calcium. They also inhibit starch retrogradation, improving the nutritional and sensory properties of food [ 73 ]. For the production of XOs, the enzyme complex must have low exoxylanase or β-xylosidase activity, to prevent the production of high amounts of xylose, which has inhibitory effects on XO production [ 74 , 75 ].
3.3. Xylanase assays
The xylanase activity is often assayed based on measurement of reducing sugar released during the course of hydrolysis of xylan, by DNS or Nelson-Somogyi methods. Due to absent of standardization, Bailey et al. [ 76 ] compared the measurement of xylanase activity by twenty different laboratories. According to the author, the major source of variation between apparent xylanase activities was probably the substrate chosen, although small differences in protocols were also significant. After standardization of substrate and method, the interlaboratory standard variation of the results decreased from 108% to 17% from the mean. Others researchers use the 4-o-methylglucuronoxylan covalently dyed with Remazol Brilliant Blue (RBB xylan) as substrate, and the xylanase is assayed based on the release of the dyed fragments [ 77 ]. There are also available some commercial methods for xylanase assays, as the fluorescence-based method EnzChek® Ultra Xylanase Assay Kit (Invitrogen, Carlsbad, CA) or the Xylazyme tablet (Megazyme, Bray, Ireland), which employs azurine-crosslinked arabinoxylan (AZCLArabinoxylan) as substrate and its hydrolysis by xylanase produces water soluble dyed fragments.
3.4. Producing microorganisms
Microorganisms, in particular, have been regarded as a good source of useful enzymes because they multiply at extremely high rates and synthesize biologically active products that can be controlled by humans. In recent years, there has been a phenomenal increase in the use of enzymes as industrial catalysts. These enzymes offer advantages over the use of conventional chemical catalysts for numerous reasons: they exhibit high catalytic activity and a high degree of substrate specificity, they can be produced in large amounts, they are highly biodegradable, they pose no threat to the environment and they are economically viable [ 4 ].
In this context, microbial xylanases are the preferred catalysts for xylan hydrolysis, due to their high specificity, mild reaction conditions, negligible substrate loss and side product generation. Xylanases derived from microorganisms have many potential applications in the food, feed, and paper pulp industries [ 10 , 12 , 78 ]. Complete xylanolytic enzyme systems, which including all of these activities, have been found to be widespread among fungi [ 20 , 24 ], actinomycetes [ 79 ] and bacteria [ 12 ], and some of the most important xylanolytic enzyme producers include Aspergillus, Trichoderma, Streptomyces, Phanerochaetes, Chytridiomycetes, Ruminococcus, Fibrobacteres, Clostridia and Bacillus [ 12 , 78 , 80 , 81 ]. The ecological niches of these microorganisms are diverse and widespread and typically include environments where plant materials accumulate and deteriorate, as well as in the rumen of ruminants [ 78 , 82 , 83 ].
Although there have been many reports on microbial xylanases since the 1960s, the prime focus has been on plant pathology related studies [ 84 ]. Only during the 1980’s did the use of xylanases for biobleaching begin to be tested [ 85 ]. Since 1982, several microorganisms, including fungi and bacteria, have been reported to readily hydrolyze xylans by synthesizing 1,4-β-D endoxylanases (E.C. 3.2.18) and β-xylosidases (EC.3.2.1.37) [ 86 ]. Table 1 presents a list of some of the xylanase-producing microorganisms and their activities.
The production of xylanases must be improved by finding more potent fungal or bacterial strains or by inducing mutant strains to excrete greater amounts of the enzymes. Moreover, the level of microbial enzyme production is influenced by a variety of nutritional and physiological factors, such as the supply of carbon and nitrogen, physical circumstances and chemical conditions [ 98 ].
| 18,895 IU/g | pH 7.0; 30 °C | Soya oil cake and casein peptone | [ ] | |
| P12–137 | 27.8 IU/mL | pH 7.2; 28 °C | Wheat bran and KNO | [ ] |
| 8,237 IU/g | pH 6.0; 40 °C | Corn cob and wheat bran and (NH ) SO | [ ] | |
| 39.7 IU/mL | 30 °C | Oat spelt xylan, urea, peptone and yeast extract | [ ] | |
| 7.5 IU/mL | pH 6.5; 30 °C | Wheat bran | [ ] | |
| 33.1 IU/mL | 30 °C | Oat spelt xylan, urea, peptone and yeast extract | [ ] | |
| PPI | 16.0 IU/mL | pH 5.0; 28 °C | Oat and urea | [ ] |
| strain L2 | 1.13 IU/mL | 50 °C | Avicel (PH 105) from Serva (Heidelberg, Germany) | [ ] |
| 1,469.4 IU/g | pH 4.5; 30 °C | Wheat straw and NaNO | [ ] | |
| D1 | 8.4 IU/mL | pH 9.0; 45 °C | Bagasse hydrolysates | [ ] |
| strain Ib 24D | 1,447.0 IU/mL | pH 7.5; 28 °C | Tomato pomace | [ ] |
| J18 | 18,580.0 IU/g | pH 6.9; 50 °C | Wheat straw and yeast extract | [ ] |
Review of xylanases-producing microorganisms.
3.4.1. Fungi
Filamentous fungi are particularly interesting producers of xylanases and other xylan-degrading enzymes because they excrete the enzymes into the medium and their enzyme levels are much higher than those of yeast and bacteria. In addition to xylanases, fungi produce several auxiliary enzymes required for the degradation of substituted xylan [ 2 ].
The fungal genera Trichoderma , Aspergillus , Fusarium , and Pichia are considered great producers of xylanases [ 99 ]. White-rot fungi have also been shown to produce extracellular xylanases that act on a wide range of hemicellulosic materials, are useful as food sources [ 100 ] and produce metabolites of interest to the pharmaceutical, cosmetic, and food industries [ 78 ]. White-rot basidiomycetes normally secrete large amounts of these enzymes to degrade lignocellulosic materials. For example, Phanerochaete chrysosporium produces high levels of α-glucuronidase [ 101 ], and Coriolus versicolor produces a complex xylanolytic combination of enzymes [ 102 ]. Xylanase is also produced by Cuninghamella subvermispora when growing on plant cell-wall polysaccharides or on wood chips [ 103 ].
Fungal xylanases are generally associated with celluloses [ 104 ]. On cellulose these strains produce both cellulase and xylanase, which may be due to traces of hemicellulose present in the cellulosic substrates [ 105 ]; however, selective production of xylanase may be possible using only xylan as the carbon source. The mechanisms that govern the formation of extracellular enzymes with regards to the carbon sources present in the medium are influenced by the availability of precursors for protein synthesis. Therefore, in some fungi, growing the cells on xylan uncontaminated by cellulose under a lower nitrogen/carbon ratio may be a possible strategy for producing xylanolytic systems free of cellulases [ 106 ]. Another major problem associated with fungi is the reduced xylanase yield in fermenter studies. Agitation is normally used to maintain the medium homogeneity, but the shearing forces in the fermenter can disrupt the fragile fungal biomass, leading to the reported low productivity. Higher rates of agitation may also lead to hyphal disruption, further decreasing the xylanase activity [ 50 ].
3.4.2. Bacteria
Xylanases have been reported in Bacillus , Streptomyces and other bacterial genera that do not have any role related to plant pathogenicity [ 86 ]. The extreme thermophile Rhodothermus marinus has been reported to produce α-L-arabinofuranosidase [ 107 ], and two different polypeptides with α-arabinofuranosidase activity from Bacillus polymyxa were characterized at the gene level for the production of α-arabinofuranosidases [ 108 ].
Bacteria, just like many other industrial enzymes, have fascinated researchers due to their alkaline-thermostable xylanase-producing trait [ 33 ]. The optimum pH of bacterial xylanases are, in general, slightly higher than the optimal pH of fungal xylanases [ 109 ], which is a suitable characteristic in most industrial applications, especially the paper and pulp industries. Noteworthy producers of high levels of xylanase activity at an alkaline pH and high temperature are Bacillus spp. [ 33 ]. When considering only temperature, a handful of xylanases that show optimum activity at higher temperatures have been reported from various microorganisms. These include Geobacillus thermoleovorans , Streptomyces sp. S27, Bacillus firmus , Actinomadura sp. strain Cpt20 and Saccharopolyspora pathunthaniensis S582, all of which produce xylanases that show activity between 65 and 90 °C [ 8 ]. One xylanase, reported from Thermotoga sp. [ 110 ], has been shown to exhibit a temperature optima between 100 and 105 °C.
3.5. Production of xylanases under SSF and SmF
Xylanases are produced by either solid-state or submerged fermentation [ 5 ]. Although most xylanase manufacturers produce these enzymes using submerged fermentation (SmF) techniques (nearly for 90% of the total xylanase sales worldwide) [ 2 ], the enzyme productivity via solid-state fermentation (SSF) is normally much higher than that of submerged fermentation [ 5 ]. The growing interest in using solid-state fermentation (SSF) techniques to produce a wide variety of enzymes, including xylanases from fungal origins, is primarily due to the economic and engineering advantages of this process [ 111 ].
The advantages of SSF processes over SmF include a low cultivation cost for the fermentation, lower risk of contamination [ 1 ], improved enzyme stability, mimicking the natural habit of the fungus, production of enzymes with higher specific activities, generation of a protein-enriched byproduct, and easier downstream processing of the enzymes produced [ 112 ]. SSF conditions are especially suitable for the growth of fungi, as these organisms are able to grow at relatively low water activities, contrary to most bacteria and yeast, which will not proliferate under these culture conditions [ 113 ].
On the contrary, submerged fermentation allows better control of the conditions during fermentation [ 114 ]. The submerged fermentation of aerobic microorganisms is a well-known and widely used method for the production of cellulase and xylanase [ 115 ]. In general, SmF is the preferred method of production when the preparations require more purified enzymes, whereas synergistic effects from a battery of xylan-degrading enzymes can easily be found in preparations obtained by SSF using complex substrates, though the latter is commonly sought in applications aimed at improving animal feed [ 113 ].
The choice of the substrate is of great importance for the selection of the fermentation process and the successful production of xylanases. In this context, purified xylans can be excellent substrates because the low molecular weight compounds derived from them are the best xylanase inducers. The use of such substrates has led to increased yields of xylanase production and a selective induction of xylanases, with concomitantly low cellulase activity in a number of microorganisms. However, for large-scale processes other alternatives have to be considered due to the cost of such substrates. Some lignocellulolytic substrates such as barley husk, corn cobs, hay, wheat bran or straw have been compared in relation to pure substrates, and many have performed significantly better than isolated xylans (or celluloses) with respect to the yields of xylanase in large-scale production processes. Solid-state fermentation processes are practical for complex substrates, including agricultural, forestry and food processing residues and wastes, which are used as inducing carbon sources for the production of xylanases [ 113 ]. The use of abundantly available and cost-effective agricultural residues, such as wheat bran, corn cobs, rice bran, rice husks, and other similar substrates, to achieve higher xylanase yields via SSF allows the reduction of the overall manufacturing cost of biobleached paper. This has facilitated the use of this environmentally friendly technology in the paper industry [ 1 ].
3.6. Cloning and expression of xylanases
To meet specific industrial needs, an ideal xylanase should have specific properties, such as stability over a wide range of pH values and temperatures, high specific activity, and strong resistance to metal cations and chemicals [ 116 ]. Other specifications include cost-effectiveness, eco-friendliness, and ease of use [ 32 ]. Therefore, most of the reported xylanases do not possess all of the characteristics required by industry [ 8 ].
Native enzymes are not sufficient to meet the demand, due to low yields and incompatibility of the standard industrial fermentation processes [ 35 ]. Therefore, molecular approaches must be implemented to design xylanases with the required characteristics [ 8 ]. Heterologous expression is the main tool for the production of xylanases at the industrial level [ 35 ]. Protein engineering (alteration or modification of existing proteins) by recombinant DNA technology could be useful in improving the specific characteristics of existing xylanases [ 8 ]. Genetic engineering and recombinant DNA technology allow the large-scale expression of xylanases in homologous or heterologous protein-expression hosts. As industrial applications require cheaper enzymes, the elevation of expression levels and efficient secretion of xylanases are vital for ensuring the viability of the process [ 23 ].
An increasing number of publications have described numerous xylanases from several sources and the cloning, sequencing, mutagenesis and crystallographic analysis of these enzymes [ 12 ]. The available amino acid sequence data, X-ray crystallographic data, molecular dynamics and computational design of xylanases provide information that authenticates the relationship between the structure and function of xylanases. All of these methods aid in the design of xylanases that are required in industrial processes, such as improvement of the stability of xylanases at higher temperatures and alkaline pHs [ 8 ].
To attempt these processes for commercial purposes, genes encoding several xylanases have been cloned in homologous and heterologous hosts [ 12 , 48 ]. Recombinant xylanases have shown equivalent or better properties than the native enzymes, and the xylanase genes from anaerobic microorganisms have also been expressed successfully in hosts that can be employed in the fermentation industry [ 35 ].
3.6.1. Expression in bacteria
Escherichia coli is known for its ease of manipulation, inexpensive growth conditions, simple techniques required for transformation and accumulation of high levels of product in the cell cytoplasm; therefore, this organism has become the most widely used expression host [ 117 ]. Despite Escherichia coli’s use as a good cloning host for recombinant proteins, it does not provide efficient and functional expression of many xylanases [ 23 , 24 ], and not all genes are easily expressed in E. coli [ 117 ]. This problem may be due to the repetitive appearance of rare codons and the requirement for specific translational modifications, such as disulfide-bond formation and glycosylation [ 23 ]. Therefore, this microorganism is useful for the detailed study of xylanase gene structure and for the improvement of the enzymes via protein engineering [ 35 ].
Lactobacillus species and Bacillus subitilis have been attractive hosts for the production of heterologous proteins, obtaining higher expression levels than E. coli [ 23 , 118 ]. B. subtilis and Lactobacillus are gram-positive and perform N-glycosilation [ 119 ]. Their primary interest in industry and research, is due to the fact that are non-toxic and are generally recognized as safe (GRAS) [ 23 , 118 ]. Members of the genus Bacillus , unlike E. coli , do not contain endotoxins (lipopolysaccharides), which are difficult to remove from many proteins during the purification process. The secretory production could also be advantageous in industrial production [ 33 ].
3.6.2. Expression in yeast
Heterologous protein expression in yeast systems is highly attractive because they provide additional benefits over bacterial expression systems. Among these benefits are the ability to perform eukaryotic post-translational modifications, the ability to grow to very high cell densities and the ability to secrete proteins into the fermentation media. Moreover, yeast are free of toxins and the majority have GRAS status [ 23 ].
Saccharomyces cerevisiae secretes high amounts of xylanases into the culture medium. Because it has already been established as an industrial microorganism, it can be used conveniently for the industrial production of xylanases at low costs [ 35 ]. Pichia pastoris has also emerged as an excellent host for the commercial production of xylanases due to very high expression under its own promoters [ 35 ]. However, the success of this methylotrophic yeast, similar to Hansenula polymorpha , is reached with the promoters of alcohol oxidase, an enzyme involved in the methanol-utilization pathway [ 23 ]. Therefore, these promoters have limited use at the large scale due to the health and fire hazards of methanol [ 35 ].
3.6.3. Expression in filamentous fungi
Filamentous fungi are capable producers of xylanases, via both heterologous and homologous gene expression, and reach high expression yields with their own promoters [ 35 ]. Filamentous fungi have already undergone intricate strain improvement for high-level protein secretion and are feasible when using the native xylanase-expressing machinery for functional expression of foreign xylanases from remote sources. The xylanase gene from P. griseofulvum has been successfully expressed in A. oryzae [ 120 ].
4. Conclusion
Xylan, the major hemicellulose component, requires the synergistic action of several hemicellulase enzymes for its complete hydrolysis to monomer sugars. The principle enzyme in this processes is endo-1,4-β-xylanase, which cleaves the glycosidic bonds between xylosides, generating short xylooligosaccharides. The majority of the studied xylanases have been classified into the GH10 or GH11 families, whereas studies of the xylanases in families 5, 7, 8 and 43 are still emerging.
The conversion of xylan to useful products represents part of our efforts to strengthen the overall economics of the processing of lignocellulosic biomass and to develop new means of energy production from renewable resources. Among these products are xylanases, enzymes that have a wide range of important industrial applications. Therefore, in the future, new methods will be developed for easier and cheaper production of these enzymes to fulfill the demands of various industries. In this context, the use of lignocellulosic agricultural waste for the production of these enzymes by either submerged or solid-sate fermentation has been very attractive, in addition to molecular techniques that are being tested to improve the enzyme’s characteristics and increase its expression rates. Moreover, as the native enzyme does not fulfill all of the process requirements, bioprospecting for new genes, rational engineering and directed evolution of known genes are powerful tools that can be used to improve these enzymes.
Acknowledgments
- 1. Beg Q K, Kapoor M, Mahajan L, Hoondal G S. Microbial xylanases and their industrial applications: a review. Applied Microbiology and Biotechnology 2001;56: 326–338.
- 2. Polizeli M L T M, Rizzatti C S, Monti R, Terenzi H F, Jorge J, Amorim D S. Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology 2005;67: 577–91.
- 3. Haltrich D, Nidetzky B, Kulbe K D, Steiner W, Župančič S. Production of fungal xylanases. Bioresource Technology 1996;58: 137–161.
- 4. Gote M. Isolation, purification and characterization of thermostable-galactosidase from Bacillus stearothermophilus (NCIM-5146). University of Pune; 2004.
- 5. Agnihotri S, Dutt D, Tyagi C H, Kumar A, Upadhyaya J S. Production and biochemical characterization of a novel cellulase-poor alkali-thermo-tolerant xylanase from Coprinellus disseminatus SW-1 NTCC 1165. World Journal of Microbiology and Biotechnology 2010;26: 1349–1359.
- 6. Collins T, Meuwis M A, Stals I, Claeyssens M, Feller G, Gerday C. A novel family 8 xylanase, functional and physicochemical characterization. The Journal of Biological Chemistry 2002;277: 35133–35139.
- 7. Pellerin P, Gosselin M, Lepoutre J P, Samain E, Debeire P. Enzymatic production of oligosaccharides from corncob xylan. Enzyme and Microbial Technology 1991;13: 617–621.
- 8. Verma D, Satyanarayana T. Molecular approaches for ameliorating microbial xylanases. Bioresource Technology 2012;17: 360–367.
- 9. Schulze E. Information regarding chemical composition of plant cell membrane. Ber Dtsch Chem Ges 1891; 24:2277–2287.
- 10. Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiology Reviews 2005;29: 3–23.
- 11. Shallom D, Shoham Y. Microbial hemicellulases. Current Opinion in Microbiology 2003; 6:219–228.
- 12. Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiology Reviews 1999;23: 411–456.
- 13. Li K, Azadi P, Collins R, Tolan J, Kim J, Eriksson K. Relationships between activities of xylanases and xylan structures. Enzyme and Microbial Technology 2000;27: 89–94.
- 14. Singh S, Madlala A M, Prior B A. Thermomyces lanuginosus : properties of strains and their hemicellulases. FEMS Microbiology Reviews 2003;27: 3–16.
- 15. Chanda S K, Hirst E L, Jones J K N, Percival E G V. The constitution of xylan from esparto grass. J. Chem. Soc. 1950;12889–12897.
- 16. Eda S, Ohnishi A, Kato K. Xylan isolated from the stalk of Nicotiana tabacum . Agric. Biol. Chem. 1976;40: 359–364.
- 17. Barry V, Dillon T. Occurrence of xylans in marine algae. Nature 1940;146: 620-620.
- 18. Nunn J R, Parolis H, Russel I. Polysaccharides of the red algae Chaetangium erinaceum . Part I: Isolation and characterization of the water-soluble xylan. Carbohydrate Research. 1973;26: 169–180.
- 19. Percival E G V, Chanda S K. The xylan of Rhodymenia palmata , Nature 1950;166: 787–788.
- 20. Sunna A, Antranikian G. Xylanolytic enzymes from fungi and bacteria. Critical Reviews in Biotechnology 1997;17: 39–67.
- 21. Voragen A G J, Gruppen H, Verbruggen M A, Vietor R J. Characterization of cereals arabinoxylans, in: Xylan and Xylanases, Elsevier, Amsterdam; 1992.
- 22. Ferreira-Filho E X. The xylan-degrading enzyme system. Brazilian Journal of Medical and Biological Research 1994;27: 1093–1109.
- 23. Juturu V, Wu J C. Microbial xylanases: Engineering, production and industrial applications. Biotechnology Advances 2011; doi:10.1016/j.biotechadv.2011.11.006.
- 24. Belancic A, SCARPA J, Peirano A, Diaz r, Steiner J, Eyzayuirre J. Penicillium purpurogenum produces several xylanases: purification and properties of two of the enzymes. Journal of Biotechnology 1995;41: 71–79.
- 25. Wong K K Y, Saddler J N. Applications of hemicellulases in the food, feed, and pulp and paper industries, in: M.P. Coughlan, G.P. Hazlewood (Eds.), Hemicelluloses and Hernicellulases, Portland Press, London; 1993.
- 26. Coughlan G P, Hazlewood M P. β-1,4-D-xylan-degrading enzyme system: Biochemistry, molecular biology, and applications. Biotechnol. Appl. Biochem. 1993;17: 259–289.
- 27. Hatanaka K. Incorporation of Fluorous Glycosides to Cell Membrane and Saccharide Chain Elongation by Cellular Enzymes. Top Curr Chem 2012;308: 291–306.
- 28. Wong K K, Tan L U, Saddler J N. Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiological Reviews 1988; 52: 305–17.
- 29. Jeffries T W. Biochemistry and genetics of microbial xylanases. Current Opinion in Biotechnology. 1996;7: 337–342.
- 30. Henrissat B, Coutinho P M. Classification of glycoside hydrolases and glycosyltransferases from hyperthermophiles. Methods in Enzymology 2001;330: 183–201.
- 31. Cantarel B L, Coutinho P M, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Research 2009; 37:233–238.
- 32. Taibi Z, Saoudi B, Boudelaa M, Trigui H, Belghith H, Gargouri A. Purification and biochemical characterization of a highly thermostable xylanase from Actinomadura sp . strain Cpt20 isolated from poultry compost. Applied Biochemistry and Biotechnology 2012; 166:663–679.
- 33. Subramaniyan S, Prema P. Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Critical Reviews in Biotechnology 2002;22: 33–64.
- 34. Coutinho P M, Henrissat B. Carbohydrate-active enzymes Server; 1999.
- 35. Ahmed S, Riaz S, Jamil A. Molecular cloning of fungal xylanases: an overview. Applied Microbiology and Biotechnology 2009;84: 19–35.
- 36. Buchert J, Tenkanen M, Kantelinen A, Viikari L. Application of xylanases in the pulp and paper industry. Bioresource Technology 1995;50: 65–72.
- 37. Biely P, Vrsanská M, Tenkanen M, Kluepfel D. Endo-β-1,4-xylanase families: differences in catalytic properties. Journal of Biotechnology 1997;57: 151–166.
- 38. Lo Leggio L, Kalogiannis S, Bhat M K, Pickersgill R W. High resolution structure and sequence of T. aurantiacus xylanase I: implications for the evolution of thermostability in family 10 xylanases and enzymes with (β)alpha-barrel architecture. Proteins 1999;36: 295–306.
- 39. Paës G, Berrin J G, Beaugrand J. GH11 xylanases: Structure/function/properties relationships and applications. Biotechnology Advances 2012;30: 564–592.
- 40. Faulds C B, Mandalari G, Lo Curto R B, Bisignano G, Christakopoulos P, Waldron K W. Synergy between xylanases from glycoside hydrolase family 10 and family 11 and a feruloyl esterase in the release of phenolic acids from cereal arabinoxylan. Applied Microbiology and Biotechnology 2006;71: 622–629.
- 41. Davies G J, Wilson K S, Henrissat B. Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochemical Journal 1997;321: 557–559.
- 42. Maslen S L, Goubet F, Adam A, Dupree P, Stephens E. Structure elucidation of arabinoxylan isomers by normal phase HPLC-MALDI-TOF/TOF-MS/MS. Carbohydrate Research 2007; 342: 724–35.
- 43. Dodd D, Cann I K O. Enzymatic deconstruction of xylan for biofuel production. Global Change Biology Bioenergy 2009;1: 2–17.
- 44. Ryttersgaard C, Lo Leggio L, Coutinho P M, Henrissat B, Larsen S. Aspergillus aculeatus β-1,4-galactanase: substrate recognition and relations to other glycoside hydrolases in clan GH-A. Biochemistry 2002;41: 15135–15143.
- 45. Larson S B, Day J, Paulina A, De B, Keen N T, Mcpherson A. First Crystallographic Structure of a Xylanase from Glycoside Hydrolase Family 5: Implications for Catalysis 2003;8411–8422.
- 46. Viikari L, Kantelinen A, Sundquist J, Linko M. Xylanases in bleaching: From an idea to the industry. FEMS Microbiology Reviews 1994;13: 335–350.
- 47. Bajpai P. Biotechnology for Pulp and Paper Processing. Springer US, Boston, MA; 2012.
- 48. Pérez J, Muñoz-Dorado J, de la Rubia T, Martínez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview., International Microbiology: the Official Journal of the Spanish Society for Microbiology 2002;5: 53–63.
- 49. Onysko K. Biological bleaching of chemical pulps: a review. Biotechnology Advances 1993;11: 179–198.
- 50. Subramaniyan S, Prema P. Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiology Letters 2000;183: 1–7.
- 51. Chidi S B, Godana B, Ncube I, Rensburg E J V, Abotsi E K. Production , purification and characterization of celullase-free xylanase from Aspergillus terreus UL 4209, Journal of Biotechnology 2008;7: 3939–3948.
- 52. Madlala A, Bissoon S, Singh S, Christov L. Xylanase-induced reduction of chlorine dioxide consumption during elemental chlorine-free bleaching of different pulp types. Biotechnology Letters 2001;23: 345–351.
- 53. Chipeta Z A, Du Preez J C, Szakacs G, Christopher L. Xylanase production by fungal strains on spent sulphite liquor. Applied Microbiology and Biotechnology 2005;69: 71–78.
- 54. Taherzadeh M J, Karimi K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. International Journal of Molecular Sciences 2008;9: 1621–1651.
- 55. Taherzadeh M J, Karimi K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources 2007;2: 707–738.
- 56. Viikari L, Vehmaanperä J, Koivula A. Lignocellulosic ethanol: From science to industry, Biomass and Bioenergy 2012;1–12.
- 57. Olsson L, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme and Microbial Technology 1996;18: 312–331.
- 58. Lee J. Biological conversion of lignocellulosic biomass to ethanol. Journal of Biotechnology 1997;56: 1–24.
- 59. Chandrakant P, Bisaria V S. Simultaneous Bioconversion of Cellulose and Hemicellulose to Ethanol. Crit Rev Biotechnol 2008;18: 295-331.
- 60. Sreenath H K, Santhanam K. The use of commercial enzymes in white grape juice clarification. Journal of Fermentation and Bioengineering 1992;73: 241–243.
- 61. Parajó J C, Domínguez H, Domínguez J. Biotechnological production of xylitol. Part 1: Interest of xylitol and fundamentals of its biosynthesis. Bioresource Technology 1998;65: 191–201.
- 62. Sirisansaneeyakul S. Screening of yeasts for production of xylitol from D-xylose. Journal of Fermentation and Bioengineering 1995;80: 565–570.
- 63. Soleimani M, Tabil L, Panigrahi S, Alberta E. Bio-production of a Polyalcohol (Xylitol) from Lignocellulosic Resources: A Review Written for presentation at the CSBE/SCGAB 2006 Annual Conference, Society; 2006.
- 64. Maat J, Roza M, Verbakel J, Stam H, DaSilra M, Egmond M. Xylanases and their application in bakery, in: J. Visser, G. Beldman, M. VanSomeren, A. Voragen (Eds.), Xylans and Xylanases, Elsevier, Amsterdam; 1992.
- 65. Collins T, Hoyoux A, Dutron A, Georis J, Genot B, Dauvrin T. Use of glycoside hydrolase family 8 xylanases in baking. Journal of Cereal Science 2006;43: 79–84.
- 66. Paridon P A, Boonman J C P, Selten G C M, Geerse C, Barug D, de Bot P H M, Hemke G. The application of fungal endoxylanase in poulty diets, in: Visser J, Beldman G, Kusters-van Someren M A, Voragen A G J, (Eds.), Xylans and Xylanases, Elsevier, Amsterdam; 1992.
- 67. Bedford M, Classen H. The influence of dietary xylanase on intestinal viscosity and molecular weight distribution of carbohydrates in rye-fed broiler chick, in: J. Visser, G. Beldman, M. VanSomeren, A. Voragen (Eds.), Xylans and Xylanases, Elsevier, Amsterdam; 1992.
- 68. Dervilly-pinel G, Saulnier L. Experimental evidence for a semi-flexible conformation 2001;330: 365–372.
- 69. Dervilly G, Leclercq C, Zimmermann D, Roue C, Thibault J, Saulnier L. Isolation and characterization of high molar mass water-soluble arabinoxylans from barley and barley malt. Carbohydr. Polym 2002;47: 143–149.
- 70. Roberfroid M B. Health benefits of non-digestible oligosaccharides. Advances in Experimental Medicine and Biology 1997;427: 211–219.
- 71. Collins M D, Gibson G R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. The American Journal of Clinical Nutrition 1999;69: 1052S–1057S.
- 72. Vázquez M, Alonso J, Domı́nguez H, Parajó J. Xylooligosaccharides: manufacture and applications. Trends in Food Science & Technology 2000;11: 387–393.
- 73. Voragen A G J. Technological aspects of functional carbohydrates. Trends in Food Science & Technology 1998;9: 328–335.
- 74. Akpinar O, Erdogan K, Bostanci S. Enzymatic production of Xylooligosaccharide from selected agricultural wastes. Food and Bioproducts Processing 2009;87: 145–151.
- 75. Vázquez M, Alonso J, Domínguez H, Parajó J. Enzymatic processing of crude xylooligomer solutions obtained by autohydrolysis of eucalyptus wood. Food Biotechnology 2002;16: 91–105.
- 76. Bailey M J, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnology 1992;23: 257–271.
- 77. Biely P, Mislovicová D, Toman R. Soluble chromatogenic substrates for the assay of endo-1,4-β-xylanases and endo-1,4-β-glucanases. Analytical Biochemistry 1985;144: 142–146.
- 78. Qinnghe C, Xiaoyu Y, Tiangui N, Cheng J, Qiugang M. The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Process Biochemistry 2004;39: 1561–1566.
- 79. Elegir G, Szakács G, Jeffries T W. Purification, Characterization, and Substrate Specificities of Multiple Xylanases from Streptomyces sp. Strain B-12-2. Applied and Environmental Microbiology 1994;60: 2609–2615.
- 80. Wubah D A, Akin D E, Borneman W S. Biology, fiber-degradation, and enzymology of anaerobic zoosporic fungi. Critical Reviews in Microbiology 1993;19: 99–115.
- 81. Matte A, Forsberg C W. Purification, characterization, and mode of action of endoxylanases 1 and 2 from Fibrobacter succinogenes S85. Applied and Environmental Microbiology 1992;58: 157–168.
- 82. Prade R A. Xylanases: from biology to biotechnology. Biotechnology & Genetic Engineering Reviews 1996;13: 101–131.
- 83. Krause D O, Denman S E, Mackie R I, Morrison M, Rae A L, Attwood G T. Opportunities to improve fiber degradation in the rumen: microbiology, ecology, and genomics. FEMS Microbiology Reviews 2003;27: 663–693.
- 84. Lebeda A, Luhova L, Sedlarova M, Jancova D. The role of enzymes in plant-fungal pathogens interactions. Zeitschrift Für Pflanzenkrankheiten Und Pflanzenschutz 2001;108: 89–111.
- 85. Viikari L, Ranua M, Kantelinen A, Sundiquist J, Linko M. Biotechnology in the pulp and paper industry, in: Bleaching with Enzymes, Proc. 3rd Int. Con. Stockholm; 1986.
- 86. Esteban R, Villanueva I R, Villa T G. β-D-Xylanases of Bacillus circulans WL-12, Can. J. Microbiol. 1982;28: 733–739.
- 87. Assamoi A A, Destain J, Thonart P. Xylanase Production by Penicillium canescens on Soya Oil Cake in Solid-State Fermentation. Applied Biochemistry and Biotechnology 2010; 160:50-62.
- 88. Coman G, Bahrim G. Optimization of xylanase production by Streptomyces sp . P12-137 using response surface methodology and central composite design. Annals of Microbiology 2011;61: 773–779.
- 89. Ge Y, Lili H, Fuliang Z. Study on the Solid-state Fermentation Conditions for Producing Thermostable Xylanase Feed in a Pressure Pulsation Bioreactor. Advanced Materials Research 2011;236-238: 72–76.
- 90. Palaniswamy M, Vaikuntavasan B, Ramaswamy P. Isolation, identification and screening of potential xylanolytic enzyme from litter degrading fungi. African Journal of Biotechnology 2008;7: 1978–1982.
- 91. Knob A, Carmona E C. Xylanase Production by Penicillium sclerotiorum and its Characterization. World Applied Sciences Journal 2008; 4: 277–283.
- 92. Pandey P, Pandey A K. Production of cellulase-free thermostable xylanases by an isolated strain of Aspergillus niger PPI utilizing various lignocellulosic wastes. World Journal of Microbiology and Biotechnology 2002;18: 281–283.
- 93. Dijkerman R, Ledeboer J, Camp H J M O D, Prins R A, Drift C V D. The Anaerobic Fungus Neocallimastix sp. Strain L2: Growth and Production of (Hemi)Cellulolytic Enzymes on a Range of Carbohydrate Substrates. Current Microbiology 1997;34: 91–96.
- 94. Arabi M I E, Jawhar M, Bakri Y. Effect of Additional Carbon Source and Moisture Level on Xylanase Production by Cochliobolus sativus in Solid Fermentation. Microbiology 2011;80: 150–153.
- 95. Bocchini D A, Oliveira O M M, Gomes E, Silva R D. Use of sugarcane bagasse and grass hydrolysates as carbon sources for xylanase production by Bacillus circulans D1 in submerged fermentation. Process Biochemistry 2005;40: 3653–3659.
- 96. Rawashdeh R, Saadoun I, Mahasneh A. Effect of cultural conditions on xylanase production by Streptomyces sp . (strain Ib 24D) and its potential to utilize tomato pomace. African Journal of Biotechnology 2005;4: 251–255.
- 97. Yang S Q, Yan Q J, Jiang Z Q, Li L T, Tian H M, Wang Y Z. High-level of xylanase production by the thermophilic Paecilomyces themophila J18 on wheat straw in solid-state fermentation. Bioresource Technology 2006;97: 1794–800.
- 98. Nagar S, Gupta V K, Kumar D, Kumar L, Kuhad R C. Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. Journal of Industrial Microbiology & Biotechnology 2010;37: 71–83.
- 99. Adsul M G, Ghule J E, Shaikh H, Singh R, Bastawde K B, Gokhale D V. Enzymatic hydrolysis of delignified bagasse polysaccharides. Carbohydrate Polymers 2005;62: 6–10.
- 100. Buswell J A, Chang S T. Biomass and extracellular hydrolytic enzyme production by six mushroom species grown on soybean waste. Biotechnology Letters 1994;16: 1317–1322.
- 101. Castanares A, Hay A J, Gordon A H, McCrae S I, Wood T M. D-Xylan-degrading enzyme system from the fungus Phanerochaete chrysosporium : Isolation and partial characterisation of an α-(4-O-methyl)-D-glucuronidase. Journal of Biotechnology 1995;43: 183-194
- 102. Abd El-Nasser N H, Helmy S M, El-Gammal A A. Formation of enzymes by biodegradation of agricultural wastes with white rot fungi. Polymer Degradation and Stability 1997;55: 249–255.
- 103. de Souza-Cruz P B, Freer J, Siika-Aho M, Ferraz A. Extraction and determination of enzymes produced by Ceriporiopsis subvermispora during biopulping of Pinus taeda wood chips. Enzyme and Microbial Technology 2004;34: 228–234.
- 104. Steiner W, Lafferty R M, Gomes I, Esterbauer H. Studies on a wild type strain of Schizophyllum commune : Cellulase and xylanase production and formation of the extracellular polysaccharide schizophyllan. Biotechnol. Bioeng. 1987;30: 169–178.
- 105. Gilbert H J, Hazlewood G P. Bacterial cellulases and xylanases. J Gen Microbiol. 1993;139: 187–194.
- 106. Biely P. Biotechnological potential and production of xylanolytic systems free of cellulases, ACS Symp. Ser. 1991;460: 408–416.
- 107. Gomes J, Gomes I, Steiner W. Thermolabile xylanase of the Antarctic yeast Cryptococcus adeliae : production and properties. Extremophiles 2000;4: 227–235.
- 108. Morales P, Sendra J M, Perez-Gonzalez J A. Purification and characterisation of an arabinofuranosidase from Bacillus polymyxa expressed in Bacillus subtilis . Appl. Microbiol. Biotechnol. 1995;44: 112–117.
- 109. Khasin A, Alchanati I, Shoham Y. Purification and characterization of a thermostable xylanase from Bacillus stearothermophilus T-6. Appl. Environ. Microbiol. 1993;59: 1725–1730.
- 110. Yoon H, Han N S, Kim C H. Expression of Thermotoga maritima Endo-β-1,4-xylanase Gene in E. coli and Characterization of the Recombinant Enzyme. Agric. Chem. Biotechnol. 2004;47: 157–160.
- 111. Pandey A, Selvakumar P, Soccol C R, Nigam P. Solid state fermentation for the production of industrial enzymes. Current Science 1999;77: 149–162.
- 112. Considine P J, Coughlan M P. Enzyme System for Lignocellulose Degradation, in: M.P. Coughlan (Ed.), Enzyme System for Lignocellulose Degradation, Elsevier Applied Science, London; 1989.
- 113. Corral, O L, Villaseñor-Ortega F. Xylanases. Advances in Agricultural and Food Biotechnology 2006;305-322.
- 114. Frost G M, Moss D M. Production of enzymes by fermentation, in: Rehm H J, Reed G (eds.,) Biotechnology; 1987.
- 115. Garcia-Kirchner O, Muñoz-Aguilar M, Pérez-Villalva R, Huitrón-Vargas C. Mixed submerged fermentation with two filamentous fungi for cellulolytic and xylanolytic enzyme production. Applied Biochemistry and Biotechnology 2002;1105–1114.
- 116. Qiu Z, Shi P, Luo H, Bai Y, Yuan T, Yang P. A xylanase with broad pH and temperature adaptability from Streptomyces megasporus DSM 41476, and its potential application in brewing industry. Enzyme and Microbial Technology 2010;46: 506–512.
- 117. Jhamb K, Sahoo D K. Production of soluble recombinant proteins in Escherichia coli : effects of process conditions and chaperone co-expression on cell growth and production of xylanase. Bioresource Technology 2012; doi: http://dx.doi.org/10.1016/j.biortech.2012.07.011.
- 118. Bron S, Bolhuis A, Tjalsma H, Holsappel S, Venema G, van Dijl J. Protein secretion and possible roles for multiple signal peptidases for precursor processing in bacilli. Journal of Biotechnology 1998;64: 3–13.
- 119. Upreti R K, Kumar M, Shankar V. Review Bacterial glycoproteins: Functions, biosynthesis and applications. Proteomics 2003;3: 363–379.
- 120. Nevalainen K M H, Te’o V S J, Bergquist P L. Heterologous protein expression in filamentous fungi. Trends in Biotechnology 2005;23: 468–74.
© 2013 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Sustainable degradation of lignocellulosic biomass.
Edited by Anuj Chandel
Published: 15 May 2013
By Zhijia Liu and Benhua Fei
3389 downloads
By Larissa Canilha, Rita de Cássia Lacerda Brambilla ...
5220 downloads
By Ayla Sant’Ana da Silva, Ricardo Sposina Sobral Tei...
5487 downloads
IntechOpen Author/Editor? To get your discount, log in .
Discounts available on purchase of multiple copies. View rates
Local taxes (VAT) are calculated in later steps, if applicable.
Support: [email protected]
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Awards & Prizes
- Thematic Issues
- Virtual Special Issues
- FEMS Journals
- FEMS Microbiology Ecology
- FEMS Microbiology Letters
- FEMS Yeast Research
- Pathogens and Disease
- FEMS Microbes
- Author Guidelines
- Submission Site
- Open Access
- Calls for Papers
- About FEMS Microbiology Reviews
- About the Federation of European Microbiological Societies
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
1 introduction, 2 the substrate: xylan, 3 xylanases: multiplicity and multiple-domains, 4 classification of xylanases, 5 glycoside hydrolase families 5, 7, 8, 10, 11 and 43, 6 extremophilic xylanases, 7 application of xylanases: extremophilic xylanases, 8 concluding remarks, acknowledgements.
- < Previous
Xylanases, xylanase families and extremophilic xylanases
- Article contents
- Figures & tables
- Supplementary Data
Tony Collins, Charles Gerday, Georges Feller, Xylanases, xylanase families and extremophilic xylanases, FEMS Microbiology Reviews , Volume 29, Issue 1, January 2005, Pages 3–23, https://doi.org/10.1016/j.femsre.2004.06.005
- Permissions Icon Permissions
Xylanases are hydrolytic enzymes which randomly cleave the β 1,4 backbone of the complex plant cell wall polysaccharide xylan. Diverse forms of these enzymes exist, displaying varying folds, mechanisms of action, substrate specificities, hydrolytic activities (yields, rates and products) and physicochemical characteristics. Research has mainly focused on only two of the xylanase containing glycoside hydrolase families, namely families 10 and 11, yet enzymes with xylanase activity belonging to families 5, 7, 8 and 43 have also been identified and studied, albeit to a lesser extent. Driven by industrial demands for enzymes that can operate under process conditions, a number of extremophilic xylanases have been isolated, in particular those from thermophiles, alkaliphiles and acidiphiles, while little attention has been paid to cold-adapted xylanases. Here, the diverse physicochemical and functional characteristics, as well as the folds and mechanisms of action of all six xylanase containing families will be discussed. The adaptation strategies of the extremophilic xylanases isolated to date and the potential industrial applications of these enzymes will also be presented.
Xylanases are glycosidases ( O -glycoside hydrolases, EC 3.2.1.x) which catalyze the endohydrolysis of 1,4-β- d -xylosidic linkages in xylan. They are a widespread group of enzymes, involved in the production of xylose, a primary carbon source for cell metabolism and in plant cell infection by plant pathogens, and are produced by a plethora of organisms including bacteria, algae, fungi, protozoa, gastropods and anthropods [1] . First reported in 1955 [2] , they were originally termed pentosanases, and were recognized by the International Union of Biochemistry and Molecular Biology (IUBMB) in 1961 when they were assigned the enzyme code EC 3.2.1.8. Their official name is endo-1,4-β-xylanase, but commonly used synonymous terms include xylanase, endoxylanase, 1,4-β- d -xylan-xylanohydrolase, endo-1,4-β- d -xylanase, β-1,4-xylanase and β-xylanase.
In the present review, the diversity of xylanases, their substrate, action and function, their importance in industry, classification into families and adaptation to various extreme environments are discussed. Special emphasis is paid to the ‘new’ xylanase containing families, highlighting their similarities and differences to the better known family 10 and 11 members, as well as to the peculiarities and interests of these hitherto scantily reviewed enzymes. In addition, the adaptation strategies, characteristics and industrial potential of extremophilic xylanases will be discussed.
The substrate of xylanases, xylan, is a major structural polysaccharide in plant cells, and is the second most abundant polysaccharide in nature, accounting for approximately one-third of all renewable organic carbon on earth [1] . Xylan constitutes the major component of hemicellulose; a complex of polymeric carbohydrates including xylan, xyloglucan (heteropolymer of d -xylose and d -glucose), glucomannan (heteropolymer of d -glucose and d -mannose), galactoglucomannan (heteropolymer of d -galactose, d -glucose and d -mannose) and arabinogalactan (heteropolymer of d -galactose and arabinose) [3] . This, together with cellulose (1,4-β-glucan) and lignin (a complex polyphenolic compound) make up the major polymeric constituents of plant cell walls [4] . Within the cell wall structure, all three constituents interact via covalent and non-covalent linkages, with the xylan being found at the interface between the lignin and cellulose where it is believed to be important for fiber cohesion and plant cell wall integrity [5] .
Xylan is found in large quantities in hardwoods from angiosperms (15–30% of the cell wall content) and softwoods from gymnosperms (7–10%), as well as in annual plants (<30%) [6] . It is typically located in the secondary cell wall of plants, but is also found in the primary cell wall, in particular in monocots [7] . A complex, highly branched heteropolysaccharide, it varies in structure between different plant species, and the homopolymeric backbone chain of 1,4-linked β- d -xylopyranosyl units can be substituted to varying degrees with glucuronopyranosyl, 4- O -methyl- d -glucuronopyranosyl, α- l -arabinofuranosyl, acetyl, feruloyl and/or p -coumaroyl side-chain groups [ 4 , 8 ] ( Fig. 1 ). Wood xylan exists as O -acetyl-4- O -methylglucuronoxylan in hardwoods and as arabino-4- O -methylglucuronoxylan in softwoods, while xylans in grasses and annual plants are typically arabinoxylans [4] . Linear unsubstituted xylan has also been reported, e.g., in esparto grass [9] , tobacco [10] and certain marine algae [ 11 , 12 ], with the latter containing xylopyranosyl residues linked by both 1,3-β and 1,4-β linkages [ 12 , 13 ]. The degree of polymerisation in xylans is also variable, with, for example, hardwood and softwood xylans generally consisting of 150–200 and 70–130 β-xylopyranose residues, respectively [4] .
![literature review xylanase (a) Structure of xylan and the sites of its attack by xylanolytic enzymes. The backbone of the substrate is composed of 1,4- β-linked xylose residues. Ac., Acetyl group; α-araf., α-arabinofuranose; α-4-O-Me-GlcUA, α-4-O-methylglucuronic acid; pcou., p-coumaric acid; fer., ferulic acid. (b) Hydrolysis of xylo-oligosaccharide by β-xylosidase. Adapted from [18]. Figures were prepared with CS Chemdraw Ultra version 6.0.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/29/1/10.1016_j.femsre.2004.06.005/1/m_FMR_3_f1.jpeg?Expires=1726711691&Signature=W-nxJ7dcMccOS~iAt9yyEPoetQsGSXnmR5Rk2JYSaA-LUXFm-DSJ-~77TWQbqVVhWsAdfYlMkpGWkyZ3wq5cuT9M1QblSFzXprcCS86uaSSd04QA3jqtd6DpLRVmow7rmudxMPBrayLD7CqHbMpsCeC9Ime60UlNnjPnaYNs3mzEYFu-IWm9VJVBxsAU8QpBVY~~MgXhTMFMnYKPtELz1DTHUDhR2l-8GEJEzpE3e5RcpZiLfGFBXmkpN57WupLsjklHwrrj01V-oIetxmhVny6IyBs5KSIouYqBFzchr28p7tVBgZ~vd0Ex6QWbZSrKhYfvnwLPwypbQ0UB7FkOUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(a) Structure of xylan and the sites of its attack by xylanolytic enzymes. The backbone of the substrate is composed of 1,4- β-linked xylose residues. Ac., Acetyl group; α-araf., α-arabinofuranose; α-4- O -Me-GlcUA, α-4- O -methylglucuronic acid; pcou., p -coumaric acid; fer., ferulic acid. (b) Hydrolysis of xylo-oligosaccharide by β-xylosidase. Adapted from [18] . Figures were prepared with CS Chemdraw Ultra version 6.0.
Due to its heterogeneity and complexity, the complete hydrolysis of xylan requires a large variety of cooperatively acting enzymes [ 14–16 ] ( Fig. 1 ). Endo-1,4-β- d -xylanases (EC 3.2.1.8) randomly cleave the xylan backbone, β- d -xylosidases (EC 3.2.1.37) cleave xylose monomers from the non-reducing end of xylo-oligosaccharides and xylobiose while removal of the side groups is catalysed by α- l -arabinofuranosidases (EC 3.2.1.55), α- d -glucuronidases (EC 3.2.1.139), acetylxylan esterases (EC 3.1.1.72), ferulic acid esterases (EC 3.1.1.73) and p -coumaric acid esterases (EC 3.1.1.-). Indeed, complete xylanolytic enzyme systems, including all of these activities, have been found to be quite widespread among fungi [ 17 , 18 ], actinomycetes [19] and bacteria [18] , and some of the most important xylanolytic enzyme producers include the Aspergilli, Trichodermi, Streptomycetes, Phanerochaetes, Chytridiomycetes, Ruminococci, Fibrobacteres, Clostridia and Bacilli [ 16 , 18 , 20 , 21 ]. The ecological niches of these micro-organisms are diverse and widespread and typically include environments where plant material accumulate and deteriorate, as well as in the rumen of ruminants [ 1 , 16 , 22 ].
In addition to the production of a variety of xylanolytic enzymes, many micro-organisms produce multiple xylanases [ 23–25 ]. These may have diverse physicochemical properties, structures, specific activities and yields, as well as overlapping but dissimilar specificities, thereby increasing the efficiency and extent of hydrolysis, but also the diversity and complexity of the enzymes. Typical examples of micro-organisms which produce xylanase isoenzymes include Aspergillus niger , which produces fifteen extracellular xylanases [26] , and Trichoderma viride , which secretes thirteen [26] . This multiplicity may be the result of genetic redundancy [7] , but cases of differential post-translational processing have also been reported [15] . The isoenzyme genes may be found as polycistronic or non-polycistronic multiple copies within the genome, and in some cases several xylanases are expressed as a distinct gene product. For example, the xylanase, β-xylosidase and acetyl esterase genes of Caldocellum saccharolyticum (now known as Caldocellulosiruptor saccharolyticus ) are polycistronic [27] , while the XynC gene product from Fibrobacter succinogenes S85 encodes two different xylanase catalytic domains [28] . Moreover, as well as multiple catalytic domains, many xylanases are also characterized by the presence of various supplementary domains. Examples include xylan binding domains [29] , cellulose binding domains [ 30 , 31 ], dockerin domains (implicated in binding to multidomain complexes produced by certain micro-organism, e.g., Clostridium thermocellum ) [ 32 , 33 ], thermostabilising domains [34] and domains for which the function has not as yet been elucidated. These domains may fold and function in an independent manner [ 35 , 36 ] and are typically separated by short junction segments enriched in hydroxyl amino acids [4] .
The vast majority of xylanases are excreted into the extracellular environment as the large size of the substrate prevents its penetration into the cell. In fact, the current belief is that xylanase production is induced by means of the products of their own action [ 6 , 15 , 37 ]. It is believed that small amounts of constitutively produced enzymes liberate xylo-oligomers which may be transported into the cell where they are further degraded by β-xylosidases, or indeed by intracellular xylanases [ 38–40 ], and where they induce further xylanase synthesis.
The heterogeneity and complexity of xylan has resulted in an abundance of diverse xylanases with varying specificities, primary sequences and folds, and hence has lead to limitations with the classification of these enzymes by substrate specificity alone. Wong et al. [7] classified xylanases on the basis of their physicochemical properties and proposed two groups: those with a low molecular weight (<30 kDa) and basic p I , and those with a high molecular weight (>30 kDa) and acidic p I . However, several exceptions to this pattern have been found [ 18 , 21 ] and approximately 30% of presently identified xylanases, in particular fungal xylanases, cannot be classified by this system.
Later, a more complete classification system was introduced [41] which allowed the classification of not only xylanases, but glycosidases in general (EC 3.2.1.x), and which has now become the standard means for the classification of these enzymes. This system is based on primary structure comparisons of the catalytic domains only and groups enzymes in families of related sequences [42] . The initial classification grouped cellulases and xylanases into 6 families (A–F) [41] , which was updated to 77 families in 1999 (1–77) [42] and which continues to grow as new glycosidase sequences are identified. At the time of writing, 96 glycoside hydrolase families exist (see the carbohydrate–active enzyme CAZY server at http://afmb.cnrs-mrs.fr/˜cazy/CAZY/ [43] ), with approximately one-third of these families being polyspecific, i.e., contain enzymes with diverse substrate specificities. As the structure and molecular mechanism of an enzyme are related to its primary structure, this classification system reflects both structural and mechanistic features. Enzymes within a particular family have a similar three-dimensional structure [42] and similar molecular mechanism [44] and it has also been suggested that they may have a similar specificity of action on small, soluble, synthetic substrates [45] . Furthermore, divergent evolution has resulted in some of the families having related three-dimensional structures and thus the grouping of families into higher hierarchical levels, known as clans, has been introduced [46] . Presently, 14 different clans have been proposed (GH-A to GH-N), with most clans encompassing two to three families, apart from clan GH-A which currently encompasses 17 families.
Within this classification system, xylanases are normally reported as being confined to families 10 (formerly F) and 11 (formerly G) [ 6 , 16 , 18 , 47–49 ]. Interestingly, a search of the appropriate databases (e.g., CAZY [43] ) using the enzyme classification number EC 3.2.1.8 indicates that enzymes with xylanase activity are also found in families 5, 7, 8, 16, 26, 43, 52 and 62. A closer look at the available literature however, shows that only those sequences classified in families 5, 7, 8, 10, 11 and 43 ( Table 1 ) contain truly distinct catalytic domains with a demonstrated endo-1,4-β-xylanase activity. Those sequences reported for families 16, 52 and 62 appear, in fact, to be bifunctional enzymes containing two catalytic domains; a family 10 or 11 xylanase domain as well as a second glycosidase domain. For example, a Ruminococcus flavefaciens enzyme contains an amino-terminal family 11 xylanase and a carboxy-terminal family 16 lichenase and is thus classified in both families 11 and 16 [50] . In addition, those enzymes classified in family 26 appear not to be endo-1,4-β-xylanases, but endo-1,3-β-xylanases. Thus, the current view that enzymes with xylanase activity are solely restricted to families 10 and 11 is not entirely correct and should be expanded to include families 5, 7, 8 and 43.
Glycoside hydrolase families containing enzymes with a demonstrated activity on xylan. The fold, mechanism of action and catalytic residues characteristic to each family are given
| Glycoside hydrolase family | Members with a demonstrated activity on xylan | Fold | Clan | Catalytic mechanism | General acid/base residue | Nucleophile/general base |
| 5 | 8 | (β/α) | GH-A | Retaining | Glutamate | Glutamate |
| 7 | 1 | β-Jelly roll | GH-B | Retaining | Glutamate | Glutamate |
| 8 | 4 | (α/α) | GH-M | Inverting | Glutamate | Aspartate |
| 10 | 127 | (β/α) | GH-A | Retaining | Glutamate | Glutamate |
| 11 | 173 | β-Jelly roll | GH-C | Retaining | Glutamate | Glutamate |
| 43 | 1 | 5-Blade β-propeller | GH-F | Inverting | Glutamate | Aspartate |
| Glycoside hydrolase family | Members with a demonstrated activity on xylan | Fold | Clan | Catalytic mechanism | General acid/base residue | Nucleophile/general base |
| 5 | 8 | (β/α) | GH-A | Retaining | Glutamate | Glutamate |
| 7 | 1 | β-Jelly roll | GH-B | Retaining | Glutamate | Glutamate |
| 8 | 4 | (α/α) | GH-M | Inverting | Glutamate | Aspartate |
| 10 | 127 | (β/α) | GH-A | Retaining | Glutamate | Glutamate |
| 11 | 173 | β-Jelly roll | GH-C | Retaining | Glutamate | Glutamate |
| 43 | 1 | 5-Blade β-propeller | GH-F | Inverting | Glutamate | Aspartate |
a Putative catalytic residues only, these have not been conclusively confirmed.
5.1 Catalytic mechanisms
Members of families 5, 7, 8, 10, 11 and 43 differ in their physico-chemical properties, structure, mode of action and substrate specificities. Similarities do however exist, for example, families 5 and 10 are both classified in clan GH-A, thus indicating a similar three-dimensional fold. Furthermore, families 5, 7, 10 and 11 contain enzymes which catalyse hydrolysis with retention of anomeric configuration with two glutamate residues being implicated in the catalytic mechanism in all cases [43] . This indicates a double-displacement mechanism, in which a covalent glycosyl-enzyme intermediate is formed and subsequently hydrolysed via oxocarbenium-ion-like transition states [ 51–53 ] ( Fig. 2(a) ). Two carboxylic acid residues suitably located in the active site (approximately 5.5 Å apart) are involved in the formation of the intermediate; one acts as a general acid catalyst by protonating the substrate, while the second performs a nucleophilic attack which results in the departure of the leaving group and the formation of the α-glycosyl enzyme intermediate (inversion β to α). In the second step, the first carboxylate group now functions as a general base, abstracting a proton from a nucleophilic water molecule which attacks the anomeric carbon. This leads to a second substitution in which the anomeric carbon again passes via an oxocarbenium-ion-like transition state to give rise to a product with the β configuration (inversion α to β). Thus the overall result is a retention of the configuration at the anomeric centre.
![literature review xylanase General mechanisms for (a) retaining and (b) inverting glycosidases. Adapted from [51]. Figures were prepared with CS Chemdraw Ultra version 6.0.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/29/1/10.1016_j.femsre.2004.06.005/1/m_FMR_3_f2.jpeg?Expires=1726711691&Signature=WZH9KCf55KWhZT8szfnlcCYziRZIICMFAzZL6Yb7vzbgVOQq8hpZjfZcuJJihTWrA4Q2wCPec-FRvaIiU3nZiehoQ3ViWq7IE550m4BcUIkM~iZvAI~Vzyj4ZB1vsPGeLtP10KcCbnMActDpAnF8qxAIft~QcB6yVKUwgDQc9naEesnX9ja7YjNcygyWGJgdgVYb56m5LUatKa6oZkSuJ75gU~IH5VyfajJk-HE~aikDq~sKJjjD4WWbDCqOs7Jdy3IsYSvcP1NBr-kGKbxd6bEkqYVbXhKXfP-SDpD5-z02eJtG5FrQBzoAtzQBdvFlru6CEPWEa019wG~okHAwbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
General mechanisms for (a) retaining and (b) inverting glycosidases. Adapted from [51] . Figures were prepared with CS Chemdraw Ultra version 6.0.
In contrast, enzymes in families 8 and 43 typically operate with inversion of the anomeric centre and a glutamate and aspartate are believed to be the catalytic residues [ 43 , 54 ]. Inverting enzymes function by a single displacement reaction ( Fig. 2(b) ) in which one carboxylate provides for a general acid-catalyzed leaving group departure and the second functions as a general base, activating a nucleophilic water molecule to attack the anomeric carbon, thereby cleaving the glycosidic bond and leading to an inversion of the configuration at the anomeric carbon [ 51–53 ]. Typically the distance between the two residues is around 9.5 Å so as to allow for accommodation of the water molecule between the anomeric carbon and the general base [ 51–53 ]. However, Alzari et al. [55] and Guérin et al. [56] have shown that this distance is 7.5 Å in the inverting endoglucanase CelA and have suggested that the distance between the two catalytic residues is less constrained in inverting than in retaining enzymes.
5.2 Glycoside hydrolase family 5
At the time of writing, family 5 (formerly family A) of the glycoside hydrolases consists of 467 sequences with varying activities, including: endoglycosylceramidase (EC 3.2.1.123), cellulase (EC 3.2.1.4), licheninase (EC 3.2.1.73), β-mannosidase (EC 3.2.1.25), glucan 1,3-β-glucosidase (EC 3.2.1.58), glucan endo-1,6-β-glucosidase (EC 3.2.1.75), mannan endo-1,4-β-mannosidase (EC 3.2.1.58), cellulose 1,4-β-cellobiosidase (EC 3.2.1.91), endo-1,6-β-galactanase (EC 3.2.1.-), 1,3-β-mannanase (EC 3.2.1.-) and endo-1,4-β-xylanase (EC 3.2.1.8) [43] . This is the largest glycoside hydrolase family and only seven amino acid residues, including the nucleophile and the general acid/base residue, are strictly conserved among all members. It is a rather diverse group of enzymes, with structural alignments indicating rms deviations of 1.25 ± 0.12 Å between equivalent residues in its members [57] and a further classification of this family into nine subfamilies has even been suggested [58] .
Eight enzymes with a demonstrated activity on xylan have been reported in this family to date ( Table 2 ) and a number of other putative xylanases, identified by sequence similarity during genome sequencing programs, have been identified in Clostridium acetobutylicum ATCC 824 (2 putative xylanases) [59] , Leptosphaeria maculans [43] , Xanthomonas axonopodis pv. citri str. 306 (2 putative xylanases) [60] , Xanthomonas campestris pv. campestris str. ATCC 33913 [60] , Bacillus subtilis str. 168 [61] and Bacteroides thetaiotaomicron VPI-5482 [62] . Xylanase activity has also been apparently demonstrated for enzymes from Aeromonas puncata ( caviae ) W-61, Erwinia ( Pectobacterium ) chrysanthemi P860219, Meloidogyne incognita and Ruminococcus albus 7 [43] but it appears that no literature has been published in direct relation to these. In total, 20 putative xylanolytic entries can be proposed.
Organisms producing family 5 enzymes with a demonstrated activity on xylan
| Organism | Reference |
| ( ) 23 | |
| S85 | |
| SY3 | |
| ( ) | [ , ] |
| ( ) D1 | [ , , ] |
| ( ) SR120A | [ , , ] |
| ( ) ME-1 |
| Organism | Reference |
| ( ) 23 | |
| S85 | |
| SY3 | |
| ( ) | [ , ] |
| ( ) D1 | [ , , ] |
| ( ) SR120A | [ , , ] |
| ( ) ME-1 |
Although characterisation of these enzymes is incomplete, large variations in the catalytic properties are already evident, this being in keeping with the poor sequence identity between some of these enzymes. Those enzymes from Prevotella ruminicola 23 [63] , Clostridium cellulovorans [64] , F. succinogenes S85 [65] and R. albus SY3 [66] were all found to have carboxymethyl cellulase as well as xylanase activities, while those from Trichoderma reesei (also known as Hypocrea jecorina ) [ 67 , 68 ], Erwinia chrysanthemi D1 [ 57 , 69–71 ] and E. chrysanthemi SR120A [69] appear to be specific for xylan. XynD from Aeromonas punctata ME-1 was found to be active on xylan but was not apparently tested on other substrates [72] . Furthermore, a search of the InterPRO database ( http://www.ebi.ac.uk/interpro , [73] ) indicates that only the four carboxymethyl cellulose active enzymes (i.e., those from P. ruminicola 23, C. cellulovorans, F. succinogenes S85 and R. albus SY3) give hits with family 5 signatures; the remaining 16 sequences give hits with family 30 or with other non-glycoside hydrolase family signatures. Moreover, these four enzymes have relatively high amino acid sequence similarity with family 5 members, while the majority of the remaining sequences have homology with both family 5 and family 30 enzymes. This has already been noted for the E. chrysanthemi and A. punctata ME-1 enzymes where it was suggested that these enzymes be classified into a new family (or subfamily) situated between families 5 and 30 [ 70 , 72 ]. In addition, structural analysis of the family 5 xylanase, XynA from E. chrysanthemi showed that, as would be expected for a family 5 enzyme, the catalytic domain displayed a common (β/α) 8 barrel fold ( Fig. 3(a) ) [57] . However, while the β-barrels aligned well with those of another family 5 enzyme, the α-helices and loops were altered, showing differences in the positioning, orientation and length. Moreover, structural alignment with a number of family 5 and 10 enzymes showed that this enzyme is nearly as structurally different to the family 5 enzymes as are the family 10 enzymes [57] .
![literature review xylanase Representative structures of enzymes from various glycoside hydrolase families. (a) Structure of the family 5 enzyme, XynA, from Erwinia chrysanthemi. The (β/α)8 barrel structure of the catalytic domain and the β9-barrel of the small domain are shown [57]. (b) Structure of the family 8 xylanase, pXyl, from Pseudoalteromonas haloplanktis TAH3a. The (α/α)6 barrel structure of the catalytic domain is shown in two perpendicular views [82]. (c) Structure of the Streptomyces lividans xylanase showing the typical family 10-fold [95]. (d) Structure of the Trichoderma reesei family 11 xylanase showing the typical family 11-fold [127]. (e) Structure of the Trichoderma reesei family 7 non-specific EGI [131]. (f) Structure of the Cellvibrio japonicus family 43 α-l-arabinanase [54]. Figures were prepared with Swiss-Pdb Viewer v3.7b2 [205].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/29/1/10.1016_j.femsre.2004.06.005/1/m_FMR_3_f3.jpeg?Expires=1726711691&Signature=vSuW2v1Xr~Hofi~Cf-UNDwWqKhDYG0bJmJX9pvUjaWqgUdgIFEQ0tJVHbnQge9cw0viHbvE~sc9Su7B0GqGYJptojzOB6D4EjHbQNZYe7ypGKpi4T8ls2CHEQPuIGkpbJMwRdWpj2NHlwxrzKtzFUW00WOEDurtvkpsbmDUF5fcHBtnWNtlUv1LXu0DGtM0ZjcRb2YFmfdZGf~jJio15aFMezyob1zVN1PI5n0EDq9GdFD2xEfESRPEnSVz1kMqrXatIaURJZv5UlpoZmDoH0sT-N3j~i3yZLsb1pvhTi~qfpT-h0tPkIonel-u4xrJlQAVmKr2SQH-WQRN2uNe9qA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Representative structures of enzymes from various glycoside hydrolase families. (a) Structure of the family 5 enzyme, XynA, from Erwinia chrysanthemi . The (β/α) 8 barrel structure of the catalytic domain and the β 9 -barrel of the small domain are shown [57] . (b) Structure of the family 8 xylanase, pXyl, from Pseudoalteromonas haloplanktis TAH3a. The (α/α) 6 barrel structure of the catalytic domain is shown in two perpendicular views [82] . (c) Structure of the Streptomyces lividans xylanase showing the typical family 10-fold [95] . (d) Structure of the Trichoderma reesei family 11 xylanase showing the typical family 11-fold [127] . (e) Structure of the Trichoderma reesei family 7 non-specific EGI [131] . (f) Structure of the Cellvibrio japonicus family 43 α- l -arabinanase [54] . Figures were prepared with Swiss-Pdb Viewer v3.7b2 [205] .
Of the four family 5 enzymes exhibiting carboxymethyl cellulase and xylanase activities, only the P. ruminicola 23 xylanase was found to have highest activity on xylan, with the carboxymethyl cellulase activity being only 18% of this [63] . In contrast, EngB from C. cellulovorans was found to be most active on lichenan, with approximately 15% activity on carboxymethylcellulose and 14% on xylan. No activity was detected on microcrystalline cellulose, avicel, polygalacturonic acid, mannan, laminaran, p -nitrophenyl β-cellobioside, p -nitrophenyl β- d -glucoside or p -nitrophenyl β- d -xyloside [64] . Unfortunately, as with the P. ruminicola 23 xylanase, the endoglucanase isolated from Fibrobacter succinogenes S85 was not tested with lichenan, this enzyme was found to have highest activity on carboxymethyl cellulose, as well as activity on oat spelt xylan (71% as compared to CMC), p -nitrophenyl cellobioside (5.3%), cellobiose (3.5%), p -nitrophenyl lactoside (2.27%) and p -nitrophenyl glucoside (0.27%) [65] .
The action patterns of the xylan specific family 5 enzymes with sequence similarities to family 30 enzymes also appear to vary from one to another. Only those enzymes from A. punctata ME-1 [72] , E. chrysanthemi D1 [ 57 , 70 , 71 ] and T. reesei [ 67 , 68 ] have been characterized in this respect, and while the first does appear to be an endoxylanase, producing xylotriose and higher xylo-oligosaccharides from birchwood xylan, the second appears to be a type of ‘appendage-dependent xylanase’ or glucuronoxylanase [74] and the third an exoxylanase. Substrate specificity studies showed that the xylanase from E. chrysanthemi D1 requires d -glucuronic acid (or its 4- O -methyl derivative) substituents on the xylan backbone to enable cleavage of 1,4-β xylosidic bonds and that it cleaves between the first and second xylose residues on the non-reducing terminal side of the substituted residue [71] . On the other hand, XYN IV from T. reesei is active on unsubstituted and substituted xylans and attacks at the first glycosidic linkage from the reducing end, producing mainly xylose, but also xylobiose and low amount of higher xylo-oligosaccharides [ 67 , 68 ]. Furthermore, the activity of this enzyme is affected by substituents on the xylan main chain and it is unable to cleave linkages next to substituted residues. Hydrolysis studies have shown that the shortest substituted fragments formed from glucuronoxylan and arabinoxylan are substituted xylotrioses, with the substitution being found on the internal xylose residue. Indeed these products are shorter than those produced by the family 7 EGI [75] produced by the same micro-organism as well as the family 11 enzymes, but, in agreement with members of this latter family and with the family 5 XynA from E. chrysanthemi D1 they contain a single xylose at the non-reducing end.
Of the above mentioned enzymes, only the structure of Xyn A from E. chrysanthemi has been determined (1.42 Å resolution) [57] while crystallisation and preliminary X-ray analysis (at 2.2 Å resolution) of the T. reesei XYN IV has also recently been reported [76] . Xyn A is composed of two domains, the larger domain contains the catalytic site and displays a (β/α) 8 barrel fold while the small domain probably functions as a xylan binding domain and has a β 9 -barrel fold ( Fig. 3(a) ). The two domains are connected by two linker peptides as well as 11 hydrogen bonds and hydrophobic interactions [57] . The β-barrel of the catalytic domain of Xyn A is elliptical in shape and the active site is formed by an acidic cleft situated on the carboxy-terminal side of the β-strands near the larger face of the molecule. This 8-fold α/β barrel structure is indeed the most frequently encountered fold and has also been described for members of families 1, 2, 10, 17, 26, 30, 35, 39, 42, 50, 51, 53, 59, 72, 79 and 86 [43] . This type of fold was originally described for triose-phosphate isomerase (TIM barrel) and as a common characteristic of all these structures is the positioning of the glutamate residues implicated in the catalysis on the carboxy-terminal of β-sheets 4 and 7, these were originally termed the 4/7 superfamily. Nowadays they are better known as clan GH-A.
5.3 Glycoside hydrolase family 8
Family 8 (formerly family D) is mainly composed of cellulases (EC 3.2.1.4), but also contains chitosanases (EC 3.2.1.132), lichenases (EC 3.2.1.73) and endo-1,4-β-xylanases (EC 3.2.1.8) [43] . It is a rapidly expanding family; from 18 members, including one xylanase, in 2001, to 61 members, including four xylanases, in 2004 ( Table 3 ). Three of the xylanases have been isolated from Bacillus sp. while the fourth is a cold-adapted enzyme isolated from the Antarctic bacterium Pseudoalteromonas haloplanktis TAH3a. Xylanase Y from the alkaliphile Bacillus halodurans C-125 was identified as part of the sequencing program of the whole genome of this organism [ 77 , 78 ] and the sequence of the xylanase from B. halodurans MIR32 is found to be identical to that for this enzyme [43] . Information is minimal and while we have demonstrated xylanase activity by these enzymes [79] , little literature has been published in direct relation to these two xylanases. Indeed the means used for their initial classification as xylanases is unclear and was probably only based on their weak isology to the cold-adapted xylanase and xylanase Y from Bacillus sp. KK-1. In contrast, the biochemical properties and substrate specificities of these latter two enzymes have been investigated [ 80–84 ]. Both have a high molecular weight (∼46 and ∼45 kDa, respectively) and while the cold-adapted xylanase also has a high p I (∼pH 9.5), this was not determined for the Bacillus sp. KK-1 xylanase. In addition, both were found to be optimally active at pH 6.5 and are solely active on xylan, being more active on birchwood than oat spelt xylan and being inactive on cellulose, carboxymethylcellulose, starch, lichenan and chitosan (this latter substrate was only tested for the cold-adapted xylanase). The cold-adapted xylanase was found to hydrolyse xylan to principally xylotriose and xylotetraose and was most active on long chain xylo-oligsaccharides. Similar to family 11 xylanases, a large substrate binding cleft containing at least six xylose binding residues, with the catalytic site in the middle, was proposed [80] . However, unlike family 10 and 11 xylanases this enzyme was found to catalyse hydrolysis with inversion of the anomeric configuration and, under the conditions used, was found to be inactive on aryl-β-glycosides of xylose, xylobiose and xylotriose.
Organisms producing family 8 enzymes with a demonstrated xylanase activity
| Organism | Reference |
| C-125 | [ , ] |
| MIR32 | |
| sp. KK-1 | |
| TAH3a | [ ] |
| Organism | Reference |
| C-125 | [ , ] |
| MIR32 | |
| sp. KK-1 | |
| TAH3a | [ ] |
This cold-adapted xylanase folds into a distorted (α/α) 6 barrel ( Fig. 3(b) ) formed by six inner and six outer α helices [ 82 , 83 ] and as such can be classified with family 48 in clan GH-M [43] . This topology has also been observed for family 9 endoglucanases, family 15 glucoamylases, family 48 cellobiohydrolases and a family 65 maltose phosphorylase [ 55 , 85 , 86 ]. However, in contrast to the common (α/α) 6 barrel proteins, the cold-adapted enzyme has an extra α-helix near the amino terminus. The globular core has an overall distorted spherical shape with a long acidic cleft running across the molecular surface at the N-terminal end of the inner helices while the proposed catalytic residues (glutamate and aspartate) are located close to each other near the middle of the cleft.
As the three-dimensional structure is believed to be comparable for members of the same family, the (α/α) 6 barrel fold described above can probably be extended to the other family 8 xylanases. Furthermore, one would expect these enzymes to operate with inversion of the anomeric configuration [ 80 , 87 ], with an aspartic and glutamic acid residue as proton acceptor and proton donor, respectively [ 56 , 83 ].
5.4 Glycoside hydrolase family 10
This family consists of endo-1,4-β-xylanases (EC 3.2.1.8), endo-1,3-β-xylanases (EC 3.2.1.32) and cellobiohydrolases (EC 3.2.1.91) [43] . The major enzymes of this family are endo-1,4-β-xylanases, however, substrate specificity studies have revealed that these may not be entirely specific for xylan and may also be active on low molecular mass cellulose substrates [ 88 , 89 ], in particular on aryl-cellobiosides [ 90 , 91 ] and certain cello-oligosaccharides [ 45 , 88 ]. In effect, it has been found that the replacement of one or two xylose residues by glucose is normally tolerated by the xylanases of this family, with this generally resulting in a lowered catalytic efficiency [90] . In consonance with family 11 xylanases, but in contrast to the cold-adapted family 8 xylanase, members of this family are also capable of hydrolyzing aryl β-glycosides of xylobiose and xylotriose at the aglyconic bond [ 90 , 92 , 93 ]. Furthermore, these enzymes are highly active on short xylo-oligosaccharides, thereby indicating small substrate binding sites [90] . In effect, crystal structure analyses, kinetic analyses of activity on xylo-oligosaccharides of various sizes and end product analyses have indicated that family 10 xylanases typically have four to five substrate binding sites [ 90 , 94 , 95 ]. Hydrolysis studies have also shown that most family 10 xylanases can attack the xylosidic linkage on the non-reducing end of a substituted residue or 1,3-β bond, but can only cleave at the third xylosidic linkage after a substituted residue and the second after a 1,3-β bond [90] . This indicates that the subsites on the non-reducing side (i.e., subsites −1, −2) are more specific than those on the reducing side (subsite +1) of the cleavage site.
Members of this family typically have a high molecular mass, a low p I and display an (α/β) 8 barrel fold [ 40 , 43 , 58 , 95–105 ] ( Fig. 3(c) ) ( Table 4 ). The structure has been likened to a ‘salad bowl’, with one face of the molecule having a large radius (approximately 45 Å) due to an elaborate loop architecture, while the opposite face, which consists of simple α/β turns, has a radius of approximately 30 Å. This is similar to the fold described for family 5 enzymes and both are members of clan GH-A. Indeed, these two families are quite closely related and in addition to sharing a common fold they have the same type of catalytic mechanism and share several common residues [ 57 , 106 ]. However, the family 10 xylanases are a more closely related family and have a high percentage of spatially equivalent and identical residues as well as much smaller rms deviations between equivalent residues (0.95 ± 0.11 Å) in its members [57] .
Family 10 xylanases for which structural coordinates are available
| Protein | Organism | PDB accession code (s) | Ref. |
| Xylanase (Xyn 10A) | 1EXP, 1FH7, 1FH8, 1FH9, 1FHD, 1J01, 2EXO, 2HIS, 2XYL | ||
| Xylanase A (Xyn 10A) | 1CLX, 1E5N, 1XYS | ||
| Xylanase F (Xyn 10C) | 1US2, 1US3 | ||
| Xylanase C (Xyn 10B) | 1UQY, 1UQZ, 1UR1, 1UR2 | ||
| Xylanase Z | NCIB 10682 | 1XYZ | |
| Xylanase T-6 | T-6 | 1HIZ, 1R85, 1R86, 1R87 | [ , ] |
| Xylanase (Xyn A2) | T-6 | 1N82 | |
| Xylanase A (Xyn A) | BT2246 | 1B30, 1B31, 1B3V, 1B3W, 1B3X, 1B3Y, 1B3Z, 1BG4 | |
| Xys 1 | JM8 | 1NQ6 | |
| Xylanase A | 1E0V, 1E0W, 1E0X, 1XAS, 1OD8 | ||
| β-1,4-Xylanase | E-86 | 1ISV, 1ISW, 1ISX, 1ISY, 1ISZ, 1ITO, 1XYF | |
| Xylanase | 1FXM, 1GOK, 1GOM, 1GOO, 1GOQ, 1GOR, 1I1W, 1I1X, 1K6A, 1TAX, 1TIX, 1TUX | [ , ] | |
| Xylanase B | 1VBR, 1VBU |
| Protein | Organism | PDB accession code (s) | Ref. |
| Xylanase (Xyn 10A) | 1EXP, 1FH7, 1FH8, 1FH9, 1FHD, 1J01, 2EXO, 2HIS, 2XYL | ||
| Xylanase A (Xyn 10A) | 1CLX, 1E5N, 1XYS | ||
| Xylanase F (Xyn 10C) | 1US2, 1US3 | ||
| Xylanase C (Xyn 10B) | 1UQY, 1UQZ, 1UR1, 1UR2 | ||
| Xylanase Z | NCIB 10682 | 1XYZ | |
| Xylanase T-6 | T-6 | 1HIZ, 1R85, 1R86, 1R87 | [ , ] |
| Xylanase (Xyn A2) | T-6 | 1N82 | |
| Xylanase A (Xyn A) | BT2246 | 1B30, 1B31, 1B3V, 1B3W, 1B3X, 1B3Y, 1B3Z, 1BG4 | |
| Xys 1 | JM8 | 1NQ6 | |
| Xylanase A | 1E0V, 1E0W, 1E0X, 1XAS, 1OD8 | ||
| β-1,4-Xylanase | E-86 | 1ISV, 1ISW, 1ISX, 1ISY, 1ISZ, 1ITO, 1XYF | |
| Xylanase | 1FXM, 1GOK, 1GOM, 1GOO, 1GOQ, 1GOR, 1I1W, 1I1X, 1K6A, 1TAX, 1TIX, 1TUX | [ , ] | |
| Xylanase B | 1VBR, 1VBU |
5.5 Glycoside hydrolase family 11
In contrast to all other families hitherto discussed, this family is monospecific, it consists solely of xylanases. Moreover, these xylanases are ‘true xylanases’ as they are exclusively active on d -xylose containing substrates. They have a lower catalytic versatility than family 10 xylanases and indeed the products of their action can be further hydrolyzed by the family 10 enzymes [ 90 , 92 ]. Like family 10 xylanases, these enzymes can hydrolyze aryl β-glycosides of xylobiose and xylotriose at the aglyconic bond, but in contrast to this family they are inactive on aryl cellobiosides. Furthermore, substituents or β 1,3 linkages represent a more serious hindrance to their activity, resulting in the production of larger products than family 10 xylanases [90] . Hydrolysis studies indicate that aldopentauronic acid, with an unsubstituted xylose residue at the non-reducing end [ 90 , 107 ], and an isomeric xylotetraose, with the 1,3-β bond at the non-reducing end [108] , are the smallest acidic and mixed linkage fragments liberated from heteroxylans and rhodymenan, respectively. Others have, however, suggested that this isomeric xylotetraose may contain a 1,4-β bond at the non-reducing end, with the 1,3-β linkage occurring subsequent to this bond [ 90 , 109 ]. In further contrast to the family 10 xylanases, but in common with the family 8 cold-adapted xylanase, these enzymes are most active on long chain xylo-oligosaccharides and indeed it has been found that they have larger substrate binding clefts, e.g., family 11 xylanases from Schizophyllum commune and A. niger have at least seven subsites [ 110 , 111 ], while, as has already been stated, family 10 enzymes are reported to have four to five subsites [ 90 , 94 , 95 ]. Further differences between family 10 and 11 xylanases include their stereochemistry of protonation and the effect of ω -epoxyalkyl glycosides of xylose and xylo-oligosaccharides on their activity; family 10 xylanases are anti -protonators and are unaffected by the ω -epoxyalkyl glycosides while family 11 xylanases are syn -protonators and are inactivated by the ω -epoxyalkyl glycosides [ 88 , 112 , 113 ].
Family 11 enzymes are generally characterized by a high p I , a low molecular weight, a double displacement catalytic mechanism, two glutamates acting as the catalytic residues and a β-jelly roll fold structure [ 114–127 ] ( Table 5 ) ( Fig. 3(d) ). The structure consists principally of β-pleated sheets formed into a two-layered trough that surrounds the catalytic site. Two [48] , or perhaps three [128] , β-sheets are present and the hydrophobic faces of these are packed against each other to form the hydrophobic core of the protein. Only one α-helix is present and this is typically packed against the hydrophobic face of the second β-sheet. This type of structure has also been described for family 12 endoglucanases and thus both families have been grouped into the same clan, clan GH-C.
Family 11 xylanases for which structural coordinates are available
| Protein | Organism | PDB accession code(s) | Ref. |
| Xylanase C | 1BK1 | ||
| Xylanase 1 | 1UKR | ||
| Xylanase | AC13 | 1H4G, 1H4H, 1QH6, 1QH7 | |
| Xylanase A | 1BCX, 1BVV, 1C5H, 1C5I, 1HV0, 1HV1, 1XNB, 1XNC, 2BVV | ||
| Xylanase | B230 | 1IGO | |
| Xylanase A | str. 168 | 1AXK | |
| Xyn 11A | 1H1A | ||
| Xylanase XynB | Rt46B.1 | 1F5J | |
| Xyn 11A | 1M4W | ||
| Xylanase | 1PVX | ||
| Xylanase | sp. S38 | 1HIX | |
| Xylanase | lanuginosus | 1YNA | |
| Xylanase | E58 ( E58) | 1XND | |
| Xylanase 1 | ( | 1XYN | |
| Xylanase 2 | ( ) | 1ENX, 1RED, 1REE, 1REF, 1XYO, 1XYP |
| Protein | Organism | PDB accession code(s) | Ref. |
| Xylanase C | 1BK1 | ||
| Xylanase 1 | 1UKR | ||
| Xylanase | AC13 | 1H4G, 1H4H, 1QH6, 1QH7 | |
| Xylanase A | 1BCX, 1BVV, 1C5H, 1C5I, 1HV0, 1HV1, 1XNB, 1XNC, 2BVV | ||
| Xylanase | B230 | 1IGO | |
| Xylanase A | str. 168 | 1AXK | |
| Xyn 11A | 1H1A | ||
| Xylanase XynB | Rt46B.1 | 1F5J | |
| Xyn 11A | 1M4W | ||
| Xylanase | 1PVX | ||
| Xylanase | sp. S38 | 1HIX | |
| Xylanase | lanuginosus | 1YNA | |
| Xylanase | E58 ( E58) | 1XND | |
| Xylanase 1 | ( | 1XYN | |
| Xylanase 2 | ( ) | 1ENX, 1RED, 1REE, 1REF, 1XYO, 1XYP |
5.6 Glycoside hydrolase families 7 and 43
To date, only one enzyme exhibiting xylanase activity has been identified and studied in each of these families and thus their importance as xylanase containing families is unclear. In addition, neither enzyme studied is a true xylanase; the family 7 enzyme, EGI (Cel7B) from T. reesei , is a non-specific endo-β-1,4-glucanase (EC 3.2.1.4) [ 75 , 129–131 ] and the family 43 XYND from Paenibacillus polymyxa has both xylanase and α- l -arabinofuranosidase activities [132] .
The family 7 endoglucanase I (EGI) from T. reesei is not produced during growth on xylan [75] and while its activity on cellulose (hydroxyethylcellulose) is only slightly higher than that on xylan (beechwood and grass), its activity on cello-oligosaccharides (G 3 , G 5 ) is 10-fold higher than that on xylo-oligosaccharides (X 3 , X 5 ) [130] . Hydrolyses of both these substrates takes place in the same active site but a shift in the bond cleavage frequency appears to occur towards the non-reducing end linkages in xylo-oligosaccharides [ 75 , 130 ]. This enzyme has characteristics in common with both family 10 and 11 xylanases. As for the former family, it has a high molecular weight and low p I as well as a small substrate binding site, approximately four subsites, with the catalytic site in the middle. On the other hand, like family 11 xylanases, this enzyme displays a β-jelly roll fold [131] ( Fig. 3(e) ) and produces aldopentauronic acid and isomeric xylotetraose as the shortest acidic and mixed linkage fragments from glucuronoxylan and rhodymenan, respectively [75] . However, even though the core structures of this enzyme and the family 11 xylanases are similar, differences, such as: variations in the location, length and orientation of the structural elements outside of this core, the presence of four short helical segments as opposed to one in the family 11 xylanases and differences in the type and conformation of the amino acid residues lining the active site, results in family 7 enzymes being classified in clan GH-B with family 16 enzymes and not in clan GH-C with the family 11 enzymes.
The family 43 enzyme (XYND) has a molecular weight of 64 kDa and was found clustered with a lichenase gene, with only 155bp separating the two genes, in Paenibacillus polymyxa . Unfortunately however, additional studies of the physicochemical or functional characteristics of this enzyme have not been carried out. Further putative family 43 xylanases in Caldicellulosiruptor sp. [ 133 , 134 ], C. acetobutylicum [59] , Bifidobacterium longum [135] and Bacillus sp. [61] have also been inferred on the basis of sequence homology to the above enzyme but xylanase activity has apparently not been confirmed by functional analysis. Indeed, members of this family have not been as thoroughly studied as some of the other glycoside hydrolase families and the structure of only one member has been determined, indicating that members of this family may display a five-blade β-propeller fold ( Fig. 3(f) ). Furtherrmore, a glutamate and aspartate in the centre of a long V-shaped surface groove formed across the face of the propeller have been suggested as the catalytic residues [54] . The family is grouped with family 62 in clan GH-F [ 43 , 54 ] and, as has also been demonstrated for family 8 enzymes, its members are believed to catalyze hydrolysis via the single displacement mechanism.
The preponderance of xylanases studied are of fungal or bacterial origin and in the majority of cases are found to be optimally active at, or near, mesophilic temperatures (approximately 40–60 °C) [ 16 , 18 ] and neutral (in particular for bacterial xylanases) or slightly acidic (in particular for fungal xylanases) pHs. Nevertheless, xylanases have also been reported which are not only stable, but active, at the extremes of pH and temperature. Indeed, xylanases active at temperatures ranging from 5 to 105 °C [ 4 , 80 , 81 ], pH's from 2 to 11 [ 4 , 114 , 136 ] and NaCl concentrations as high as 30%[ 137 , 138 ] have been reported. These are produced by micro-organisms which have colonized environments that may be said to be extreme from an anthropocentric point of view and which produce enzymes adapted to these extreme habitats. Of the extremophilic xylanases, the thermophiles, alkaliphiles and acidophiles have been the most extensively studied while cold-adapted xylanases have been much less investigated.
6.1 Thermophiles
A number of thermophilic (optimal growth at 50–80 °C) and hyperthermophilic (optimal growth at >80 °C) xylanase producing micro-organisms have been isolated from a variety of sources, including terrestrial and marine solfataric fields, thermal springs, hot pools and self-heating decaying organic debris [ 6 , 128 , 139–142 ]. The majority of the xylanases produced have been found to belong to families 10 and 11, with as yet, no reported studies of thermophilic xylanases belonging to any of the other glycoside hydrolase families. Interestingly, the gene for the thermostable xylanase (half-life of 8 minutes at 100 °C) from the extreme thermophilic archaeon Thermococcus zilligii [143] has thus far proven refractory to cloning with family 10 and 11 consensus primers [141] , suggesting that this enzyme may belong to one of the other less well studied glycoside hydrolase families described in this paper (i.e., families 5, 7, 8 or 43) or indeed to another as yet unknown xylanase family.
Family 10 xylanases have been isolated from various thermophilic and hyperthermophilic organisms, including Thermotoga sp. [ 34 , 144 ], Caldicellulosiruptor sp. [145] , Rhodothermus marinus [146] , Bacillus stearothermophilus [147] , Thermoascus aurantiacus [58] and C. thermocellum [58] . Indeed, a family 10 xylanase, XynA from Thermotoga sp. strain FjSS3-B.1 is one of the most thermostable xylanases reported to date with an apparent optimum temperature for activity of 105 °C and a half-life of 90 minutes at 95 °C [148] . While less frequent, family 11 thermophilic xylanases have also been isolated, with those from Thermomyces lanuginosus [ 6 , 149 ], Paecilomyces varioti [122] , Caldicellulosiruptor sp Rt69B.1. [133] , Dictyoglomus thermophilum [121] , Chaetomium thermophilum [120] , Nonomuraea flexuosa [120] and Bacillus strain D3 [ 128 , 150 ] being the most thoroughly investigated. Those from Nonomuraea flexuosa and Dictyoglomus thermophilum are among the most stable, with apparent temperature optima of 80 and 85 °C, respectively. In addition to the above mentioned xylanase producing bacteria a number of xylanase producing hyperthermophilic archaea have also been recently reported: Thermococcus zilligii [143] , Pyrococcus furiosus [143] , Sulfolobus solfataricus [140] , Pyrodictium abyssi [ 151 , 152 ] and a number of Thermofilum strains [153] .
Crystal structure analyses, sequence alignments and mutagenesis studies have indicated that mesophilic and thermophilic xylanases are very similar and that enhanced stability is probably due to an array of minor modifications, with many xylanases using unique strategies to improve their thermostability. These modifications include: an increase in the number of salt bridges and hydrogen bonds [ 120 , 124 ], an improved internal packing [120] , an increased number of charged surface residues [154] , the presence, often as tandem repeats, of thermostabilising domains [ 34 , 144 , 155 ], and/or the introduction of disulphide bridges, in particular at the N- or C- termini or in the α-helix regions [ 122 , 156 , 157 ]. Recently, the thermostabilising role of calcium on a modular family 10 xylanase was demonstrated [146] while the Bacillus D3 xylanase was also shown to use a very unique adaptation strategy. Here a series of surface aromatic residues form clusters or “sticky patches” between pairs of molecules and these intermolecular hydrophobic interactions are believed to contribute to the thermostability of this enzyme [ 128 , 150 ]. Collectively, or singly, all of the above mentioned modifications could improve the network of interactions within the protein, thereby leading to a more rigid and stable enzyme.
A number of comprehensive structural studies of thermal adaptation for family 10 and 11 xylanases have allowed identification of specific adaptation strategies for each family. For example, a comparison of the thermophilic xylanases from Thermoascus aurantiacus and C.thermocellum with mesophilic family 10 xylanases indicated that the thermostability in this family is a consequence of an improved hydrophobic packing, a favorable interaction of charged side chains with the helix dipoles as well as an increased proline content in the N-termini of helices [58] . In contrast, a recent comparative structural analysis of 5 thermophilic and 7 mesophilic family 11 enzymes suggested that a general thermostabilising adaptation in this family is a higher threonine to serine ratio (threonine has a high β-forming propensity), an increased number of residues in the β-strands and frequently an additional β-strand B1 at the N-terminus [120] . It is evident that the structural differences between the families are the basis for this difference in adaptation strategies; family 10 enzymes have a high α-helix content (approximately 40%) [95] while family 11 enzymes have a high β-sheet content (greater than 50%) [120] .
6.2 Psychrophiles
Even though cold-temperature environments are the most abundant on earth [158] , only a small number of cold-adapted, or psychrophilic, xylanase producers have been identified. These encompass a wide range of organisms; two gram negative bacteria ( Pseudoalteromonas haloplanktis TAH3a [ 80–83 ] and Flavobacterium frigidarium sp. nov. [159] ), a gram positive bacterium ( Clostridium strain PXYL1 [160] ), a yeast isolate ( Cryptococcus adeliae [161] ), krill ( Euphasia superba [162] ), a number of fungi ( Penicillium sp., Alternaria alternata and Phoma sp. 2 [163] ) and a number of basidiomycetes (e.g., Coprinus psychromorbidus [164] ). All have been isolated from the Antarctic environment, but, apart from the bacterial family 8 xylanase from Pseudoalteromonas haloplanktis TAH3a (pXyl) and the Cryptococcus adeliae family 10 xylanase (X B ), studies of the xylanases produced are minimal. Indeed, only these two xylanases and two xylanases, xylanases A and B, from Antarctic krill have been purified and characterized.
In accordance with most other psychrophilic enzymes investigated to date [ 165–167 ], the common features of the psychrophilic xylanases studied are a low temperature optimum, high catalytic activities at low temperatures and poor stability ( Fig. 4 ). Indeed, comparative studies of pXyl and X B with mesophilic xylanases showed that these enzymes have a higher catalytic activity at low and moderate temperatures, having, respectively, 10 and 3 times higher activity at 5 °C and 3 and 2 times higher activity at 30 °C [80] . Moreover, all psychrophilic enzymes studied display high catalytic activity at low temperatures. At 5 °C, activity of pXyl is 60% of the maximum while xylanases A and B from Euphasia superba display, respectively, approximately 30% and 40% of their maximum activity. In comparison, a mesophilic xylanase showed less than 5% of its maximum activity at this temperature [80] . Likewise, the apparent optimal temperatures for activity of pXyl, X B and the microfungal xylanases, which are, respectively, approximately 25, 9 and 10–30 °C lower than that of the mesophilic reference xylanases used, gives further evidence of the adaptation to cold environments of these enzymes. Poor thermal stability of the psychrophilic xylanases studied is indicated by short half-lives (e.g., at 55 °C pXyl has a 12 times shorter half-life of inactivation than a mesophilic xylanase) and low denaturation temperatures (pXyl shows a 10 °C and X B a 14 °C decrease in melting temperature compared to mesophilic reference xylanases) while a lower chemical stability of the cold-adapted family 8 xylanase is demonstrated by short half-lives of guanidine hydrochloride inactivation and unfolding [81] .
![literature review xylanase (a) Unfolding as monitored by differential scanning calorimetry at a scan rate of 1 K min−1 and (b) thermodependence of activity of the cold-adapted family 8 xylanase pXyl (circles, solid lines), the mesophilic family 11 xylanase Xyl1 [206] (squares, dashed lines) and the thermophilic family 8 endoglucanase CelA [55, 207] (triangles, short dashed lines). Baseline subtracted DSC data have been normalized for protein concentration. The lower molecular weight of the mesophilic enzyme gives rise to the observed low calorimetric enthalpy.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/29/1/10.1016_j.femsre.2004.06.005/1/m_FMR_3_f4.jpeg?Expires=1726711691&Signature=btoYl8V1BNfDhC8p2EzA3BxOJwQ7Ogn47Bpdgp2ivxZZPc34a1cEIYYeS9nnfkQ5jKD6Mygu9cDP5A3sTPV9prZTCyFYY5T45P-~fjxGuVeKaXf422ufhju0B25bhRZqrzYpDKg35m-OJGy9lE1EPUHzlycHzl1kSbjXtzcbd0k9GmqU5ELo-6zc1GLKZQMZEO0OcPFvfnZtT4L1UYQkE33VJ0YNlAx4ueZfOiUlWBY3Nllku7tpdjz1jgRyxUF256W~RPaxGW8WECTqKmB7vGKQlr~6BBzo21sVZsHxl8MJcSJAOpMIZIHdwTd-36zyKR4yTNPk2nT31Pwezk5Cag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(a) Unfolding as monitored by differential scanning calorimetry at a scan rate of 1 K min −1 and (b) thermodependence of activity of the cold-adapted family 8 xylanase pXyl (circles, solid lines), the mesophilic family 11 xylanase Xyl1 [206] (squares, dashed lines) and the thermophilic family 8 endoglucanase CelA [ 55 , 207 ] (triangles, short dashed lines). Baseline subtracted DSC data have been normalized for protein concentration. The lower molecular weight of the mesophilic enzyme gives rise to the observed low calorimetric enthalpy.
Fluorescence monitoring of acrylamide quenching indicated that, in addition to a reduced stability and increased low temperature activity, the family 8 cold-adapted xylanase has an increased flexibility compared to a thermophilic homologous enzyme (CelA from C. thermocellum ) [81] . Indeed, this supports the hypothesis that efficient catalysis at low temperatures is brought about by an increased flexibility of the molecular edifice, thereby allowing the molecular motions necessary for activity in the low temperature, low energy environment but also leading to the observed reduced stability.
At the structural level, it was found that, when compared to a thermophilic and mesophilic homolog, respectively, the family 8 and yeast cold-adapted xylanases are both distinguished by a number of discrete modifications which could give rise to a decrease in the stability, and hence an increase in the flexibility, of the molecular structure. The Pseudoalteromonas haloplanktis TAH3a xylanase is characterised by a reduced number of salt-bridges and an increased exposure of hydrophobic residues [ 82 , 83 ], while the family 10 yeast xylanase is characterized by a less compact hydrophobic packing, the loss of one salt bridge and a destabilization of the helix macrodipoles [161] . Indeed it can be seen that these modifications are an extension of those observed between thermophilic and mesophilic xylanases, however, further analysis of psychrophilic xylanases, especially comparative studies with more closely related and better characterised homologs are necessary to better understand temperature adaptation in these enzymes. In particular, further comparative studies of family 10 and 11 psychrophilic xylanases would be most beneficial.
6.3 Alkaliphiles and acidophiles
While the majority of natural environments on earth are essentially neutral, with pH values of between 5 and 9, habitats with extreme pHs are also common, in particular in geothermal regions, carbonate laden soils, soda deserts and soda lakes such as found in Egypt (Wadi Natrun), the African Rift valley (Lakes Magadi and Nakuru in Kenya), Central Asia, Western USA (Yellowstone National Park) and Southern Europe (Vulcano Island, Italy), Indeed, xylanase producing alkaliphilic micro-organisms, which typically grow optimally at pH values above 9, and acidophiles, which grow optimally between pH 1 and 5, have been isolated from these environments [ 136 , 168 , 169 ] and also from such sources as kraft pulp [170] , pulp and paper industry wastes [171] , decomposing organic matter [172] , faeces [168] , plant sources [173] , soils [ 136 , 174 ] and even from neutral environments where they are found coexisting with neutrophilic micro-organisms [168] .
The first report of a xylanase produced by an alkaliphilic micro-organism was as early as 1973 for a xylanase from Bacillus sp. C-59-2 [175] and since this initial finding a number of xylanases have been isolated from various acidophilic and alkaliphilic micro-organisms. These include family 10 and 11 xylanases from a number of Bacillus sp. [ 116 , 147 , 174 ], Trichoderma sp. [ 126 , 127 , 176 ], Aspergillus sp. [ 114 , 115 ], Penicillium sp. [136] , Acidobacterium sp. [177] and Cryptococcus sp. [178] . In addition, family 8 xylanases have been isolated from alkalophilic B. halodurans C-125 [ 77 , 78 ] and B. halodurans MIR32 [43] , however analysis of the amino-acid sequences indicates that these may be intracellular enzymes and thus may not be adapted to the environment of their hosts.
Many of the alkaliphilic microorganisms studied have been found to produce xylanases with pH optima in the near neutral region but with relatively high activities being retained in alkaline conditions. In addition, a number of xylanases with more alkaline pH optima have also been isolated and one the most alkaliphilic xylanases reported to date is XylB from Bacillus sp. AR-009, which has a pH optimum of pH 9–10 [169] . Other highly alkaliphilic xylanases include xylanase J from Bacillus sp. strain 41M-1 [174] and a xylanase from Bacillus pumilus 13 a [172] , both of which have a pH optimum of 9. Much fewer acidophilic than alkaliphilic xylanases have been studied and the most important of these are the family 10 and 11 members from T. reesei [126] , A. niger [115] , Aspergillus kawachii [ 114 , 179 ], Cryptococcus sp. S-2 [178] and Penicillium sp. 40 [136] . The latter three of these are among the most acidophilic of the studied xylanases with a pH optimum of 2 and stability over a broad pH range; the Penicillium sp. 40 xylanase is stable from pH 2 to pH 5 [136] and the A. kawachii XynC is stable at pH 1–9 [ 114 , 179 ].
The pH activity profiles of enzymes are highly dependent on the p K a s of the catalytic residues which are themselves dependent on the local environment and hence on the nature of the amino-acids in the vicinity of the catalytic residues. A recent study of a family 11 xylanase showed that, in general, residues that contribute positive charges and hydrogen bonds serve to lower the p K a values with shorter bonds having a more pronounced effect. The chemical nature of the donor is also important, with COOH being more effective than OH and CONH 2 [180] . In contrast to this, neighbouring carboxyl groups can either lower or raise the p K a values of the catalytic glutamic acids depending upon the electrostatic linkage of the residues involved in the interaction [180] . In fact it has been noted that family 11 acidophilic xylanases have an aspartic acid residue hydrogen bonded to the general acid/base catalyst which is replaced by an asparagine in the xylanases active under more alkaline conditions [ 114 , 126 , 181 , 182 ]. This residue influences the pH dependence of activity and mutation of this aspartic acid to its amide derivative in the A. kawachii acidophilic xylanase resulted in an upward shift of the pH optimum from pH 2 to a pH of 5. Indeed, tertiary structure analysis of this enzyme [114] as well as of the family 11 xylanases from T. reesei [126] and A. niger [115] indicated that adaptation to low pH is brought about by an increase in negative charge and a substitution and reorientation of residues, in particular aromatic residues, in the active sites. In contrast, a random mutagenesis study of a Neocallimastix patriciarum xylanase indicated that an increased negative charge and increased hydrophobicity increased the pH optimum of this enzyme [183] .
Stability at the extremes of pH appears to be characterized by a spatially biased distribution of charged residues. The acidophilic and acid stable xylanase from A. kawachii , for example, is characterized by a concentration of acidic residues on its surface [114] which are believed to reduce electrostatic repulsion of the positively charged residues at low pHs. In contrast, enzymes stable in alkaline conditions are typically characterized by a decreased number of acidic residues and an increased number of arginines. Furthermore, a recent comparative structural study of family 11 enzymes suggests a correlation between pH activity/stability and the number of salt bridges, with acidophilic xylanases having much less of these interactions than their alkaliphilic homologs [120] . Indeed it was even suggested that adaptation to high pH may occur via a similar mechanism to adaptation to high temperatures [120] .
Global markets for industrial enzymes grew from €1 billion in 1995 [184] to almost €2 billion in 2001 [185] and continue to increase as new enzymes and applications are discovered. In the grain-processing enzymes sector alone (which currently accounts for approximately 25–28% of total enzyme sales) an increase in market value from €510 million in 2001 to €760 million in 2010 has been forecasted [185] . Presently the technical industries, dominated by the detergent, starch, textile and fuel alcohol industries, account for the majority of the total enzymes market, with the feed and food enzymes together totaling only about 35%. Recently however, sales in some of the major technical industries has stagnated (3% drop in 2001) while sales in both the food and feed industries are increasing, with annual growth rates of approximately 4–5% being forecasted [185] .
Hydrolases constitute approximately 75% of the markets for industrial enzymes, with the glycosidases, including cellulases, amylases and hemicellulases, constituting the second largest group after proteases [186] . Xylanases constitute the major commercial proportion of hemicellulases but represent only a small percentage of the total enzyme sales. The sales figures are expected to increase however, as these enzymes have attracted increasing attention due to their potential for use in several applications. In effect, the United States Patent and Trademark Office ( http://www.uspto.gov/ ) lists 468 patents introduced since 2001 with reference to xylanases (search field = all fields).
Xylanases have potential applications in a wide range of industrial processes, covering all three sectors of industrial enzymes markets [186] and some of the most important of these are listed in Table 6 . Other less well documented putative applications include: in brewing, to increase wort filterability and reduce haze in the final product [187] ; in coffee extraction and in the preparation of soluble coffee [7] ; in detergents [188] ; in the protoplastation of plant cells [4] ; in the production of pharmacologically active polysaccharides for use as antimicrobial agents [107] or antioxidants [189] ; in the production of alkyl glycosides for use as surfactants [190] ; and in the washing of precision devices and semiconductors [191] . The xylanases are frequently utilized alone, but are more commonly used in conjunction with other enzymes and in particular with other hydrolases, but also with proteases, oxidases, isomerases etc.
Potential applications for xylanases
| Market | Industry | Application | Function | Reference |
| Food | Fruit and vegetable processing, brewing, wine production. | Fruit and vegetable juices, nectars and purees, oils (e.g., olive oil, corn oil) and wines | Improves maceration and juice clarification, reduces viscosity. Improves extraction yield and filtration, process performance and product quality. | [ , , ] |
| Baking | Dough and bakery products | Improves elasticity and strength of the dough, thereby allowing easier handling, larger loaf volumes and improved bread texture. | [ , , ] | |
| Feed | Animal feeds. | Monogastric (swine and poultry) and ruminant feeds | Decreases the content of non-starch polysaccharides, thereby reducing the intestinal viscosity and improving the utilization of proteins and starch. Improves animal performance, increases digestability and nutritive value of poorly degradable feeds, e.g., barley and wheat. | [ , ] |
| Technical | Paper and pulp | Biobleaching of kraft pulps | Reduces chlorine consumption and toxic discharges. | [ , ] |
| Bio-mechanical pulping | Facilitates the pulping process and reduces the use of mechanical pulping methods, hence reduces energy consumption. | |||
| Bio-modification of fibers | Improves fibrillation and drainage properties of pulp, hence improving the process efficiency and the paper strength. | |||
| Bio-de-inking | Facilitates the de-inking process and reduces the use of alkali. | [ , ] | ||
| Starch | Starch-gluten separation | Reduces batter viscosity, improves gluten agglomeration and process efficiency. | ||
| Textiles | Retting of flax, jute, ramie, hemp, etc. | Enzymatic retting, reduces/replaces chemical retting methods. | [ , , ] | |
| Bioremediation/Bioconversion | Treatment of agricultural, municipal and food industry wastes | Treatment/recycling of wastes. Production of fermentable products, renewable fuel (bioethanol) and fine chemicals. | [ , , ] |
| Market | Industry | Application | Function | Reference |
| Food | Fruit and vegetable processing, brewing, wine production. | Fruit and vegetable juices, nectars and purees, oils (e.g., olive oil, corn oil) and wines | Improves maceration and juice clarification, reduces viscosity. Improves extraction yield and filtration, process performance and product quality. | [ , , ] |
| Baking | Dough and bakery products | Improves elasticity and strength of the dough, thereby allowing easier handling, larger loaf volumes and improved bread texture. | [ , , ] | |
| Feed | Animal feeds. | Monogastric (swine and poultry) and ruminant feeds | Decreases the content of non-starch polysaccharides, thereby reducing the intestinal viscosity and improving the utilization of proteins and starch. Improves animal performance, increases digestability and nutritive value of poorly degradable feeds, e.g., barley and wheat. | [ , ] |
| Technical | Paper and pulp | Biobleaching of kraft pulps | Reduces chlorine consumption and toxic discharges. | [ , ] |
| Bio-mechanical pulping | Facilitates the pulping process and reduces the use of mechanical pulping methods, hence reduces energy consumption. | |||
| Bio-modification of fibers | Improves fibrillation and drainage properties of pulp, hence improving the process efficiency and the paper strength. | |||
| Bio-de-inking | Facilitates the de-inking process and reduces the use of alkali. | [ , ] | ||
| Starch | Starch-gluten separation | Reduces batter viscosity, improves gluten agglomeration and process efficiency. | ||
| Textiles | Retting of flax, jute, ramie, hemp, etc. | Enzymatic retting, reduces/replaces chemical retting methods. | [ , , ] | |
| Bioremediation/Bioconversion | Treatment of agricultural, municipal and food industry wastes | Treatment/recycling of wastes. Production of fermentable products, renewable fuel (bioethanol) and fine chemicals. | [ , , ] |
Many of the xylanases used in industry today appear to be of mesophilic and/or neutrophilic origin, yet enzymes from extremophilic sources may be of tremendous utility in many biotechnological processes. In particular, thermophilic enzymes could be used in applications where a cooling step would be uneconomical or where high temperatures are required to increase the bioavailability and/or solubility of substrates, to reduce viscosity and/or to reduce the risk of contamination. Acidophilic and alkaliphilic enzymes would obviously be beneficial in processes where extreme pH conditions are required or where adjustment of the pH to neutral conditions is uneconomical. On the other hand, cold-adapted xylanases would be beneficial to those processes where heating is economically counterproductive or where low temperatures are required to avoid alteration of ingredient and/or product quality (e.g., flavour, colour etc.), to avoid microbial development and fermentation and/or to avoid product denaturation. In fact, enzymes which combine a number of extremophilic characteristics may be of the most use in industry.
The major current application of xylanases is in the pulp and paper industries where the high temperature (55–70 °C) and alkaline pH of the pulp substrate requires thermo-alkaliphilic enzymes for efficient biobleaching [ 5 , 192 ]. Thermo-alkaliphilic or even thermo-acidophilic xylanases may also be of use in bioconversion processes where a variety of treatments, including hot water and steam explosion, alkaline, solvent or acidic pretreatments may be used prior to or simultaneous to enzyme treatment [ 193 , 194 ]. Alkaliphilic xylanases would also be required for detergent applications where high pHs are typically used [188] while a thermostable xylanase would be beneficial in animal feeds if added to the feeds before the pelleting process (typically carried out at 70–95 °C). In addition, for this latter application the enzyme must be highly active at the temperature (approximately 40 °C) and pH (approximately pH 4.8) of the digestive tract [195] . Cold adapted xylanases, which are most active at low and intermediate temperatures, could offer advantages over the currently used xylanases in many of the low to moderate temperature processes, in particular in the food industry. For example, they would be most suited for use in the baking industry as dough preparation and proofing is generally carried out at temperatures below 35 °C and indeed it has recently been demonstrated that a cold-adapted family 8 xylanase is more efficient in baking than a commonly used commercial enzyme [79] .
A large variety of enzymes with xylanase activity have been isolated and studied and can be classified in glycoside hydrolase families 5, 7, 8, 10, 11 and 43, with each of these families being characterized by a particular fold and mechanism of action. While extensive studies of family 10 and 11 xylanases have been carried out, leading to an enrichment in the understanding of these enzymes, studies of the xylanase members of the other families are sparse. Only a small number of enzymes with xylanase activity have been identified in families 5, 7, 8 and 43, however, the numbers will increase with further studies and may indeed be extended to additional families as novel enzymes from novel sources are discovered. New approaches, such as genome sequencing programs, functional and/or consensus PCR screening of environmental DNA libraries (known as metagenomics) as well as the study of extremophilic enzymes will, without doubt, further extend the current repertoire, understanding, and applications of xylanases and xylanase families.
The authors thank N. Gerardin and R. Marchand for their skillful technical assistance and Jean-Claude Marx for his help with the figures. We also wish to thank the French Institute for Polar Research for generously accommodating our research fellows at the French Antarctic station in J.S. Dumont d'Urville, Terre Adelie. This work was supported by the ‘Région Wallonne’ (contracts FIRST Europe ‘objective 3’ R0202/215266 and BIOVAL 981/3860), the European Union (network contract CT970131) and the ‘Fonds National de la Recherche Scientifique’ (contracts 2.4515.00 and 2.4536.04).
Prade R.A. ( 1995 ) Xylanases: from biology to biotechnology . Biotech. Genet. Eng. Rev . 13 , 100 – 131 .
Google Scholar
Whistler R. Masek E. ( 1955 ) Enzymatic hydrolysis of xylan . J. Am. Chem. Soc . 77 , 1241 – 1243 .
Shallom D. Shoham Y. ( 2003 ) Microbial hemicellulases . Curr. Opin. Microbiol . 6 , 219 – 228 .
Kulkarni N. Shendye A. Rao M. ( 1999 ) Molecular and biotechnological aspects of xylanases . FEMS Microbiol. Rev . 23 , 411 – 456 .
Beg Q.K. Kapoor M. Mahajan L. Hoondal G.S. ( 2001 ) Microbial xylanases and their industrial applications: a review . Appl. Microbiol. Biotechnol . 56 , 326 – 338 .
Singh S. Madlala A.M. Prior B.A. ( 2003 ) Thermomyces lanuginosus : properties of strains and their hemicellulases . FEMS Microbiol. Rev . 27 , 3 – 16 .
Wong K.K.Y. Tan L.U.L. Saddler J.N. ( 1988 ) Multiplicity of beta-1,4-xylanases in microorganisms: functions and applications . Microbiol. Rev . 52 , 305 – 317 .
Li K. Azadi P. Collins R. Tolan J. Kim J. Eriksson K. ( 2000 ) Relationships between activities of xylanases and xylan structures . Enzyme Microb. Technol . 27 , 89 – 94 .
Chanda, S.K., Hirst, E.L., Jones, J.K.N., Percival, E.G.V. The constitution of xylan from esparto grass. J. Chem. Soc. 1950. 12889–12897
Eda S. Ohnishi A. Kato K. ( 1976 ) Xylan isolated from the stalk of Nicotiana tabacum . Agric. Biol. Chem . 40 , 359 – 364 .
Barry, V., Dillon, T. Occurence of xylans in marine algae. Nature. 146, 1940. 620
Nunn J.R. Parolis H. Russel I. ( 1973 ) Polysaccharides of the red algae Chaetangium erinaceum . Part I: Isolation and characterization of the water-soluble xylan . Carbohydr. Res . 26 , 169 – 180 .
Percival E.G.V. Chanda S.K. ( 1950 ) The xylan of Rhodymenia palmata . Nature 166 , 787 – 788 .
Puls J. Schmidt O. Granzow C. ( 1987 ) Glucuronidase in two microbial xylanolytic systems . Enzyme Microb. Technol . 9 , 83 – 88 .
Biely P. ( 1985 ) Microbial xylanolytic systems . Trends Biotechnol . 3 , 286 – 290 .
Subramaniyan S. Prema P. ( 2002 ) Biotechnology of microbial xylanases: enzymology, molecular biology, and application . Crit. Rev. Biotechnol . 22 , 33 – 64 .
Belancic A. Scarpa J. Peirano A. Diaz R. Steiner J. Eyzaguirre J. ( 1995 ) Penicillium purpurogenum produces several xylanases: purification and properties of two of the enzymes . J. Biotechnol . 41 , 71 – 79 .
Sunna A. Antranikian G. ( 1997 ) Xylanolytic enzymes from fungi and bacteria . Crit. Rev. Biotechnol . 17 , 39 – 67 .
Elegir G. Szakacs M. Jeffries T.W. ( 1994 ) Purification, characterization and substrate specificities of multiple xylanases from Streptomyces sp. strain B-12-2 . Appl. Environ. Microbiol . 60 , 2609 – 2615 .
Wubah D.A. Akin D.E. Borneman W.S. ( 1993 ) Biology, fiber-degradation, and enzymology of anaerobic zoosporic fungi . Crit. Rev. Microbiol . 19 , 99 – 115 .
Matte A. Forsberg C.W. ( 1992 ) Purification, characterization, and mode of action of endoxylanases 1 and 2 from Fibrobacter succinogenes S85 . Appl. Environ. Microbiol . 58 , 157 – 168 .
Krause D.O. Denman S.E. Mackie R.I. Morrison M. Rae A.L. Attwood G.T. McSweeney C.S. ( 2003 ) Opportunities to improve fiber degradation in the rumen: microbiology, ecology, and genomics . FEMS Microbiol. Rev . 27 , 663 – 693 .
Gilbert H.J. Hazlewood G.P. ( 1993 ) Bacterial cellulases and xylanases . J. Gen. Microbiol . 139 , 187 – 194 .
Gilbert H.J. Sullivan D.A. Jenkins G. Kellett L.E. Minton N.P. Hall J. ( 1988 ) Molecular cloning of multiple xylanase genes from Pseudomonas fluorescens subsp. cellulosa . J. Gen. Microbiol . 134 ( Pt 12 ), 3239 – 3247 .
Yang R.C. MacKenzie C.R. Bilous D. Narang S.A. ( 1989 ) Identification of two distinct Bacillus circulans xylanases by molecular cloning of the genes and expression in Escherichia coli . Appl. Environ. Microbiol . 55 , 568 – 572 .
Biely P. Markovic O. Mislovicova D. ( 1985 ) Sensitive detection of endo-1,4-beta-glucanases and endo-1,4-beta-xylanases in gels . Anal. Biochem . 144 , 147 – 151 .
Luthi E. Love D.R. McAnulty J. Wallace C. Caughey P.A. Saul D. Bergquist P.L. ( 1990 ) Cloning, sequence analysis, and expression of genes encoding xylan-degrading enzymes from the thermophile Caldocellum saccharolyticum . Appl. Environ. Microbiol . 56 , 1017 – 1024 .
Zhu H. Paradis F.W. Krell P.J. Phillips J.P. Forsberg C.W. ( 1994 ) Enzymatic specificities and modes of action of the two catalytic domains of the XynC xylanase from Fibrobacter succinogenes S85 . J. Bacteriol . 176 , 3885 – 3894 .
Irwin D. Jung E.D. Wilson D.B. ( 1994 ) Characterization and sequence of a Thermomonospora fusca xylanase . Appl. Environ. Microbiol . 60 , 763 – 770 .
Black G.W. Rixon J.E. Clarke J.H. Hazlewood G.P. Ferreira L.M. Bolam D.N. Gilbert H.J. ( 1997 ) Cellulose binding domains and linker sequences potentiate the activity of hemicellulases against complex substrates . J. Biotechnol . 57 , 59 – 69 .
Millward-Sadler S.J. Davidson K. Hazlewood G.P. Black G.W. Gilbert H.J. Clarke J.H. ( 1995 ) Novel cellulose-binding domains, NodB homologues and conserved modular architecture in xylanases from the aerobic soil bacteria Pseudomonas fluorescens subsp. cellulosa and Cellvibrio mixtus . Biochem. J . 312 ( Pt 1 ), 39 – 48 .
Hayashi H. Takagi K.I. Fukumura M. Kimura T. Karita S. Sakka K. Ohmiya K. ( 1997 ) Sequence of xynC and properties of XynC, a major component of the Clostridium thermocellum cellulosome . J. Bacteriol . 179 , 4246 – 4253 .
Grepinet O. Chebrou M.C. Beguin P. ( 1988 ) Purification of Clostridium thermocellum xylanase Z expressed in Escherichia coli and identification of the corresponding product in the culture medium of C. thermocellum . J. Bacteriol . 170 , 4576 – 4581 .
Winterhalter C. Heinrich P. Candussio A. Wich G. Liebl W. ( 1995 ) Identification of a novel cellulose-binding domain within the multidomain 120 kDa xylanase XynA of the hyperthermophilic bacterium Thermotoga maritima . Mol. Microbiol . 15 , 431 – 444 .
Black G.W. Rixon J.E. Clarke J.H. Hazlewood G.P. Theodorou M.K. Morris P. Gilbert H.J. ( 1996 ) Evidence that linker sequences and cellulose-binding domains enhance the activity of hemicellulases against complex substrates . Biochem. J . 319 ( Pt 2 ), 515 – 520 .
Gilkes N.R. Henrissat B. Kilburn D.G. Miller R.C. Jr. Warren R.A. ( 1991 ) Domains in microbial 4-glycanases: sequence conservation, function, and enzyme families . Microbiol. Rev . 55 , 303 – 315 .
Defaye J. Guillot J.M. Biely P. Vrsanska M. ( 1992 ) Positional isomers of thioxylobiose, their synthesis and inducing ability for d-xylan-degrading enzymes in the yeast Cryptococcus albidus . Carbohydr. Res . 228 , 47 – 64 .
Fontes C.M. Gilbert H.J. Hazlewood G.P. Clarke J.H. Prates J.A. McKie V.A. Nagy T. Fernandes T.H. Ferreira L.M. ( 2000 ) A novel Cellvibrio mixtus family 10 xylanase that is both intracellular and expressed under non-inducing conditions . Microbiology 146 ( Pt 8 ), 1959 – 1967 .
Shulami S. Gat O. Sonenshein A.L. Shoham Y. ( 1999 ) The glucuronic acid utilization gene cluster from Bacillus stearothermophilus T-6 . J. Bacteriol . 181 , 3695 – 3704 .
Teplitsky A. Shulami S. Moryles S. Shoham Y. Shoham G. ( 2000 ) Crystallization and preliminary X-ray analysis of an intracellular xylanase from Bacillus stearothermophilus T-6 . Acta Crystallogr. D: Biol. Crystallogr . 56 ( Pt 2 ), 181 – 184 .
Henrissat B. Claeyssens M. Tomme P. Lemesle L. Mornon J.P. ( 1989 ) Cellulase families revealed by hydrophobic cluster analysis . Gene 81 , 83 – 95 .
Henrissat B. Coutinho P.M. ( 2001 ) Classification of glycoside hydrolases and glycosyltransferases from hyperthermophiles . Methods Enzymol . 330 , 183 – 201 .
Coutinho, P.M. and Henrissat, B. (1999) Carbohydrate-active enzyme server (CAZY) at URL: http://afmb.cnrs-mrs.fr/˜cazy/CAZY/
Gebler J. Gilkes N.R. Claeyssens M. Wilson D.B. Beguin P. Wakarchuk W.W. Kilburn D.G. Miller R.C. Jr. Warren R.A. Withers S.G. ( 1992 ) Stereoselective hydrolysis catalyzed by related beta-1,4-glucanases and beta-1,4-xylanases . J. Biol. Chem . 267 , 12559 – 12561 .
Claeyssens M. Henrissat B. ( 1992 ) Specificity mapping of cellulolytic enzymes: classification into families of structurally related proteins confirmed by biochemical analysis . Protein Sci . 1 , 1293 – 1297 .
Bourne Y. Henrissat B. ( 2001 ) Glycoside hydrolases and glycosyltransferases: families and functional modules . Curr. Opin. Struct. Biol . 11 , 593 – 600 .
Bergquist P.L. Gibbs M.D. Morris D.D. Thompson D.R. Uhl A.M. Daniel R.M. ( 2001 ) Hyperthermophilic xylanases . Methods Enzymol . 330 , 301 – 319 .
Torronen A. Rouvinen J. ( 1997 ) Structural and functional properties of low molecular weight endo-1,4-beta-xylanases . J. Biotechnol . 57 , 137 – 149 .
Jeffries T.W. ( 1996 ) Biochemistry and genetics of microbial xylanases . Curr. Opin. Biotechnol . 7 , 337 – 342 .
Flint H.J. Martin J. McPherson C.A. Daniel A.S. Zhang J.X. ( 1993 ) A bifunctional enzyme, with separate xylanase and beta(1,3-1,4)-glucanase domains, encoded by the xynD gene of Ruminococcus flavefaciens . J. Bacteriol . 175 , 2943 – 2951 .
Rye C.S. Withers S.G. ( 2000 ) Glycosidase mechanisms . Curr. Opin. Chem. Biol . 4 , 573 – 580 .
Zechel D.L. Withers S.G. ( 2000 ) Glycosidase mechanisms: anatomy of a finely tuned catalyst . Acc. Chem. Res . 33 , 11 – 18 .
McCarter J.D. Withers S.G. ( 1994 ) Mechanisms of enzymatic glycoside hydrolysis . Curr. Opin. Struct. Biol . 4 , 885 – 892 .
Nurizzo D. Turkenburg J.P. Charnock S.J. Roberts S.M. Dodson E.J. McKie V.A. Taylor E.J. Gilbert H.J. Davies G.J. ( 2002 ) Cellvibrio japonicus alpha-l-arabinanase 43A has a novel five-blade beta-propeller fold . Nat. Struct. Biol . 9 , 665 – 668 .
Alzari P.M. Souchon H. Dominguez R. ( 1996 ) The crystal structure of endoglucanase CelA, a family 8 glycosyl hydrolase from Clostridium thermocellum . Structure 4 , 265 – 275 .
Guerin D.M. Lascombe M.B. Costabel M. Souchon H. Lamzin V. Beguin P. Alzari P.M. ( 2002 ) Atomic (0.94 Å) resolution structure of an inverting glycosidase in complex with substrate . J. Mol. Biol . 316 , 1061 – 1069 .
Larson S.B. Day J. Barba de la Rosa A.P. Keen N.T. McPherson A. ( 2003 ) First crystallographic structure of a xylanase from glycoside hydrolase family 5: implications for catalysis . Biochemistry 42 , 8411 – 8422 .
Lo Leggio L. Kalogiannis S. Bhat M.K. Pickersgill R.W. ( 1999 ) High resolution structure and sequence of T. aurantiacus xylanase I: implications for the evolution of thermostability in family 10 xylanases and enzymes with (beta)alpha-barrel architecture . Proteins 36 , 295 – 306 .
Nolling J. Breton G. Omelchenko M.V. Makarova K.S. Zeng Q. Gibson R. Lee H.M. Dubois J. Qiu D. Hitti J. Wolf Y.I. Tatusov R.L. Sabathe F. Doucette-Stamm L. Soucaille P. Daly M.J. Bennett G.N. Koonin E.V. Smith D.R. ( 2001 ) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum . J. Bacteriol . 183 , 4823 – 4838 .
da Silva A.C. Ferro J.A. Reinach F.C. Farah C.S. Furlan L.R. Quaggio R.B. Monteiro-Vitorello C.B. Van Sluys M.A. Almeida N.F. Alves L.M. do Amaral A.M. Bertolini M.C. Camargo L.E. Camarotte G. Cannavan F. Cardozo J. Chambergo F. Ciapina L.P. Cicarelli R.M. Coutinho L.L. Cursino-Santos J.R. El-Dorry H. Faria J.B. Ferreira A.J. Ferreira R.C. Ferro M.I. Formighieri E.F. Franco M.C. Greggio C.C. Gruber A. Katsuyama A.M. Kishi L.T. Leite R.P. Lemos E.G. Lemos M.V. Locali E.C. Machado M.A. Madeira A.M. Martinez-Rossi N.M. Martins E.C. Meidanis J. Menck C.F. Miyaki C.Y. Moon D.H. Moreira L.M. Novo M.T. Okura V.K. Oliveira M.C. Oliveira V.R. Pereira H.A. Rossi A. Sena J.A. Silva C. De Souza R.F. Spinola L.A. Takita M.A. Tamura R.E. Teixeira E.C. Tezza R.I. Trindade dos Santos M. Truffi D. Tsai S.M. White F.F. Setubal J.C. Kitajima J.P. ( 2002 ) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities . Nature 417 , 459 – 463 .
Kunst F. Ogasawara N. Moszer I. Albertini A.M. Alloni G. Azevedo V. Bertero M.G. Bessieres P. Bolotin A. Borchert S. Borriss R. Boursier L. Brans A. Braun M. Brignell S.C. Bron S. Brouillet S. Bruschi C.V. Caldwell B. Capuano V. Carter N.M. Choi S.K. Codani J.J. Connerton I.F. Danchin A. ( 1997 ) The complete genome sequence of the gram-positive bacterium Bacillus subtilis . Nature 390 , 249 – 256 .
Xu J. Bjursell M.K. Himrod J. Deng S. Carmichael L.K. Chiang H.C. Hooper L.V. Gordon J.I. ( 2003 ) A genomic view of the human– Bacteroides thetaiotaomicron symbiosis . Science 299 , 2074 – 2076 .
Whitehead T.R. ( 1993 ) Analyses of the gene and amino acid sequence of the Prevotella ( Bacteroides ) ruminicola 23 xylanase reveals unexpected homology with endoglucanases from other genera of bacteria . Curr. Microbiol . 27 , 27 – 33 .
Foong F. Hamamoto T. Shoseyov O. Doi R.H. ( 1991 ) Nucleotide sequence and characteristics of endoglucanase gene engB from Clostridium cellulovorans . J. Gen. Microbiol . 137 ( Pt 7 ), 1729 – 1736 .
Cho K.K. Kim S.C. Woo J.H. Bok J.D. Choi Y.J. ( 2000 ) Molecular cloning and expression of a novel family A endoglucanase gene from Fibrobacter succinogenes S85 in Escherichia coli . Enzyme Microb. Technol . 27 , 475 – 481 .
Poole D.M. Hazlewood G.P. Laurie J.l. Barker P.J. Gilbert H.J. ( 1990 ) Nucleotide sequence of the Ruminococcus albus SY3 endoglucanase genes celA and celB . Mol. Gen. Genet . 223 ( 2 ), 217 – 230 .
Saloheimo, M., Siika-aho, M., Tenkanen, M. and Penttila, M.E. (2003) Novel xylanase from Trichoderma reesei , method for production thereof, and methods employing this enzyme. In: United States Patent Application 20030054518
Tenkanen, M., Burgermeister, M., Vrsanska, M., Biely, P., Saloheimo, M., Siika-aho, M. (2003) A novel xylanase XYN IV from Trichoderma reesei and its action on different xylans. In: Recent Advances in Enzymes in Grain Processing (Courtin, C.M., Veraverbeke, W.S., Delcour, J.A., Eds.), pp.41–46 Kat. Univ. Leuven, Leuven.
Braun E.J. Rodrigues C.A. ( 1993 ) Purification and properties of an endoxylanase from a corn stalk rot strain of Erwinia chrysanthemi . Phytopathology 83 , 332 – 338 .
Keen N.T. Boyd C. Henrissat B. ( 1996 ) Cloning and characterization of a xylanase gene from corn strains of Erwinia chrysanthemi . Mol. Plant Microbe Interact . 9 , 651 – 657 .
Hurlbert J.C. Preston J.F. 3rd ( 2001 ) Functional characterization of a novel xylanase from a corn strain of Erwinia chrysanthemi . J. Bacteriol . 183 , 2093 – 2100 .
Suzuki T. Ibata K. Hatsu M. Takamizawa K. Keiichi K. ( 1997 ) Cloning and expression of a 58-kDa xylanase VI gene ( xynD ) of Aeromonas caviae ME-1 in Escherichia coli which is not categorized as a family F or family G xylanase . J. Fermen. Bioeng . 84 , 86 – 89 .
Mulder N.J. Apweiler R. Attwood T.K. Bairoch A. Barrell D. Bateman A. Binns D. Biswas M. Bradley P. Bork P. Bucher P. Copley R.R. Courcelle E. Das U. Durbin R. Falquet L. Fleischmann W. Griffiths-Jones S. Haft D. Harte N. Hulo N. Kahn D. Kanapin A. Krestyaninova M. Lopez R. Letunic I. Lonsdale D. Silventoinen V. Orchard S.E. Pagni M. Peyruc D. Ponting C.P. Selengut J.D. Servant F. Sigrist C.J. Vaughan R. Zdobnov E.M. ( 2003 ) The InterPro Database, 2003 brings increased coverage and new features . Nucleic Acids Res . 31 , 315 – 318 .
Nishitani K. Nevins D.J. ( 1991 ) Glucuronoxylan xylanohydrolase. A unique xylanase with the requirement for appendant glucuronosyl units . J. Biol. Chem . 266 , 6539 – 6543 .
Biely, P., Vrsanska, M., Kremnicky, L., Tenkanen, M., Poutanen, K., Hayn, M. (1993) Catalytic properties of endo-b-1,4-xylanases of Trichoderma reesei . In: Trichoderma reesei Cellulases and Other Hydrolases (Suominen, P., Reinikainen, T., Eds.), pp.125–135 Fagepaino Oy, Helsinki.
Parkkinen T. Hakulinen N. Tenkanen M. Siika-aho M. Rouvinen J. ( 2004 ) Crystallization and preliminary X-ray analysis of a novel Trichoderma reesei xylanase IV belonging to glycoside hydrolase family 5 . Acta Crystallogr. D: Biol. Crystallogr . 60 , 542 – 544 .
Takami H. Nakasone K. Takaki Y. Maeno G. Sasaki R. Masui N. Fuji F. Hirama C. Nakamura Y. Ogasawara N. Kuhara S. Horikoshi K. ( 2000 ) Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis . Nucleic Acids Res . 28 , 4317 – 4331 .
Takami H. Horikoshi K. ( 2000 ) Analysis of the genome of an alkaliphilic Bacillus strain from an industrial point of view . Extremophiles 4 , 99 – 108 .
Dutron, A., Georis, J., Genot, B., Dauvrin, T., Collins, T., Hoyoux, A. and Feller, G. (2004) Use of family 8 enzymes with xylanolytic activity in baking. In: World Intellectual Property Organization, PCT, WO 2004/023879 A1
Collins T. Meuwis M.A. Stals I. Claeyssens M. Feller G. Gerday C. ( 2002 ) A novel family 8 xylanase: functional and physico-chemical characterization . J. Biol. Chem . 277 , 35133 – 35139 .
Collins T. Meuwis M.A. Gerday C. Feller G. ( 2003 ) Activity, stability and flexibility in glycosidases adapted to extreme thermal environments . J. Mol. Biol . 328 , 419 – 428 .
Van Petegem F. Collins T. Meuwis M.A. Gerday C. Feller G. Van Beeumen J. ( 2002 ) Crystallization and preliminary X-ray analysis of a xylanase from the psychrophile Pseudoalteromonas haloplanktis . Acta Crystallogr. D: Biol. Crystallogr . 58 , 1494 – 1496 .
Van Petegem F. Collins T. Meuwis M.A. Gerday C. Feller G. Van Beeumen J. ( 2003 ) The structure of a cold-adapted family 8 xylanase at 1.3 Å resolution. Structural adaptations to cold and investgation of the active site . J. Biol. Chem . 278 , 7531 – 7539 .
Yoon K.H. Yun H.N. Jung K.H. ( 1998 ) Molecular cloning of a Bacillus sp. KK-1 xylanase gene and characterization of the gene product . Biochem. Mol. Biol. Int . 45 , 337 – 347 .
Parsiegla G. Juy M. Reverbel-Leroy C. Tardif C. Belaich J.P. Driguez H. Haser R. ( 1998 ) The crystal structure of the processive endocellulase CelF of Clostridium cellulolyticum in complex with a thiooligosaccharide inhibitor at 2.0 Å resolution . EMBO J . 17 , 5551 – 5562 .
Egloff M.P. Uppenberg J. Haalck L. Van Tilbeurgh H. ( 2001 ) Crystal structure of maltose phosphorylase from Lactobacillus brevis : unexpected evolutionary relationship with glucoamylases . Structure (Camb) 9 , 689 – 697 .
Fierobe H.P. Bagnara-Tardif C. Gaudin C. Guerlesquin F. Sauve P. Belaich A. Belaich J.P. ( 1993 ) Purification and characterization of endoglucanase C from Clostridium cellulolyticum . Catalytic comparison with endoglucanase A . Eur. J. Biochem . 217 , 557 – 565 .
Biely, P. (2003) Diversity of microbial endo-b-1,4-xylanases. In: Applications of Enzymes to Lignocellulosics (Mansfield, S.D., Saddler, J.N., Eds.), pp.361–380 American chemical Society, Washington.
Gilkes N.R. Claeyssens M. Aebersold R. Henrissat B. Meinke A. Morrison H.D. Kilburn D.G. Warren R.A. Miller R.C. Jr. ( 1991 ) Structural and functional relationships in two families of beta-1,4-glycanases . Eur. J. Biochem . 202 , 367 – 377 .
Biely P. Vrsanska M. Tenkanen M. Kluepfel D. ( 1997 ) Endo-beta-1,4-xylanase families: differences in catalytic properties . J. Biotechnol . 57 , 151 – 166 .
Van Tilbeurgh H. Claeyssens M. ( 1985 ) Detection and differentiation of cellulase components using low molecular mass fluorogenic substrates . FEBS Lett . 187 , 283 – 288 .
Biely P. Kluepfel D. Morosoli R. Shareck F. ( 1993 ) Mode of action of three endo-beta-1,4-xylanases of Streptomyces lividans . Biochim. Biophys. Acta 1162 , 246 – 254 .
Haas H. Herfurth E. Stoffler G. Redl B. ( 1992 ) Purification, characterization and partial amino acid sequences of a xylanase produced by Penicillium chrysogenum . Biochim. Biophys. Acta 1117 , 279 – 286 .
Biely P. Kratky Z. Vrsanska M. ( 1981 ) Substrate-binding site of endo-1,4-beta-xylanase of the yeast Cryptococcus albidus . Eur. J. Biochem . 119 , 559 – 564 .
Derewenda U. Swenson L. Green R. Wei Y. Morosoli R. Shareck F. Kluepfel D. Derewenda Z.S. ( 1994 ) Crystal structure, at 2.6-Å resolution, of the Streptomyces lividans xylanase A, a member of the F family of beta-1,4-d-glycanases . J. Biol. Chem . 269 , 20811 – 20814 .
White A. Withers S.G. Gilkes N.R. Rose D.R. ( 1994 ) Crystal structure of the catalytic domain of the beta-1,4-glycanase cex from Cellulomonas fimi . Biochemistry 33 , 12546 – 12552 .
Harris G.W. Jenkins J.A. Connerton I. Cummings N. Lo Leggio L. Scott M. Hazlewood G.P. Laurie J.I. Gilbert H.J. Pickersgill R.W. ( 1994 ) Structure of the catalytic core of the family F xylanase from Pseudomonas fluorescens and identification of the xylopentaose-binding sites . Structure 2 , 1107 – 1116 .
Pell G. Taylor E.J. Gloster T.M. Turkenburg J.P. Fontes C.M. Ferreira L.M. Nagy T. Clark S.J. Davies G.J. Gilbert H.J. ( 2004 ) The mechanisms by which family 10 glycoside hydrolases bind decorated substrates . J. Biol. Chem . 279 , 9597 – 9605 .
Pell G. Szabo L. Charnock S.J. Xie H. Gloster T.M. Davies G.J. Gilbert H.J. ( 2004 ) Structural and biochemical analysis of Cellvibrio japonicus xylanase 10C: how variation in substrate-binding cleft influences the catalytic profile of family GH-10 xylanases . J. Biol. Chem . 279 , 11777 – 11788 .
Dominguez R. Souchon H. Spinelli S. Dauter Z. Wilson K.S. Chauvaux S. Beguin P. Alzari P.M. ( 1995 ) A common protein fold and similar active site in two distinct families of beta-glycanases . Nat. Struct. Biol . 2 , 569 – 576 .
Mechaly A. Teplitsky A. Belakhov V. Baasov T. Shoham G. Shoham Y. ( 2000 ) Overproduction and characterization of seleno-methionine xylanase T-6 . J. Biotechnol . 78 , 83 – 86 .
Schmidt A. Schlacher A. Steiner W. Schwab H. Kratky C. ( 1998 ) Structure of the xylanase from Penicillium simplicissimum . Protein Sci . 7 , 2081 – 2088 .
Canals A. Vega M.C. Gomis-Ruth F.X. Diaz M. Santamaria R.R. Coll M. ( 2003 ) Structure of xylanase Xys1delta from Streptomyces halstedii . Acta Crystallogr. D: Biol. Crystallogr . 59 , 1447 – 1453 .
Fujimoto Z. Kuno A. Kaneko S. Yoshida S. Kobayashi H. Kusakabe I. Mizuno H. ( 2000 ) Crystal structure of Streptomyces olivaceoviridis E-86 beta-xylanase containing xylan-binding domain . J. Mol. Biol . 300 , 575 – 585 .
Natesh R. Bhanumoorthy P. Vithayathil P.J. Sekar K. Ramakumar S. Viswamitra M.A. ( 1999 ) Crystal structure at 1.8 A resolution and proposed amino acid sequence of a thermostable xylanase from Thermoascus aurantiacus . J. Mol. Biol . 288 , 999 – 1012 .
Ryttersgaard C. Lo Leggio L. Coutinho P.M. Henrissat B. Larsen S. ( 2002 ) Aspergillus aculeatus beta-1,4-galactanase: substrate recognition and relations to other glycoside hydrolases in clan GH-A . Biochemistry 41 , 15135 – 15143 .
Christakopoulos P. Katapodis P. Kalogeris E. Kekos D. Macris B.J. Stamatis H. Skaltsa H. ( 2003 ) Antimicrobial activity of acidic xylo-oligosaccharides produced by family 10 and 11 endoxylanases . Int. J. Biol. Macromol . 31 , 171 – 175 .
Ntarima, P. (2000) Les xylanases des familles 10 et 11: différentiation et caractérisation, Laboratory of Biochemistry, University of Gent, Gent, pp. 96
Katapodis P. Vrsanska M. Kekos D. Nerinckx W. Biely P. Claeyssens M. Macris B.J. Christakopoulos P. ( 2003 ) Biochemical and catalytic properties of an endoxylanase purified from the culture filtrate of Sporotrichum thermophile . Carbohydr. Res . 338 , 1881 – 1890 .
Bray M.R. Clarke A.J. ( 1992 ) Action pattern of xylo-oligosaccharide hydrolysis by Schizophyllum commune xylanase A . Eur. J. Biochem . 204 , 191 – 196 .
Vrsanska M. Gorbacheva I.V. Kratky Z. Biely P. ( 1982 ) Reaction pathways of substrate degradation by an acidic endo-1,4-beta-xylanase of Aspergillus niger . Biochim. Biophys. Acta 704 , 114 – 122 .
Heightman T.D. Vasella A. ( 1999 ) Recent insights into inhibition, structure, and mechanism of configuration-retaining glycosidase . Angew. Chem. Int. Ed . 38 , 750 – 770 .
Ntarima P. Nerinckx W. Klarskov K. Devreese B. Bhat M.K. Van Beeumen J. Claeyssens M. ( 2000 ) Epoxyalkyl glycosides of d-xylose and xylo-oligosaccharides are active-site markers of xylanases from glycoside hydrolase family 11, not from family 10 . Biochem. J . 347 ( Pt 3 ), 865 – 873 .
Fushinobu S. Ito K. Konno M. Wakagi T. Matsuzawa H. ( 1998 ) Crystallographic and mutational analyses of an extremely acidophilic and acid-stable xylanase: biased distribution of acidic residues and importance of Asp37 for catalysis at low pH . Protein Eng . 11 , 1121 – 1128 .
Krengel U. Dijkstra B.W. ( 1996 ) Three-dimensional structure of Endo-1,4-beta-xylanase I from Aspergillus niger , molecular basis for its low pH optimum . J. Mol. Biol . 263 , 70 – 78 .
Sabini E. Wilson K.S. Danielsen S. Schulein M. Davies G.J. ( 2001 ) Oligosaccharide binding to family 11 xylanases: both covalent intermediate and mutant product complexes display (2,5)B conformations at the active. centre . Acta Crystallogr. D: Biol. Crystallogr . 57 , 1344 – 1347 .
Wakarchuk W.W. Campbell R.L. Sung W.L. Davoodi J. Yaguchi M. ( 1994 ) Mutational and crystallographic analyses of the active site residues of the Bacillus circulans xylanase . Protein Sci . 3 , 467 – 475 .
Oakley A.J. Heinrich T. Thompson C.A. Wilce M.C. ( 2003 ) Characterization of a family 11 xylanase from Bacillus subtillis B230 used for paper bleaching . Acta Crystallogr. D: Biol. Crystallogr . 59 , 627 – 636 .
Ay J. Gotz F. Borriss R. Heinemann U. ( 1998 ) Structure and function of the Bacillus hybrid enzyme GluXyn-1: native-like jellyroll fold preserved after insertion of autonomous globular domain . Proc. Natl. Acad. Sci. USA 95 , 6613 – 6618 .
Hakulinen N. Turunen O. Janis J. Leisola M. Rouvinen J. ( 2003 ) Three-dimensional structures of thermophilic beta-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa . Comparison of twelve xylanases in relation to their thermal stability . Eur. J. Biochem . 270 , 1399 – 1412 .
McCarthy A.A. Morris D.D. Bergquist P.L. Baker E.N. ( 2000 ) Structure of XynB, a highly thermostable beta-1,4-xylanase from Dictyoglomus thermophilum Rt46B.1, at 1.8 Å resolution . Acta Crystallogr. D: Biol. Crystallogr . 56 ( Pt 11 ), 1367 – 1375 .
Kumar P.R. Eswaramoorthy S. Vithayathil P.J. Viswamitra M.A. ( 2000 ) The tertiary structure at 1.59 Å resolution and the proposed amino acid sequence of a family-11 xylanase from the thermophilic fungus Paecilomyces varioti bainier . J. Mol. Biol . 295 , 581 – 593 .
Wouters J. Georis J. Engher D. Vandenhaute J. Dusart J. Frere J.M. Depiereux E. Charlier P. ( 2001 ) Crystallographic analysis of family 11 endo-beta-1,4-xylanase Xyl1 from Streptomyces sp. S38 . Acta Crystallogr. D: Biol. Crystallogr . 57 , 1813 – 1819 .
Gruber K. Klintschar G. Hayn M. Schlacher A. Steiner W. Kratky C. ( 1998 ) Thermophilic xylanase from Thermomyces lanuginosus : high-resolution X-ray structure and modeling studies . Biochemistry 37 , 13475 – 13485 .
Campbell, R.L., Rose, D.R., Wakarchuk, W.W., To, R.J., Sung, Z., Yagushi, M. (1993) High resolution structures of xylanases from Bacillus circulans and Trichoderma harzianum identify a new folding pattern and implications for the atomic basis of the catalysis. Foundation for biotechnical and industrial fermentation research. In: Trichoderma reesei Cellulases and Other Hydrolases (Souminen, P., Reikainen, T., Eds.), pp.63–72 Espoo, Finland.
Torronen A. Rouvinen J. ( 1995 ) Structural comparison of two major endo-1,4-xylanases from Trichoderma reesei . Biochemistry 34 , 847 – 856 .
Torronen A. Harkki A. Rouvinen J. ( 1994 ) Three-dimensional structure of endo-1,4-beta-xylanase II from Trichoderma reesei : two conformational states in the active site . EMBO J . 13 , 2493 – 2501 .
Harris G.W. Pickersgill R.W. Connerton I. Debeire P. Touzel J.P. Breton C. Perez S. ( 1997 ) Structural basis of the properties of an industrially relevant thermophilic xylanase . Proteins 29 , 77 – 86 .
Penttila M. Lehtovaara P. Nevalainen H. Bhikhabhai R. Knowles J. ( 1986 ) Homology between cellulase genes of Trichoderma reesei : complete nucleotide sequence of the endoglucanase I gene . Gene 1692 , 253 – 263 .
Biely P. Vrsanska M. Claeyssens M. ( 1991 ) The endo-1,4-beta-glucanase I from Trichoderma reesei . Action on beta-1,4-oligomers and polymers derived from d-glucose and d-xylose . Eur. J. Biochem . 200 , 157 – 163 .
Kleywegt G.J. Zou J.Y. Divne C. Davies G.J. Sinning I. Stahlberg J. Reinikainen T. Srisodsuk M. Teeri T.T. Jones T.A. ( 1997 ) The crystal structure of the catalytic core domain of endoglucanase I from Trichoderma reesei at 3.6 Å resolution, and a comparison with related enzymes . J. Mol. Biol . 272 , 383 – 397 .
Gosalbes M.J. Perez-Gonzalez J.A. Gonzalez R. Navarro A. ( 1991 ) Two beta-glycanase genes are clustered in Bacillus polymyxa : molecular cloning, expression, and sequence analysis of genes encoding a xylanase and an endo-beta-(1,3)-(1,4)-glucanase . J. Bacteriol . 173 , 7705 – 7710 .
Morris D.D. Gibbs M.D. Ford M. Thomas J. Bergquist P.L. ( 1999 ) Family 10 and 11 xylanase genes from Caldicellulosiruptor sp. strain Rt69B.1 . Extremophiles 3 , 103 – 111 .
Gibbs M.D. Reeves R.A. Farrington G.K. Anderson P. Williams D.P. Bergquist P.L. ( 2000 ) Multidomain and multifunctional glycosyl hydrolases from the extreme thermophile Caldicellulosiruptor isolate Tok7B.1 . Curr. Microbiol . 40 , 333 – 340 .
Schell M.A. Karmirantzou M. Snel B. Vilanova D. Berger B. Pessi G. Zwahlen M.C. Desiere F. Bork P. Delley M. Pridmore R.D. Arigoni F. ( 2002 ) The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract . Proc. Natl. Acad. Sci. USA 99 , 14422 – 14427 .
Kimura T. Ito J. Kawano A. Makino T. Kondo H. Karita S. Sakka K. Ohmiya K. ( 2000 ) Purification, characterization, and molecular cloning of acidophilic xylanase from Penicillium sp.40 . Biosci. Biotechnol. Biochem . 64 , 1230 – 1237 .
Waino M. Ingvorsen K. ( 2003 ) Production of beta-xylanase and beta-xylosidase by the extremely halophilic archaeon Halorhabdus utahensis . Extremophiles 7 , 87 – 93 .
Wejse P.L. Ingvorsen K. Mortensen K.K. ( 2003 ) Purification and characterisation of two extremely halotolerant xylanases from a novel halophilic bacterium . Extremophiles 7 , 423 – 431 .
Vieille C. Zeikus G.J. ( 2001 ) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability . Microbiol. Mol. Biol. Rev . 65 , 1 – 43 .
Cannio R. Di Prizito N. Rossi M. Morana A. ( 2004 ) A xylan-degrading strain of Sulfolobus solfataricus : isolation and characterization of the xylanase activity . Extremophiles 8 , 117 – 124 .
Sunna A. Bergquist P.L. ( 2003 ) A gene encoding a novel extremely thermostable1,4-beta-xylanase isolated directly from an environmental DNA sample . Extremophiles 7 , 63 – 70 .
Sunna A. Moracci M. Rossi M. Antranikian G. ( 1997 ) Glycosyl hydrolases from hyperthermophiles . Extremophiles 1 , 2 – 13 .
Uhl A.M. Daniel R.M. ( 1999 ) The first description of an archaeal hemicellulase: the xylanase from Thermococcus zilligii strain AN1 . Extremophiles 3 , 263 – 267 .
Zverlov V. Piotukh K. Dakhova O. Velikodvorskaya G. Borriss R. ( 1996 ) The multidomain xylanase A of the hyperthermophilic bacterium Thermotoga neapolitana is extremely thermoresistant . Appl. Microbiol. Biotechnol . 45 , 245 – 247 .
Luthi E. Jasmat N.B. Bergquist P.L. ( 1990 ) Xylanase from the extremely thermophilic bacterium Caldocellum saccharolyticum : overexpression of the gene in Escherichia coli and characterization of the gene product . Appl. Environ. Microbiol . 56 , 2677 – 2683 .
Abou-Hachem M. Olsson F. Nordberg Karlsson E. ( 2003 ) Probing the stability of the modular family 10 xylanase from Rhodothermus marinus . Extremophiles 7 , 483 – 491 .
Khasin A. Alchanati I. Shoham Y. ( 1993 ) Purification and characterization of a thermostable xylanase from Bacillus stearothermophilus T-6 . Appl. Environ. Microbiol . 59 , 1725 – 1730 .
Simpson H.D. Haufler U.R. Daniel R.M. ( 1991 ) An extremely thermostable xylanase from the thermophilic eubacterium Thermotoga . Biochem. J . 277 ( Pt 2 ), 413 – 417 .
Schlacher A. Holzmann K. Hayn M. Steiner W. Schwab H. ( 1996 ) Cloning and characterization of the gene for the thermostable xylanase XynA from Thermomyces lanuginosus . J. Biotechnol . 49 , 211 – 218 .
Connerton I. Cummings N. Harris G.W. Debeire P. Breton C. ( 1999 ) A single domain thermophilic xylanase can bind insoluble xylan: evidence for surface aromatic clusters . Biochim. Biophys. Acta 1433 , 110 – 121 .
Andrade C.M.M.C. Pereira N. Antranikian G. ( 1999 ) Extremely thermophilic microorganisms and their polymer-hydrolytic enzymes . Rev. Microbiol . 30 , 287 – 298 .
Niehaus F. Bertoldo C. Kahler M. Antranikian G. ( 1999 ) Extremophiles as a source of novel enzymes for industrial application . Appl. Microbiol. Biotechnol . 51 , 711 – 729 .
Bragger J.M. Daniel R.M. Coolbear T. Morgan H.W. ( 1989 ) Very stable enzymes from extremely thermophilic archaebacteria and eubacteria . Appl. Environ. Microbiol . 31 , 556 – 561 .
Turunen O. Vuorio M. Fenel F. Leisola M. ( 2002 ) Engineering of multiple arginines into the Ser/Thr surface of Trichoderma reesei endo-1,4-beta-xylanase II increases the thermotolerance and shifts the pH optimum towards alkaline pH . Protein Eng . 15 , 141 – 145 .
Fontes C.M. Hall J. Hirst B.H. Hazlewood G.P. Gilbert H.J. ( 1995 ) The resistance of cellulases and xylanases to proteolytic inactivation . Appl. Microbiol. Biotechnol . 43 , 52 – 57 .
Turunen O. Etuaho K. Fenel F. Vehmaanpera J. Wu X. Rouvinen J. Leisola M. ( 2001 ) A combination of weakly stabilizing mutations with a disulfide bridge in the alpha-helix region of Trichoderma reesei endo-1,4-beta-xylanase II increases the thermal stability through synergism . J. Biotechnol . 88 , 37 – 46 .
Wakarchuk W.W. Sung W.L. Campbell R.L. Cunningham A. Watson D.C. Yaguchi M. ( 1994 ) Thermostabilization of the Bacillus circulans xylanase by the introduction of disulfide bonds . Protein Eng . 7 , 1379 – 1386 .
Sheridan P.P. Panasik N. Coombs J.M. Brenchley J.E. ( 2000 ) Approaches for deciphering the structural basis of low temperature enzyme activity . Biochim. Biophys. Acta 1543 , 417 – 433 .
Humphry D.R. George A. Black G.W. Cummings S.P. ( 2001 ) Flavobacterium frigidarium sp. nov., an aerobic, psychrophilic, xylanolytic and laminarinolytic bacterium from Antarctica . Int. J. Syst. Evol. Microbiol . 51 , 1235 – 1243 .
Akila G. Chandra T.S. ( 2003 ) A novel cold-tolerant Clostridium strain PXYL1 isolated from a psychrophilic cattle manure digester that secretes thermolabile xylanase and cellulase . FEMS Microbiol. Lett . 219 , 63 – 67 .
Petrescu I. Lamotte-Brasseur J. Chessa J.P. Ntarima P. Claeyssens M. Devreese B. Marino G. Gerday C. ( 2000 ) Xylanase from the psychrophilic yeast Cryptococcus adeliae . Extremophiles 4 , 137 – 144 .
Turkiewiz M. Kalinowska H. Zielinska M. Bielecki S. ( 2000 ) Purification and characterisation of two endo-1,4-xylanases from Antarctic krill, Euphasia superba Dana . Comp. Biol. Physiol. Part B 127 , 325 – 335 .
Bradner J.R. Sidhu R.K. Gillings M. Nevalainen K.M. ( 1999 ) Hemicellulase activity of antarctic microfungi . J. Appl. Microbiol . 87 , 366 – 370 .
Inglis G.D. Popp A.P. Selinger L.B. Kawchuk L.M. Gaudet D.A. McAllister T.A. ( 2000 ) Production of cellulases and xylanases by low-temperature basidiomycetes . Can. J. Microbiol . 46 , 860 – 865 .
Collins, T., Claverie, P., D'Amico, S., Georlette, D., Gratia, E., Hoyoux, A., Meuwis, M.A., Poncin, J., Sonan, G., Feller, G., Gerday, C., Life in the Cold: Psychrophilic Enzymes. Recent Research Developments in Proteins. Vol. 1, 2002. Transworld Research Network, Trivandrum. pp. 13–26
Feller G. Gerday C. ( 2003 ) Psychrophilic enzymes: hot topics in cold adaptation . Nat. Rev. Microbiol . 1 , 200 – 208 .
Georlette D. Blaise V. Collins T. D'Amico S. Gratia E. Hoyoux A. Marx J.C. Sonan G. Feller G. Gerday C. ( 2004 ) Some like it cold: biocatalysis at low temperatures . FEMS Microbiol. Rev . 28 , 25 – 42 .
Horikoshi K. ( 1999 ) Alkaliphiles: some applications of their products for biotechnology . Microbiol. Mol. Biol. Rev . 63 , 735 – 750 .
Gessesse A. ( 1998 ) Purification and properties of two thermostable alkaline xylanases from an alkaliphilic Bacillus sp . Appl. Environ. Microbiol . 64 , 3533 – 3535 .
Yang V.W. Zhuang Z. Elegir G. Jeffries T.W. ( 1995 ) Alkaline-active xylanase produced by an alkaliphilic Bacillus sp. isolated from kraft pulp . J. Indust. Microbiol . 15 , 434 – 441 .
Ratanakhanokchai K. Kyu K.L. Tanticharoen M. ( 1999 ) Purification and properties of a xylan-binding endoxylanase from alkaliphilic Bacillus sp. strain K-1 . Appl. Environ. Microbiol . 65 , 694 – 697 .
Duarte M.C. Pellegrino A.C. Portugal E.P. Ponezi A.N. Franco T.T. ( 2000 ) Characterization of alkaline xylanases from Bacillus pumilus . Braz. J. Microbiol . 31 , 90 – 94 .
Christakopoulos P. Nerinckx W. Kekos D. Macris B. Claeyssens M. ( 1996 ) Purification and characterization of two low molecular mass alkaline xylanases from Fusarium oxysporum F3 . J. Biotechnol . 51 , 181 – 189 .
Nakamura S. Wakabayashi K. Nakai R. Aono R. Horikoshi K. ( 1993 ) Purification and some properties of an alkaline xylanase from alkaliphilic Bacillus sp. strain 41M-1 . Appl. Environ. Microbiol . 59 , 2311 – 2316 .
Horikoshi K. Atsukawa Y. ( 1973 ) Xylanase produced by alkalophilic Bacillus no C-59-2 . Agric. Biol. Chem . 37 , 2097 – 2103 .
Torronen A. Mach R.L. Messner R. Gonzalez R. Kalkkinen N. Harkki A. Kubicek C.P. ( 1992 ) The two major xylanases from Trichoderma reesei : characterization of both enzymes and genes . Biotechnology (NY) 10 , 1461 – 1465 .
Inagaki K. Nakahira K. Mukai K. Tamura T. Tanaka H. ( 1998 ) Gene cloning and characterization of an acidic xylanase from Acidobacterium capsulatum . Biosci. Biotechnol. Biochem . 62 , 1061 – 1067 .
Iefuji H. Chino M. Kato M. Iimura Y. ( 1996 ) Acid xylanase from yeast Cryptococcus sp. S-2: purification, characterization, cloning, and sequencing . Biosci. Biotechnol. Biochem . 60 , 1331 – 1338 .
Ito K. Iwashita K. Iwano K. ( 1992 ) Cloning and sequencing of the xynC gene encoding acid xylanase of Aspergillus kawachii . Biosci. Biotechnol. Biochem . 56 , 1338 – 1340 .
Joshi M.D. Sidhu G. Nielsen J.E. Brayer G.D. Withers S.G. McIntosh L.P. ( 2001 ) Dissecting the electrostatic interactions and pH-dependent activity of a family 11 glycosidase . Biochemistry 40 , 10115 – 10139 .
Sapag A. Wouters J. Lambert C. De Ioannes P. Eyzaguirre J. Depiereux E. ( 2002 ) The endoxylanases from family 11: computer analysis of protein sequences reveals important structural and phylogenetic relationships . J. Biotechnol . 95 , 109 – 131 .
Joshi M.D. Sidhu G. Pot I. Brayer G.D. Withers S.G. McIntosh L.P. ( 2000 ) Hydrogen bonding and catalysis: a novel explanation for how a single amino acid substitution can change the pH optimum of a glycosidase . J. Mol. Biol . 299 , 255 – 279 .
Chen Y.L. Tang T.Y. Cheng K.J. ( 2001 ) Directed evolution to produce an alkalophilic variant from a Neocallimastix patriciarum xylanase . Can. J. Microbiol . 47 , 1088 – 1094 .
Godfrey, T., West, S. (1996) Industrial Enzymology, Second ed. Macmillan Press Ltd., London.
Godfrey, T. (2003) The enzymes market for grain processing. In: Recent Advances in Enzymes in Grain Processing (Courtin, C.M., Veraverbeke, W.S., Delcour, J.A., Eds.), pp.401–406 Kat. Univ. Leuven, Leuven.
Bhat M.K. ( 2000 ) Cellulases and related enzymes in biotechnology . Biotech. Adv . 18 , 355 – 383 .
Tikhomirov, D.F., Sinitsyn, A.P., Zorov, I.N., Williams, C. (2003) Non-starch polysaccharide hydrolysing microbial enzymes in grain processing. In: Recent Advances in Enzymes in Grain Processing (Courtin, C.M., Veraverbeke, W.S., Delcour, J.A., Eds.), pp.413–418 Kat. Univ. Leuven, Leuven.
Kamal Kumar, B., Balakrishnan, H. and Rele, M.V. (2004) Compatibility of alkaline xylanases from an alkaliphilic Bacillus NCL (87-6-10) with commercial detergents and proteases. J. Ind. Microbiol. Biotechnol. Epub ahead of print
Katapodis P. Vardakou M. Kalogeris E. Kekos D. Macris B.J. Christakopoulos P. ( 2003 ) Enzymic production of a feruloylated oligosaccharide with antioxidant activity from wheat flour arabinoxylan . Eur. J. Nutr . 42 , 55 – 60 .
Matsumura S. Sakiyama K. Toshima K. ( 1999 ) Preparation of octyl-B-d-xylobioside and xyloside by xylanase catalyzed direct transglycosylation reaction of xylan and octanol . Biotechnol. Lett . 21 , 17 – 22 .
Imanaka, T. and Sakurai, S. (1992) Method of washing super precision devices, semiconductors, with enzymes. United States Patent 5,078,802
Viikari L. ( 1994 ) Xylanases in bleaching: from an idea to the industry . FEMS Microbiol. Rev . 13 , 335 – 350 .
Mielenz J.R. ( 2001 ) Ethanol production from biomass: technology and commercialization status . Curr. Opin. Microbiol . 4 , 324 – 329 .
Saha B.C. ( 2003 ) Hemicellulose bioconversion . J. Ind. Microbiol. Biotechnol . 30 , 279 – 291 .
Campenhout, L.V., Somers, I., Van de Craen, S., Adams, C. (2003) In vitro test to evaluate protein degradation by feed enzymes. In: Recent Advances in Enzymes in Grain Processing (Courtin, C.M., Veraverbeke, W.S., Delcour, J.A., Eds.), pp.387–390 Kat. Univ. Leuven., Leuven.
Galante, Y.M., De Conti, A., Monteverdi, R. (1998) Application of Trichoderma enzymes in food and feed industries. In: Trichoderma and Gliocladium – Enzymes, Biological Control and Commercial Applications (Harman, G.E., Kubicek, C.P., Eds.), pp.327–342 Taylor and Francis, London.
Wong, K.K.Y., Saddler, J.N. (1993) Applications of hemicellulases in the food, feed and pulp and paper industries. In: Hemicelluloses and Hemicellulases (Coughlan, M.P., Hazlewood, G.P., Eds.), pp.127–143 Portland Press, London.
Maat, J., Roza, M., Verbakel, J., Stam, H., daSilra, M.J.S., Egmond, M.R., Hagemans, M.L.D., van Garcom, R.F.M., Hessing, J.G.M., van Derhondel, C., van Rotterdam, C. (1992) Xylanases and their application in baking. In: Xylan and Xylanases (Visser, J., Beldman, G., van Someren, M.A.K., Voragen, A.G.J., Eds.), pp.349–360 Elsevier, Amsterdam.
Mathlouthi N. Lalles J.P. Lepercq P. Juste C. Larbier M. ( 2002 ) Xylanase and beta-glucanase supplementation improve conjugated bile acid fraction in intestinal contents and increase villus size of small intestine wall in broiler chickens fed a rye-based diet . J. Anim. Sci . 80 , 2773 – 2779 .
Mathlouthi N. Mohamed M.A. Larbier M. ( 2003 ) Effect of enzyme preparation containing xylanase and beta-glucanase on performance of laying hens fed wheat/barley- or maize/soybean meal-based diets . Br. Poult. Sci . 44 , 60 – 66 .
Mathlouthi N. Juin H. Larbier M. ( 2003 ) Effect of xylanase and beta-glucanase supplementation of wheat- or wheat- and barley-based diets on the performance of male turkeys . Br. Poult. Sci . 44 , 291 – 298 .
Pala H. Mota M. Gama F.M. ( 2004 ) Enzymatic versus chemical deinking of non-impact ink printed paper . J. Biotechnol . 108 , 79 – 89 .
Frederix, S.A., Courtin, C.M., Delcour, J.A. (2003) Impact of endoxylanases with different substrate selectivity on gluten-starch separation. In: Recent Advances in Enzymes in Grain Processing (Courtin, C.M., Veraverbeke, W.S., Delcour, J.A., Eds.), pp.247–254 Kat. Univ. Leuven, Leuven.
Sharma H.S.S. ( 1987 ) Enzymatic degradation of residual non-cellulosic polysaccharides present on dew-retted flax fibers . Appl. Microbiol. Biotechnol . 26 , 2714 – 2723 .
Guex N. Peitsch M.C. ( 1997 ) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling . Electrophoresis 18 , 2714 – 2723 .
Georis J. Giannotta F. Lamotte-Brasseur J. Devreese B. Van Beeumen J. Granier B. Frere J.M. ( 1999 ) Sequence, overproduction and purification of the family 11 endo-beta-1,4-xylanase encoded by the xyl1 gene of Streptomyces sp. S38 . Gene 237 , 123 – 133 .
Beguin P. Cornet P. Aubert J.P. ( 1985 ) Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum . J. Bacteriol . 162 , 102 – 105 .
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 27 |
| February 2017 | 87 |
| March 2017 | 89 |
| April 2017 | 82 |
| May 2017 | 66 |
| June 2017 | 48 |
| July 2017 | 41 |
| August 2017 | 51 |
| September 2017 | 86 |
| October 2017 | 60 |
| November 2017 | 90 |
| December 2017 | 141 |
| January 2018 | 172 |
| February 2018 | 145 |
| March 2018 | 257 |
| April 2018 | 225 |
| May 2018 | 261 |
| June 2018 | 175 |
| July 2018 | 166 |
| August 2018 | 196 |
| September 2018 | 236 |
| October 2018 | 274 |
| November 2018 | 333 |
| December 2018 | 260 |
| January 2019 | 204 |
| February 2019 | 238 |
| March 2019 | 353 |
| April 2019 | 401 |
| May 2019 | 369 |
| June 2019 | 315 |
| July 2019 | 239 |
| August 2019 | 231 |
| September 2019 | 244 |
| October 2019 | 358 |
| November 2019 | 383 |
| December 2019 | 294 |
| January 2020 | 231 |
| February 2020 | 211 |
| March 2020 | 273 |
| April 2020 | 366 |
| May 2020 | 258 |
| June 2020 | 334 |
| July 2020 | 295 |
| August 2020 | 216 |
| September 2020 | 269 |
| October 2020 | 270 |
| November 2020 | 277 |
| December 2020 | 285 |
| January 2021 | 285 |
| February 2021 | 323 |
| March 2021 | 470 |
| April 2021 | 369 |
| May 2021 | 412 |
| June 2021 | 316 |
| July 2021 | 223 |
| August 2021 | 264 |
| September 2021 | 297 |
| October 2021 | 456 |
| November 2021 | 515 |
| December 2021 | 492 |
| January 2022 | 430 |
| February 2022 | 352 |
| March 2022 | 547 |
| April 2022 | 468 |
| May 2022 | 560 |
| June 2022 | 367 |
| July 2022 | 230 |
| August 2022 | 273 |
| September 2022 | 310 |
| October 2022 | 464 |
| November 2022 | 404 |
| December 2022 | 541 |
| January 2023 | 341 |
| February 2023 | 309 |
| March 2023 | 401 |
| April 2023 | 404 |
| May 2023 | 446 |
| June 2023 | 238 |
| July 2023 | 318 |
| August 2023 | 317 |
| September 2023 | 277 |
| October 2023 | 378 |
| November 2023 | 366 |
| December 2023 | 319 |
| January 2024 | 380 |
| February 2024 | 344 |
| March 2024 | 521 |
| April 2024 | 433 |
| May 2024 | 378 |
| June 2024 | 292 |
| July 2024 | 259 |
| August 2024 | 113 |
Email alerts
Citing articles via.
- Recommend to your Library
- Journals Career Network
Affiliations
- Online ISSN 1574-6976
- Print ISSN 0168-6445
- Copyright © 2024 Federation of European Microbiological Societies
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Microbial xylanases and their industrial applications: A review
- September 2001
- Applied Microbiology and Biotechnology 56(3-4):326-38
- 56(3-4):326-38
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Central Food Technological Research Institute

- University of Delhi
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Priyanka Nath
- Ashily Rajendra

- Attalla F El-Kott
- Muhammad Qamar Bilal

- MOL PLANT PATHOL

- Yanxin Wang

- BIORESOURCES
- Caroline T. Gilleran
- Alan Hernon

- ENVIRON SCI POLLUT R
- Guiyang Zeng

- Nguyen Xuan Hoan
- Nguyễn Huỳnh Đạt

- Fatma A. Abo Nouh

- Jovanderson J. B. da Silva

- CARBOHYD RES

- Sithes Logendra
- Ilya Raskin
- Shigeru Eda
- Akio Ohnishi

- Geeta Nanda
- Koki Horikoshi

- Anne Kantelinen

- J.C.P. Boonman
- G.C.M. Selten

- J. Schuseil
- Michael P. Coughlan

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Microbiol
- v.2012; 2012

Isolation, Purification, and Characterization of Xylanase Produced by a New Species of Bacillus in Solid State Fermentation
Rajashri d. kamble.
1 Department of Biotechnology Engineering, Tatyasaheb Kore Institute of Engineering & Technology, Warananagar Panhala, Kolhapur, Maharashtra, 416113, India
Anandrao R. Jadhav
2 Department of Microbiology, K.R.P. Kanya Mahavidyalaya, Islampur, Walwa, Sangli, Maharashtra, 415414, India
A thermoalkalophilic new species of Bacillus , similar to Bacillus arseniciselenatis DSM 15340, produced extracellular xylanase under solid state fermentation when wheat bran is used as carbon source. The extracellular xylanase was isolated by ammonium sulfate (80%) precipitation and purified using ion exchange chromatography. The molecular weight of xylanase was ~29.8 kDa. The optimum temperature and pH for the enzyme activity were 50°C and pH 8.0. The enzyme was active on birchwood xylan and little active on p -nitrophenyl xylopyranoside but not on Avicel, CMC, cellobiose, and starch, showing its absolute substrate specificity. For birchwood xylan, the enzyme gave a Km 5.26 mg/mL and Vmax 277.7 μ mol/min/mg, respectively. In addition, the xylanase was also capable of producing high-quality xylo-oligosaccharides, which indicated its application potential not only in pulp biobleaching processes but also in the nutraceutical industry.
1. Introduction
Xylan is the most abundant noncellulosic polysaccharide present in both hardwoods and annual plants and accounts for 20–35% of the total dry weight in tropical plant biomass [ 1 – 3 ]. In temperate softwoods, xylans are less abundant and may comprise about 8% of the total dry weight [ 4 ]. Xylan is found mainly in the secondary cell wall and is considered to be forming an interphase between lignin and other polysaccharides. It is likely that xylan molecules covalently link with lignin phenolic residues and also interact with polysaccharides, such as pectin and glucan. In simplest forms, xylans are linear homopolymers that contain D-xylose monomers linked through β -1, 4–glycosyl bonds [ 5 , 6 ]. Xylanase (E.C 3.2.1.8) degrades β -1, 4 xylan by cleaving β -1, 4 glycosidic linkages randomly, and the products are xylose and xylo-oligosaccharides like xylobiose [ 7 , 8 ]. Xylanases are of industrial importance, which can be used in paper manufacturing to bleach paper pulp, increasing the brightness of pulp and improving the digestibility of animal feed and for clarification of fruit juices. Applications of xylanase avoid the use of chemicals that are expensive and cause pollution [ 9 ]. Microorganisms are the rich sources of xylanases, produced by diverse genera and species of bacteria, actinomycetes, and fungi. Several species of Bacillus and filamentous fungi secrete high amounts of extracellular xylanases [ 10 ]. Xylanase secretion often associates with low or high amount of cellulases. To use xylanase for pulp treatment, it is preferable to use cellulose-free xylanases, since the cellulase may adversely affect the quality of the paper pulp [ 11 – 15 ]. The most practical approach is the screening for naturally occurring microbial strains capable of secreting cellulose-free xylanases under optimized fermentation conditions. To use xylanase prominently in bleaching process it should be stable at high temperature and alkaline pH [ 16 , 17 ].
Industrial production of enzymes on large scale is associated mainly with substrate. The use of agriculture residues as low-cost substrates for the production of industrial enzymes is a significant way to reduce production cost. The technique of fermentation using solid state substrate has the great advantage over submerged fermentation due to absence or near absence of aqueous phase that provides natural habitat for growth of microorganisms, economy of the space, simplicity of the media, no complex machinery, equipments and control systems, greater compactness of the fermentation vessel owing to a lower water volume, greater product yields, reduced energy demand, lower capital and recurring expenditures in industry, easier scale-up of processes, lesser volume of solvent needed for product recovery, superior yields, absence of foam build-up, and easier control of contamination due to the low moisture level in the system [ 10 , 18 ]. In consideration with these facts the present study aims to characterize extracellular alkalothermophilic xylanase produced by Bacillus arseniciselenatis DSM 15340 when grown in solid state fermentation. To our knowledge, this is the first report describing the production of thermoalkalophilic cellulase-free xylanase by Bacillus arseniciselenatis DSM 15340. In addition, this xylanase was found to be able to degrade xylan into xylo-oligosaccharides.
2. Materials and Methods
2.1. screening of xylanolytic strains.
Soil samples were collected from coastal areas of Mandovi, Goa, India. Enrichment was done using birchwood xylan (Sigma Chemicals, Germany) as a sole source of carbon. Twenty five bacterial cultures were screened for xylanolytic ability by adding dye-labelled substrate, for example, xylan-brilliant red 3BA in xylan agar medium [ 19 ].
2.2. Phenotypic Characteristics
Prominent selected isolate was identified on the basis of morphological, cultural, biochemical properties [ 20 ] and 16S rRNA sequencing. Culture was deposited at National Centre for Cell Sciences (NCCS), Pune, India.
2.3. Phylogenic Analysis
The partial 16S rRNA sequences were retrieved on NCBI server ( http://blast.ncbi.nlm.nih.gov/Blast.cgi ) using BLAST tool. Top 10 similar sequences were downloaded in FASTA format. Multiple alignment of sequences and calculations of levels of sequence similarity were performed by using ClustalW2 program. A phylogenetic tree obtained was analyzed for closely related organism. The evolutionary history was inferred using the neighbor-joining method [ 14 ].
2.4. Growth Conditions of Culture
The bacterial isolate was maintained in liquid medium as well as solid medium in basal salt solution (BSS) containing 0.5% xylan having pH 8.0 at 45°C and stored at 4°C.
2.5. Xylanase Production in Solid State Fermentation (SSF)
The selected strain was further tested for their abilities to produce extracellular xylanase under solid state fermentation. Wheat bran was used as the substrate. For this the strain was cultured in Erlenmeyer flasks (250 mL) containing 10 g of wheat bran moistened with 18 mL of the basal salt solution (BSS: substrate-to-moisture ratio 1 : 1. 8). After 48 h of fermentation spent; solid substrate was removed and suspended in 50 mM phosphate buffer (pH 8.0), vortexed thoroughly to extract the xylanase. The sample was centrifuged at 5000 ×g for 10 minutes at 4°C. Centrifugation will remove xylanase from substrate. Supernatant was filtered through Whatman No. 1 filter paper and the clear filtrate was used as crude xylanase preparation. Prior to centrifugation, the samples were withdrawn for determining viable number of cells by standard viable plate count technique.
2.6. Xylanase Assay
Xylanase activity was measured according to Bailey et al. [ 21 ]. A 900 μ L of 1% solubilised birchwood xylan solution was added with 100 μ L enzyme solution in a test tube. 1.5 mL DNS reagent was added and incubated at 50°C for 5 min in water bath [ 22 ]. The absorbance was measured at 540 nm. The reaction was terminated at zero time in the control tubes. The standard graph was prepared using 0–500 μ g xylose. An autozero was set in UV-VIS spectrophotometer (Hitachi, Japan) using buffer solution. One unit of xylanase activity was defined as the amount of enzyme that liberates 1 micromole of reducing sugars equivalent to xylose per minute under the assay conditions described. Solubilised xylan was prepared by stirring birchwood xylan with 1 M NaOH for six hours at room temperature followed by centrifugation and freeze drying the supernatant after neutralising the alkali with 1 M HCl.
2.7. Cellulase Assay
Cellulase activity was measured according to Ghose with necessary modifications [ 23 ]. A 900 μ L 1% carboxy methyl cellulose solution was added with 100 μ L enzyme in a test tube. 1.5 mL DNS reagent was added and incubated at 50°C for 5 min in water bath. The absorbance was measured at 540 nm. The reaction was terminated at zero time in control tubes. A standard graph was prepared using 0–500 μ g glucose. An autozero was set in spectrophotometer using buffer solution. One unit of cellulase activity was defined as the amount of enzyme that liberates 1 micromole of glucose equivalents per minute under the assay conditions.
2.8. 1,4- β -xylosidase Assay
1,4- β -xylosidase activity was measured according to Lachke [ 24 ]. A 900 μ L p -nitrophenyl β -xyloside ( ρ -NPX) solution was added with 100 μ L of appropriately diluted enzyme solution in a test tube. The mixture was incubated at 50°C for 30 min. Then 1 mL of 2 M sodium carbonate solution was added. The absorbance was measured at 410 nm. The reaction was terminated at zero time in control tubes. One unit of 1,4- β -xylosidase activity was defined as the amount of enzyme that catalyzes the formation of 1 micromole of ρ -nitrophenol per minute under assay conditions.
2.9. Determination of Total Protein Content
Total soluble protein was measured according to Lowry et al. [ 25 ]. Protein concentration was determined using bovine serum albumin (BSA) as a standard. The protein content of the chromatographic eluant was measured by monitoring the optical density at 280 nm.
2.10. Ammonium Sulphate Precipitation
Protein precipitation by salting out technique using ammonium sulphate (NH 4 (SO 4 ) 2 ) was carried out with constant gentle stirring [ 26 ]. This was left overnight and the precipitate was collected by centrifugation at 10,000 ×g for 10 min. The precipitate obtained was dissolved in phosphate buffer (50 mM, pH 8.0) and dialyzed against the same buffer for 24 h. Dialysis was carried out using cellulose tubing (molecular weight cut-off 13,000 kDa, Himedia LA393-10 MT).
2.11. Ion Exchange Chromatography
Dialyzed enzyme (2 mL) was loaded onto a anion exchange DEAE Cellulose (Sigma-Aldrich Co., USA) column. The column was packed with activated DEAE-cellulose equilibrated with 50 mM phosphate buffer (pH 8.0). The height of column was 20 cm with the 2.5 cm diameter. The protein was eluted with the 0.0 to 0.5 M NaCl gradient. The 50 fractions were collected having 5 mL volume of each fraction with the flow rate of 1 mL/min. All the steps were carried out at 4 to 8°C.
2.12. Molecular Mass Determination by SDS-PAGE
SDS-PAGE of partially purified xylanase was performed in a 12.5% acrylamide gel Laemmli [ 27 ]. Coomassie brilliant blue R-250 was used to stain the gel. The protein molecular weight markers used were of medium range containing 14.4 kDa to 94.0 kDa obtained from Bangalore GeNei, India.
2.13. Substrate Specificity
Substrate specificity of the xylanase was found by using 1% xylan, cellobiose, starch, carboxy methyl cellulose (CMC), and p -nitrophenyl xylopyranoside and Avicel as substrates.
2.14. Kinetic Parameters
Initial reaction rates using birchwood and oat spelt xylan as substrate were determined at substrate concentrations of 0.5–10 mg/mL in 50 mM phosphate buffer (pH 7.0) at 45°C. The kinetic constants, Km and Vmax, were estimated using the linear regression method of Lineweaver and Burk [ 28 ].
2.15. Identification of Hydrolysis Products
To 50 mL of birchwood xylan suspension (1% of birchwood xylan in 50 mM Phosphate buffer pH 7.0), 40 μ g of xylanase enzyme was added and incubated at 45°C. Hydrolysis products were detected by thin layer chromatography (TLC) [ 29 ]. TLC (TLC plates, 0.25 mm layers of silica gel F 254, Merck, India) was performed using the mixture of n -butanol : ethanol : H 2 O (5 : 3 : 2 by vol) as a solvent system. Compounds were detected by spraying with 50% sulphuric acid in ethanol followed by heating at 150°C for 5 min. D-xylose (X 1 ), xylobiose (X 2 ), xylotriose (X 3 ), and xylotetraose (X 4 ) were applied as standard.
2.16. Effect of Temperature on Activity and Stability
The optimum temperature for maximum xylanase activity was determined by varying the reaction temperature from 30 to 80°C. To evaluate thermal stability, 0.5 mL of the enzyme solution was incubated at 30–80°C temperatures for up to 4 h. The relative enzyme activity was recorded at 1 h interval during period of 4 h.
2.17. Effect of pH on Activity and Stability
The effect of pH on enzyme activity was determined by incubating xylanase at various pH ranging from 6.0 to 11.0. The various buffers used were 50 mM sodium phosphate (pH 6, 7), 50 mM Tris HCl (pH 8, 9), 50 mM carbonate bicarbonate buffer (pH 10), and 50 mM glycine-NaOH buffer (pH 11). To evaluate the stability of the enzyme at each pH, the purified enzyme was incubated into the respective buffer over a pH range of 6.0–11.0 for up to 4 h at optimum temperature. The relative enzyme activity was determined at 1 h interval during the 4 h period of incubation.
3. Results and Discussion
3.1. isolation and identification of bacteria.
About 25 bacterial strains, which formed clear halos around their colonies on xylan agar plates, were picked up for further studies, isolated from soil collected at selected study site. The strain that showed 33 mm zone of clearance around the colony proved its xylanolytic ability ( Figure 1 ). It was identified on the basis of various morphological and biochemical characteristics as shown in Table 1 .

Plate showing zone of clearance around colony by isolate.
Morphological, physiological, and biochemical characteristics of the isolate.
| Tests | Results | Tests | Results |
|---|---|---|---|
| Colony morphology | Growth on NaCl (%) | ||
| Shape | Circular | 2.0 | − |
| Margin | Regular | 4.0 | − |
| Elevation | Raised | 6.0 | + |
| Consistency | Moist | 8.0 | + |
| Color | Pale yellow | 10.0 | − |
| Opacity | Opaque | Anaerobic Growth | Facultative |
| Gram nature | Gram Positive | Utilization of carbohydrates | |
| Shape of the cell | Long rods | Xylose | + |
| Motility | Sluggish Motile | Lactose | + |
| Endospore position | Central | Mannitol | − |
| Growth at temperatures | Arabinose | − | |
| 10°C | − | Sucrose | − |
| 25°C | − | Glucose | − |
| 30°C | + | Fructose | + |
| 37°C | + | Melibiose | − |
| 45°C | +++ | Starch hydrolysis | + |
| 60°C | + | Gelatin hydrolysis | + |
| 70°C | – | Urea hydrolysis | − |
| Growth at pH | Esculin hydrolysis | − | |
| 5.0 | − | Casein hydrolysis | − |
| 6.0 | − | Tween 20 hydrolysis | + |
| 7.0 | + | Catalase test | + |
| 8.0 | ++ | Oxidase test | + |
| 10.0 | − | Nitrate reduction | + |
| H S production | − | ||
+: Positive; −: Negative.
The isolate was confirmed as Bacillus arseniciselenatis strain DSM-15340 with partial 16S rRNA sequencing having a length of 1499 bp nucleotide. The sequence was deposited in Gene Bank (Accession No. {"type":"entrez-nucleotide","attrs":{"text":"AJ865469","term_id":"56266617","term_text":"AJ865469"}} AJ865469 ). The phylogenetic relation of this isolate is as shown in Figure 2 . It is closely associated with Bacillus sp. AMnr. It was also isolated from soil sample collected at coastal areas of Mandovi, Goa. Shivaji et al. isolated Bacillus arseniciselenatis DSM 15340 and Bacillus arsenicus from a bore well located in the chakdah region of West Bengal, India [ 30 ].

The phylogenetic tree of Bacillus arseniciselenatis DSM 15340 (designated as “0”).
3.2. Xylanase Production in SSF
When the strain was grown on wheat bran for 3 days of incubation at pH 8.0 and 45°C, maximum xylanase production was observed, that is, 910.49 U/gram dry substance, which was absolutely free from cellulase. Several workers reported the suitability of wheat bran for xylanase production in SSF [ 31 , 32 ]. Commercial wheat bran consists of 30% cellulose, 27% hemicellulose, 21% lignin, and 8% ash [ 33 ]. Hence there was increase in possibility of cellulase contamination when grown on wheat bran. Haltrich et al. also reported that xylanases were always associated with cellulase [ 34 ]. From twenty selected strains, five were able to produce cellulase along with xylanase in SSF. This was due to the presence of cellulose in substrate wheat bran used in SSF.
3.3. Purification of Xylanase
The culture filtrate was precipitated by fractional (35–80%) ammonium sulphate saturation. Proteins precipitated within this range had maximum xylanase activity and was used for purification. Xylanase was further purified by DEAE cellulose ion exchange column. The enzyme was eluted from DEAE cellulose column at a NaCl concentration of 0.25 M ( Figure 3 ). The fractions (no. 19–25) having maximum specific activity were concentrated. Xylanase was purified 3.06-fold with a specific activity of 299.25 U/mg ( Table 2 ).

Elution profile of xylanase from DEAE-cellulose column chromatography.
Purification steps of xylanase enzyme isolated from Bacillus arseniciselenatis DSM 15340 when grown on wheat bran.
| Purification steps | Xylanase activity (U) | Total protein content (mg) | Specific activity (U/mg) | Purification fold |
|---|---|---|---|---|
| Crude filtrate | 231659 | 2376 | 97.49 | 1.0 |
| (NH ) SO precipitation | 196220 | 1460 | 134.39 | 1.37 |
| DEAE sepharose FF | 96360 | 322 | 299.25 | 3.06 |
The specific activity of xylanase produced by Bacillus pumilus was previously reported as 298 U/mg by Panbangred et al. [ 35 ].
3.4. Molecular Weight Determination
The purified enzyme showed a single-protein band on SDS-PAGE. The molecular mass of denatured xylanase, estimated from the relative mobility of proteins on SDS-PAGE, was ~29.8 kDa as shown in Figure 4 . The present results were supported by previous work. The enzyme from a fungus Plectosphaerella cucumerina had a molecular weight of 19 kDa reported by Zhang et al. [ 36 ]. Xylanase produced by Bacillus sp . strain BP-23 is of 32 kDa [ 37 ] whereas the second xylanase obtained from Bacillus firmus had a molecular weight of 45 kDa [ 38 ].

SDS-PAGE analysis of purified xylanases from Bacillus arseniciselenatis DSM 15340. Lane M: molecular markers; Lane B: Purified xylanase enzyme.
3.5. Substrate Specificity
The action of the purified xylanase towards various substrates was studied. The enzyme was active on birchwood xylan, little active on p -nitrophenyl xylopyranoside but not on Avicel, CMC, cellobiose, and starch ( Table 3 ). Purified xylanase was not active on Avicel, CMC, cellobiose, and starch even when the enzyme concentration was 5 times greater than used in normal assay and incubation period of 20 minutes rather than 5 minutes. Similarly, xylanase with absolute substrate specificity was purified from Trichoderma viride by Ujiie et al. [ 39 ]. Kanda et al. purified two different xylanases, named xyl I and III that showed no activity towards glycans, other than xylan, such as starch, pachyman, and Avicel (microcrystalline cellulose), except for the almost one twentieth activity of xyl III toward carboxymethyl cellulose (CMC) [ 40 ].
Substrate specificity of purified xylanase.
| Substrates | Xylanase activity (U/mg protein) |
|---|---|
| Birchwood xylan | 291.9 + 0.35 |
| Cellobiose | 0.0 + 0.0 |
| Starch | 0.0 + 0.0 |
| Carboxy methyl cellulose (CMC) | 0.0 + 0.0 |
| -Nitrophenyl xylopyranoside | 0.22 + 0.001 |
| Avicel | 0.0 + 0.0 |
Each value represents the mean + standard error values.
3.6. Kinetic Parameters
The kinetic parameters Km and V max of the enzyme were determined from Lineweaver-Burk double-reciprocal plots of xylanase activity at 45°C using various concentrations of birchwood xylan as substrate ( Figure 5 ). The Km and V max values of xylanase were 5.26 mg/mL and 277.7 μ mol/min/mg, respectively. Wang et al. reported that Km and V max values of xylanase isolated from Bacillus sp . NTU-06 were 3.45 mg/mL and 387.3 μ mol/min/mg, respectively [ 41 ]. Bansod et al. also reported that Km values of xylanases lie in the range from 0.5 to 19.6 mg/mL [ 42 ]. Xylanases isolated from Aeromonas cavie 171 ME-1 and Bacillus sp. strain 41m-1 showed similar values of V max 260 to 350 μ mol/min/mg protein [ 43 , 44 ].

Double reciprocal plot for determining the V max and Km values of xylanase Bacillus arseniciselenatis DSM 15340 when acted on Birchwood xylan.
3.7. Analysis of Hydrolytic Products
After 1 h of incubation of birchwood xylan with xylanase Bacillus arseniciselenatis DSM 15340, xylotriose and xylotetraose were the main products in the hydrolytic mixture along with little amount xylobiose. ( Figure 6 ). The present results indicated that xylanase cleaved the substrate to liberate mainly xylooligosaccharides, but not able to act on resulting oligosaccharides to form xylose, suggesting that it is a endoxylanase.

TLC analysis for hydrolysis products released from birchwood xylan by xylanase from Bacillus arseniciselenatis DSM 15340. S: substrate; 1: sample; X 1 : D-xylose; X 2 : xylobiose; X 3 : xylotriose; X 4 : xylotetraose.
Analysis of hydrolytic products of xylan by the xylanase of Thermoascus aurantiacus showed that xylan was degraded to various xylo-oligosaccharides without a significant accumulation of xylose [ 45 ]. Xylobiose and xylotriose were the main hydrolysis products when xylanase of Bacillus stearothermophilus reacted with oat spelt xylan and resulted oligosaccharides were then cleaved to form xylose by the β -xylosidase action [ 46 ]. The end products were xylobiose, xylotriose, xylotetraose, and higher oligosaccharides when xylan was hydrolyzed with endoxylanase of alkalophilic Bacillus sp. No. C-125. No xylose was found in the hydrolysis products when analysed by HPLC [ 47 ].
3.8. Effect of Temperature on Activity and Stability
For xylanase from Bacillus arseniciselenatis DSM 15340, activity was found to be gradually increased with increasing temperature and found significantly declined at 80°C ( Figure 7 ). 50°C was found to be the most favourable for enzyme activity. Stability of the enzyme was the most important factor in studying characteristics. In case of xylanase purified from Bacillus arseniciselenatis DSM 15340, it was more stable at temperatures 30°C and 40°C for 4 h of incubation and retained almost 93% activity. At higher temperature values xylanase stability was gradually declined ( Figure 8 ). Bernier et al. reported that multiple forms of xylanases were purified from Aeromonas sp. by Ohkoshi et al. and the properties of the three xylanases were well characterized [ 2 , 48 ]. It was found that these xylanases were most active at 50°C to 60°C. Kang et al. purified two xylanases which gave the highest activity at 50°C. They showed relatively high stabilities at 50°C temperature [ 49 ].

Effect of temperature on activity of xylanase from Bacillus arseniciselenatis DSM 15340.

Effect of temperature on stability of xylanase from Bacillus arseniciselenatis DSM 15340.
3.9. Effect of pH on Activity and Stability
pH was the most important factor to characterize the enzyme. Xylanase from Bacillus arseniciselenatis DSM 15340 showed 100% activity at pH 8.0 ( Figure 9 ). At higher pH values also, activity was 95%. With respect to stability, at all tested pH values xylanase activity was 100% activity for 1 h. pH 10.0 was the most favourable for stability and retained 60% activity for 4 h of incubation ( Figure 10 ).

Effect of pH on activity of xylanase from Bacillus arseniciselenatis DSM 15340.

Effect of pH on stability of xylanase from Bacillus arseniciselenatis DSM 15340.
Similarly, Honda et al. purified two xylanases namely xylanases N and xylanase A from Bacillus sp. No. C-125 [ 47 ]. Among these xylanases, N shows maximum activity at pH ranging from 6.0 to 7.0, while xylanase A was active at pH ranging from 6.0 to 10.0 and showed some activity at pH 12.0 also. In the present study, 100% activity was retained by Bacillus arseniciselenatis DSM 15340 xylanase for 2 h of incubation at pH 10.0. Stability at the extreme pH values may be due to charged amino acid residues. The enzymes stable in alkaline conditions were characterized by a decreased number of acidic residues and an increased number of arginines [ 50 ].
4. Conclusions
Bacillus arseniciselenatis DSM 15340 produced a thermoalkalophilic cellulose-free xylanase in higher amount when grown on solid state conditions using cheaply available agroresidual substrate wheat bran. Hence it can be used for large-scale production of xylanase using such agroresidual substrates. The purified xylanase also was capable of producing high-quality xylo-oligosaccharides, indicating its application potential not only in pulp biobleaching processes but also in the nutraceutical industry.
Acknowledgment
The authors are thankful to Dr. V. V. Kajinnii, Principal, Tatyasaheb Kore Institute of Engineering and Technology, Warananagar, India, for financial support.
- Open access
- Published: 17 August 2024
Emergency robotic surgery: the experience of a single center and review of the literature
- Graziano Ceccarelli 1 ,
- Fausto Catena 2 ,
- Pasquale Avella 3 , 4 ,
- Brian WCA Tian 5 ,
- Fabio Rondelli 1 ,
- Germano Guerra 4 ,
- Michele De Rosa 1 &
- Aldo Rocca 3 , 4
World Journal of Emergency Surgery volume 19 , Article number: 28 ( 2024 ) Cite this article
95 Accesses
1 Altmetric
Metrics details
Backgrounds
Laparoscopic surgery is widely used in abdominal emergency surgery (AES), and the possibility of extending this approach to the more recent robotic surgery (RS) arouses great interest. The slow diffusion of robotic technology mainly due to high costs and the longer RS operative time when compared to laparoscopy may represent disincentives, especially in AES. This study aims to report our experience in the use of RS in AES assessing its safety and feasibility, with particular focus on intra- and post-operative complications, conversion rate, and surgical learning curve. Our data were also compared to other experiences though an extensive literature review.
We retrospectively analysed a single surgeon series of the last 10 years. From January 2014 to December 2023, 36 patients underwent urgent or emergency RS. The robotic devices used were Da Vinci Si (15 cases) and Xi (21 cases).
36 (4.3%) out of 834 robotic procedures were included in our analysis: 20 (56.56%) females. The mean age was 63 years and 30% of patients were ≥ 70 years. 2 (5.55%) procedures were performed at night. No conversions to open were reported in this series. According to the Clavien-Dindo classification, 2 (5.5%) major complications were collected. Intraoperative and 30-day mortality were 0%.
Conclusions
Our study demonstrates that RS may be a useful and reliable approach also to AES and intraoperative laparoscopic complications when performed in selected hemodynamically stable patients in very well-trained robotic centers. The technology may increase the minimally invasive use and conversion rate in emergent settings in a completely robotic or hybrid approach.
Introduction
Abdominal Emergency Surgery (AES) can be defined as a procedure requiring to deal with an acute threat to life, organ, trauma, acute disease process, acute exacerbation of a chronic disease process, or complication of a surgical or other interventional procedure, normally within hours of decision to operate [ 1 , 2 ].
Further, “expedited surgery” refers to the clinical situation exemplified by a patient in need of prompt treatment but not in imminent danger to life or organ survival; this procedure often takes place a few days after the decision to operate [ 3 ].
Nowadays, minimally invasive laparoscopic approach to urgent abdominal surgery (cholecystitis, acute appendicectomies, bowel perforation or obstruction, etc.) represents the standard of care in many cases and recent guidelines recommend it [ 4 , 5 , 6 , 7 ].
Nevertheless, after more than 20 years from clinical introduction, Robotic Surgery (RS) represents the most important technological evolution and a revolutionary concept of computer-assisted technology in minimally invasive surgery [ 8 ]. It allows to overcome many limits of conventional laparoscopy and to expand the use of minimally invasive approaches.
Its peculiar features include a three-dimensional high-definition view, articulated instruments, tremor eradication, and improved ergonomics for surgeons, enable the performance of extremely accurate procedures (micro-sutures, fine dissections, etc.) with consequently lowering conversion rates and postoperative complications, particularly in case of challenging surgical procedures [ 9 , 10 , 11 ]. In addition, compared to traditional laparoscopic surgery, RS demonstrated shorter learning curves for several complex procedures [ 12 , 13 ]. On the other hand, the main drawbacks of robotic technology are linked to its limited diffusion also due to expensive costs [ 14 , 15 , 16 , 17 , 18 , 19 , 20 ].
Nevertheless, robotic surgical technologies have expanded and evolved over the past 20 years, bringing new devices, and improving the most established ones [ 21 , 22 ].
The spreading of robotic platforms and their easier management led to increased RS applications in all abdominal surgical specialities including upper gastrointestinal surgery [ 15 , 23 , 24 , 25 ], colorectal surgery [ 26 , 27 , 28 ], HBP surgery [ 14 , 18 , 29 , 30 , 31 ], abdominal wall surgery and many others [ 7 ].
Despite the huge diffusion of RS in all surgical fields, its application in urgent scenarios has never been investigated representing a new field of interest, with limited literature experiences [ 32 ].
So considering that our experience in RS has been implemented since 2002 and it raised from general to major complex surgery [ 33 , 34 , 35 , 36 , 37 , 38 ], we aim to set the state of art of Robotic Emergency Surgery sharing our experience through the analysis of our peri-operative outcomes and indications in RS. Furthermore, due to the limited evidence available, we have as a secondary endpoint an extensive analysis of previous literature experiences.
Study design and patient selection
We retrospectively reviewed a prospectively collected database of patients undergoing RS at General and Robotic Surgery Unit of San Giovanni Battista Hospital (Foligno, Italy) and General Surgery Unit of San Donato Hospital (Arezzo, Italy) from January 2014 to December 2023.
The patients’ data were analyzed according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [ 39 ]. All patients signed an informed consent allowing the anonymous scientific use of clinical data and images. The study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approved date: May 12, 2021).
In all participating centres, data were prospectively collected from electronic patient records.
We selected 834 consecutive robotic procedures for abdominal surgery performed by both centres. Patients were divided into two cohorts: elective surgery and urgent or emergency surgery groups.
Urgent surgery was defined as a condition requiring surgery within 72 h in stable patients, but not suitable for discharge. Moreover, emergency surgery was defined as a clinical scenario requiring within 24 h in stable patients, with a low risk of deterioration. All patients < 18 years old and affected by hemodynamical instability were excluded.
Criteria adopted to assess baseline characteristics of patients, surgical issues and technologies that allow to benefit of RS in urgent and emergency settings are summarized in Table 1 .
Furthermore, to analyze the diagnosis and intraoperative data we carried out a specialities classification as reported in Table 2 .
Implementation of the robotic surgery program and learning curve completion
Our experience with RS started in September 2002 with the da Vinci S ® platform (Intuitive Surgical, Sunnyvale, California, USA), and over time, its application in abdominal surgery grew as well as platform technologies. During the study period, the da Vinci Si ® platform (Intuitive Surgical, Sunnyvale, California, USA) and, since 2017, da Vinci Xi ® (Intuitive Surgical, Sunnyvale, California, USA) were available at our institutions.
Beginning from colorectal surgery, hiatal hernia repairs and cholecystectomies, our surgical team have gradually selected more challenging procedures by carrying out liver and pancreatic resections, oesophageal benign and malignant disorders, bariatric surgery, abdominal wall hernia repairs and nephrectomies [ 14 , 15 , 25 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 ].
All procedures were performed by a well-trained surgeon in minimally invasive surgery (G.C.) with 10 years of previous experience in RS.
36 (4.3%) out of 834 robotic procedures were included in our analysis and treated as urgent or emergent procedures.
All patients signed an informed consent allowing the anonymous scientific use of clinical data and images. The study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approved date: 12 May 2021).
The collected data included demographic characteristics such as age, sex, and preoperative comorbidities classified according to the Charlson comorbidity Index (CCI) [ 48 ]. The anesthetic risk assessment was performed thanks to the American Society of Anesthesiologists (ASA) score [ 49 ].
We reviewed pre-operative diagnosis, main and associated surgical procedures, operative time and intraoperative complications, and conversion to open rate. Postoperative complications were stratified according to the Clavien-Dindo classification [ 50 ] and were considered severe when ≥ 3. Patients were monitored until their 30th postoperative day.
Categorical variables were expressed as frequencies and percentages, while quantitative data were collected as means or medians and interquartile ranges.
Literature review
We performed literature research on the PubMed Dataset (US National Library of Medicine, http://www.ncbi.nlm.nih.gov/PubMed ), using the subsequent keywords: “robotic surgery”, “urgent surgery”, and “emergency surgery”. We selected only English studies. Original articles, case reports and case series were included, while editorials, letters, and reviews were excluded. Number of treated patients does not represent an exclusion criterion. Articles were first evaluated by title and abstract examination, then a full-text read was performed. More than 50 studies described RS in AES. An extensive analysis was performed to summarize similarities and differences among RS approaches according to abdominal surgery specialities.
Pre-, intra- e post-operative outcomes
36 (4.3%) out of 834 robotic procedures were included in our analysis. Baseline characteristics of patients are listed in Table 3 .
According to surgical procedures, Fig. 1 shows AES performed thanks to RS compared to elective surgery.
Over the cohort of 36 patients treated, 16 (44.44%) were males while 20 (56.56%) were females. The mean age was 63.20 years (range: 43–88 years): 30.55% [ 11 ] of patients were ≥ 70 years. The mean Body Mass Index was 26.68 kg/m 2 (range: 23–42). According to the ASA score, 8 (22.22%) patients were classified as ASA 3. No ASA 4 was treated. Patients’ CCI are listed in Table 3 . The da Vinci Si platform was used for the first 15 (41.67%) cases, while Xi for the last 21 (58.33%). 2 (5.55%) procedures were performed at night. No conversions to open were reported in this series. According to Clavien-Dindo grade, 2 (5.55%) major complication was collected: 1 after urgent surgery and 1 after emergency setting. Two minor complications requiring conservative treatments were observed: both complications were related to primary disorders and not to RS. Intraoperative and 30-day mortality were 0%. The mean length of stay was 4.92 days (range: 1–21). The mean follow-up was 26.53 months (range: 7–68).
Number of elective and urgent/emergency procedures ( A ) and percentage of overall surgical procedures performed in emergency settings ( B ) according to abdominal surgery specialities. Abbreviations: HPB, Hepatopancreatic and Biliary Surgery;
Our experience demonstrates the safety and feasibility of RS also in urgent and emergency abdominal settings in patients not affected by hemodynamic instability. To date, the minimally invasive approaches in emergency scenarios are mainly validated for laparoscopy, as reported by several literature experiences included in the last WSES review [ 51 ]. Despite the diagnostic role of MIS, authors demonstrated several benefits of laparoscopic approaches in hemodynamically stable patients undergone AES, including trauma. However, patient selection, surgeons’ expertise as well as specific surgical training represent crucial key points [ 51 ].
In literature, RS in AES studies is related to high-volume centres, and well-trained surgeons’ experiences and their optimal outcomes should encourage further applications and Randomized Clinical Trials [ 32 , 52 ]. We further analyzed short- and long-term outcomes of RS in AES according to specialties (Table 4 ).
Robotic surgery in emergency setting
In the literature, RS in the emergency setting is reported by a limited number of experiences, especially case reports and case series.
The urologist experience described by Capibaribe et al. [ 53 ] demonstrated the safety and efficacy of robotic treatment in the case of vesicourethral anastomotic stenosis after open radical prostatectomy, providing better continence results, without pubectomy.
Globally, the major barrier to RS adoption is due to limited device access resulting from a shared use policy by several surgical teams (gynaecologists, general surgeons, thoracic surgeons, and urologists). Furthermore, the lack of dedicated teams (surgeons, nurses, and anaesthesiologists) during the night shift might further limit RS.
On the other hand, in emergency settings, the “time-sparing” concept is largely known. Commonly, to avoid useless costs due to waste disposable instruments, a hybrid approach should be discussed by the whole surgical team: before robotic docking, a laparoscopic exploration could be the first surgical step to verify clinical environments for doing RS.
A crucial issue is represented by frequent operating table position changes, especially during explorative steps (tilting, Trendelenburg, or reverse-Trendelenburg positions) and rapid conversion to open surgery when necessary [ 54 ]. It could be underlined that quick and safe docking and undocking are performed by skilled teams and well-trained surgeons in elective procedures [ 54 , 55 ].
In the last years, the RS technologies have also impacted operative time [ 56 ]: The Xi robot represents a radical evolution from the Si robot. Literature experiences demonstrated better docking ability during Da Vinci Xi surgery when compared to previous robotic systems (Da Vinci S, Si, X) [ 57 , 58 ]. These features were due to laser targeting and improved cannula mounts that resulted in a simplified “linear” port configuration and an abbreviated docking time.
Besides, the ability to exchange the robotic camera from port-to-port increased versatility for multi-quadrant surgeries thanks to the smaller 8 mm camera [ 59 , 60 ]. The multi-quadrant operations represent challenges due to the axis of visualization shift up to 360°. This procedure requires undocking the robot and rotating it on the axis. It is crucial for many colorectal surgeries that require access to the entire abdomen such as subtotal colectomy and total proctocolectomy.
Furthermore, it was reported that the Xi system’s better fluency is also due to thinner robotic arms that reduce their collisions during surgery and synchronous movements with the operating Table [ 56 ]. In addition, Da Vinci Xi integrates the Indocyanine-Green technology that could be easily used to better identify bile duct during cholecystectomy in patients affected by acute cholecystitis, to assess organ vascularization during their resections and anastomosis, as reported in our experience.
Bianchi et al. [ 61 ] performed an extensive comparison of Da Vinci Si and Xi systems to define their advantages and disadvantages. 89 patients (64 in the Si system vs. 25 in the Xi system group) who underwent liver surgery were included. The Si system group experienced a greater total incisional length (+ 8.99 mm; p < 0.0001) due to a higher number of robotic/laparoscopic ports. Nevertheless, no differences were described regarding operative time, conversion rate, estimated blood loss, postoperative complications, mortality, use of analgesics, and costs. The authors concluded that da Vinci Xi represents an effective technological advancement.
Hill et al. [ 62 ] hypothesized that Da Vinci Xi will allow for greater efficiency and result in shorter operative times if compared to Da Vinci Si. To validate their hypothesis, the authors performed a retrospective review of patients undergoing sigmoid colon resection or Low Anterior Rectal resection. A total of 93 patients underwent sigmoid resection thanks to RS (Si, n = 52 vs. Xi, n = 41). The Xi group had significantly shorter surgical times for Low Anterior Rectal and sigmoid resection (162 vs. 238 min, p = 0.0001). Nowadays, no data are available on the Da Vinci Si and Xi comparison in AES.
However, according to the type of procedures, the mean operative times of Da Vinci Xi were superimposable to the Si group in our experience.
The robotic technology in hemodynamically stable patients could potentially reduce the conversion to open rate (0% in our short series), thanks to high-definition view and accuracy of dissection and fine micro-sutures.
In 2022, the World Society of Emergency Surgery (WSES) published a position paper on RS in AES after the literature evaluation by a steering committee and an international expert panel [ 32 ]. Ten studies (3 case reports, 3 case series, and 4 retrospective comparative cohort articles) were found and 6 statements were proposed. Experts concluded that RS can be considered safe, and feasible in selected cases represented by hemodynamically stable patients. It should be emphasized that the WSES team reported some RS drawbacks: it is mandatory to perform dedicated surgical training, RS showed longer operative times, higher costs and difficult availability and accessibility represent the main issues during night shifts [ 32 ].
These aspects probably may change in the future with RS diffusion and new robotic devices in the health market.
In our experience, the mean age of patients was 63 years. Therefore, more than 30% of patients were older than 70 years (range: 43–88 years) and it is in line with RS literature experiences that showed good outcomes also in the elderly population [ 28 , 41 ]. Nevertheless, operative time represents a crucial point in this frail cohort. Despite RS showing longer operative time when compared to open and laparoscopic surgery, this disadvantage may be offset by lower postoperative complication rates, shorter hospital stays, and lower conversion rates [ 28 , 41 , 63 , 64 , 65 ].
Another key aspect is represented by enhanced vision through near-infrared imaging. It may be useful in AES in case of tissue perfusion evaluations or biliary tree identification in challenging procedures. This feature is not routinely available in laparoscopic surgery [ 66 , 67 ].
Figure 2 shows an emergency scenario due to splenic artery aneurysm repair thanks to RS.
The robotic approach during Emergency Setting for patients affected by splenic artery aneurysms using a vessel resection and end-to-end vascular anastomosis
Robotics in emergency upper-GI and bariatric surgery
One of the earliest studies on RS in AES was published in 2012 [ [ 68 ]]: Sudan et al. experience in complex bariatric surgery involved 2 patients affected by a stomach stricture and an acute abdomen due to perforation with biliary peritonitis after biliopancreatic diversion, respectively. The perforation was treated through an initial laparoscopic investigation followed by a handsewn robotic stitch reparation of duodenal stump dehiscence.
In 2020, Cubas et al. [ 69 ] presented an RS procedure for incarcerated Morgagni Hernia in a 29-year-old male. The hernia defect (reported as 10 × 7 cm) was corrected via mesh placement. Patient discharge was possible on POD 5. No recurrence was detected at 1-year follow-up.
During the same year, Ceccarelli et al. [ 45 ] published a series of 5 patients affected by strangulated Giant Hiatal Hernia: 3 (60%) patients experienced RS while 2 (40%) laparoscopic approach. The authors described an easier incarcerated stomach management thanks to RS, maybe due to better surgeon ergonomic position and more accurate dissection preserving pleural integrity and vagus nerve.
Kim et al. [ 70 ] reported a case of robotic transthoracic repair of a right-sided traumatic diaphragmatic rupture in a 45-year-old male with a history of chronic obstructive pulmonary disease presented as a restrained driver in a low-speed motor vehicle collision. The patient was effectively operated after a 48-hour observation.
In 2021, 300 USA hospitals were involved in retrospective data collection of adult patients affected by Hiatal Hernia and treated in elective and urgent/emergency scenarios from 2015 to 2017 [ 71 ]. Data analysis revealed that laparoscopy (64%) was the most frequent approach used during AES, followed by open surgery (30%). A limited number of patients (6%) experienced RS. After cost evaluations and outcomes analysis, authors declared the technical feasibility of minimally invasive approaches when compared to open surgery due to lower cost, lower length of hospital stay, complications, and mortality.
Robinson et al. [ 72 ], in 2021, performed a statistical analysis of “in-room-to-surgery-start time” in a retrospective cohort study of 44 patients affected by emergent perforated gastrojejunal ulcers. The comparison between RS and laparoscopic (24 and 20 cases respectively) showed encouraging results for RS (25 versus 31 min, p = 0.01). Furthermore, no statistical differences were observed in terms of intra- and post-operative outcomes (operative time, complication rate, complication severity, hospital length of stay, discharge to home, and 30-day readmission). Despite RS showing higher surgical costs, authors concluded that emergency gastric perforation could be safely approached thanks to RS.
No complications were reported in all studies reported in our review [ 69 , 70 , 71 , 72 ].
Robotics in emergency colorectal surgery and appendectomies
Nowadays, emergency laparoscopy represents a safe and valid approach to colorectal disorders such as perforated diverticulitis with generalized peritonitis [ 73 ], iatrogenic colonoscopy perforations [ 74 ], bowel obstructions and anastomotic leaks management [ 75 , 76 , 77 ].
In 2012, Pedraza et al. [ 78 ] showed successful robotic colectomy due to iatrogenic colon perforation following colonoscopy.
Two years later, Felli et al. [ 79 ] described a case of an 86-year-old woman admitted to the emergency unit for massive intestinal bleeding due to ascending colon cancer. After patient resuscitation thanks to blood transfusions, surgeons carried out a robotic right colectomy. The postoperative period was uneventful.
Several series compared laparoscopic and robotic outcomes in patients who underwent elective colorectal surgery [ 80 , 81 , 82 ], suggesting the potential role of RS in this surgical field. Nevertheless, an interesting analysis was performed by Beltzer et al. [ 83 ] in 2019. 106 patients were treated for uncomplicated, complicated, or recurrent diverticulitis. The authors concluded that RS achieves better outcomes when compared to laparoscopic surgery in challenging cases (abscess or relapsing diverticulitis).
Three monocentric experiences reported by Kudsi et al. [ 84 , 85 , 86 ] showed the effectiveness of urgent RS for the treatment of obstructive transverse colon cancer, bleeding sigmoid diverticulosis and caecal volvulus.
However, RS could represent a crucial approach also in colorectal autoimmune diseases. Concerning this field, Anderson et al. [ 87 ] in 2020 reported a matched case-control study of 6 patients treated by urgent subtotal colectomy for ulcerative colitis using the robotic platform. In addition, authors compared patients who underwent RS to laparoscopic urgent procedures (6 versus 13 cases) concluding that no differences in perioperative outcomes were observed.
According to Yang et al. [ 88 ] estimation, more than 17 millions of patients were affected by appendicitis in 2019, making it the most common surgical emergency worldwide. Nevertheless, regarding urgent robotic appendectomies, only 5 literature experiences reported robotic approaches [ 89 , 90 , 91 , 92 , 93 ]. A total of 11 patients were collected and 3 (27.27%) required an appendix stump suture. No complications or conversions were reported. Moreover, Hüttenbrink et al. [ 94 ] described incidental appendicectomy during robotic prostatectomy.
Figure 3 shows our experience during RS for complicated sigmoid diverticulitis with sigmoid-bladder fistula.
Lunardi et al. [ 95 ] presented an interesting analysis of temporal trends in the use of minimally invasive surgery in Abdominal Emergency and Urgent Settings. The authors compared 89,098 emergency colectomies performed between 2013 and 2021. The increase per year for robotic colectomy was 0.9% (from 1.4% of total procedures in 2013 to 8.8% in 2021). As a result of this increase, a 0.7% decrease was registered for the open approach. Furthermore, patients who underwent RS were older, had more comorbidities and had higher BMI when compared to laparoscopic and open groups. Intraoperative outcomes were encouraging for RS: after Propensity Score Matching, a conversion rate of 25.5% (860/3,375 patients) was registered during laparoscopic surgery, while in 11.2% (379/3,375 patients) of RS cases, a conversion to open was required ( p < 0.001). After Propensity Score Marching of patients underwent Emergency surgery only, RS demonstrated advantages in terms of conversion to open (27.5% vs. 12% in laparoscopic and robotic groups respectively, p < 0.001) and post-operative LOS (7.12 vs. 6.85 days respectively, p = 0.001).
In conclusion, conventional open surgery should be recommended for unstable and frail patients who require time-critical surgery. Nevertheless, it could be underlined that stable and frail patients may benefit from an enhanced recovery after surgery associated with RS in the acute setting when compared to open surgery.
Robotic approach during emergency setting for patients affected by complicated sigmoid diverticulitis with sigmoid-bladder fistula. We performed a fistula resection and bladder suture in double-layer barbed suture
Robotics in acute cholecystitis and biliary tree diseases
Another interesting field of application in AES may be the biliary tree and gallbladder diseases including cholecystitis, Mirizzi syndromes, biliary fistulas, iatrogenic diseases and common bile duct stones.
In 2016, Kubat et al. [ 96 ] published a retrospective series of 150 consecutive robotic single-site cholecystectomies (74 versus 76 cases treated in emergency scenarios and elective settings respectively). The mean operative time for ES cohort was significantly longer (95.0 ± 4.4 versus 71.9 ± 2.6 min; p < 0.001). Both cohorts required 1 conversion to open (1.35% for the emergency group and 1.31% for the elective group). One bile duct injury (0.7%) was reported in patients treated in emergency conditions. The authors concluded that robotic single-site cholecystectomy can be performed safely and effectively in both elective and urgent scenarios with a learning curve of about 48 cases to reach acceptable perioperative outcomes.
Mirizzi syndrome represents one of the most challenging complications of cholelithiasis [ 97 , 98 , 99 ].
In 2014, Lee et al. [ 97 ] evaluated the outcomes of five patients treated by endoscopic biliary stent placement and subsequent robotic partial cholecystectomy due to Mirizzi syndrome. No conversion to open was reported and all patients experienced an uneventful postoperative course.
In 2017 Magge et al. [ 98 ] reported a 6-patient series. All cases were treated performing a combined endoscopic and robotic approaches. In 3 cases (50%) a Roux-en-Y hepatico-jejunostomy was carried out. In these challenging scenarios, RS showed relevant benefits when compared to laparoscopy, facilitating complex dissections, and reducing conversion to open rate.
The most representative cohort of patients was described by Gangemi et al. [ 100 ] in 2017. Authors compared a large series of 676 patients receiving a robotic cholecystectomy with 284 treated by conventional laparoscopy: data analysis showed a significantly lower conversion to open in RS group, especially in patients affected by acute or gangrenous cholecystitis.
A 3-patient experience was described by Milone et al. [ 101 ] in 2019, achieving good perioperative outcomes in acute cholecystitis treatment.
Major bile duct injuries after cholecystectomy require complex surgical repairs that are usually performed with a conventional open approach [ 102 ]. This field may represent an interesting application of RS to safety perform biliary anastomosis. Cubisino et al. presented a systematic review of 13 literature experiences on minimally invasive biliary anastomosis after iatrogenic bile duct injury [ 103 ]. 198 patients were included. 135 patients (63.1%) underwent laparoscopic biliary anastomosis, while 73 (36.1%) received an analogue robotic procedure. According to Strasberg’s classification [ 104 ], all Bile Duct Injuries were types D and E (E1–E5). No conversions occurred in the RS series, while 4 patients required conversion to open surgery among the laparoscopic ones. Postoperative complications were superimposable (18.7% and 19.7% in laparoscopic and robotic approaches, respectively). Nevertheless, the overall reoperation rate was 4.4%, 5.5% in laparoscopic and 2.6% in robotic repairs.
During the follow-up period (median 24.6 months), 9 patients developed an anastomotic stricture: 5 (3.70%) in laparoscopic and 4 (5.48%) in robotic series that required a redo-anastomosis in 60% and 25% respectively.
When compared to open and laparoscopic cholecystectomy in AES, RS showed an increase of 0.7% per year in Lunardi et al. cohort of 793’800 cholecystectomies [ 95 ], ranging from 2.5 to 8.8% between 2013 and 2021. It could be underlined that conversion rate and LOS were statistically lower in RS group ( p < 0.001). Despite these findings, laparoscopic cholecystectomy yet represents the preferred approach in AES.
Robotics emergencies in hernia and abdominal wall surgery
Only a few studies analyzed urgent hernia operations treated using robotic surgery.
In 2020, Bou-Ayash et al. [ 105 ] published a retrospective series of 19 patients (including 23 surgical procedures) affected by inguinal hernia, treated from 2013 to 2020. The authors concluded that the robotic approach represents a safe procedure in selected patients, with a short length of stay and a low complication rate compared to open and laparoscopic surgery.
In 2021, Kudsi et al. [ 106 ] described perioperative outcomes of RS in a 34-patient cohort treated between 2013 and 2019. All patients experienced robotic ventral and incisional hernia repair in an emergency setting. 20% of patients were classified as Clavien-Dindo I or II, while about 11% Clavien-Dindo III and IV. Only 3% of the population experienced a recurrence.
Muysoms et al. [ 107 ] performed an extensive analysis of robotic cost. They retrospective evaluate laparoscopic (272 procedures of which 6 were emergency cases) and robotic (404 procedures of which 8 were emergency cases) inguinal hernia repairs. As reported in other literature experiences, authors concluded that Robotic inguinal hernia repair was significantly ( p < 0.001) more expensive if compared to laparoscopic surgery (mean cost €2612 versus €1963, respectively). Nevertheless, in the robotic group, a larger number of patients were treated as outpatients with lower postoperative complications.
Regarding inguinal and ventral hernia repair, the analysis conducted by Lunardi et al. [ 95 ] showed encouraging data for RS approach: from 2013 to 2021 RS increased of 1.9% per year and 1.1% per year respectively. After propensity score matching, authors reported superimposable data in terms of CCI and BMI, comparing laparoscopic and robotic approaches. Nonetheless, RS showed benefits also in these fields: lower conversion rates were reported both in inguinal hernia repairs (18.1% vs. 3.8%, p < 0.001) and in ventral hernia repair (16.2% vs. 4.8%, p < 0.001). In addition, a statistically significant shorter postoperative LOS was registered in the RS group (the mean LOS in the inguinal hernia group was 3.34 vs. 3 days in laparoscopic and robotic approaches respectively, and the mean LOS in the ventral hernia group was 3.87 vs. 3.73 days, respectively).
Other abdominal emergency surgery and future perspectives
A rare indication for urgent RS was post-traumatic splenic bleeding reported by Giulianotti et al. [ 108 ].
Until now, no reports of RS in adhesive intestinal obstruction have been published.
A possible and useful application of RS is represented by telementoring and telesurgery [ 32 , 109 , 110 , 111 ]. The original aim of RS and the recent COVID-19 pandemic gave an important incentive in these directions. The advantage of telementoring and telepresence of an expert surgeon in a virtual way is nowadays possible and may be improved thanks to the modern and future highspeed internet connection (5G networks) as well as the telesurgery in ultra-remote countries, in low-volume centers and in an emergent civil or battlefield surgical scenarios [ 112 , 113 , 114 ].
The development of new modular robotic platforms may contribute to increase RS applications in emergency settings. Nowadays, several different robotic platforms are approved for human use, such as CMR Versius (Cambridge Medical Robotics, Cambridge, UK), Distalmotion Dexter (Distalmotion, Epalinges, Switzerland) and Medtronic Hugo (Medtronic Inc., Minneapolis, USA). Most of them share the opportunity of switching from a conventional laparoscopic setting to a robot-assisted one.
Limitations
The main bias of our study was represented by hospital organisations: RS devices are available in the same building as the General Surgery Unit at San Donato Hospital (Arezzo, Italy) facilitating emergency surgical procedures. On the other hand, Da Vinci Xi is situated in a separate building specifically dedicated to RS at the General and Robotic Surgery Unit of San Giovanni Battista Hospital (Foligno, Italy).
Furthermore, these findings represented a limit when the surgeons’ team wanted to perform a laparoscopic exploration to validate a minimally invasive robotic approach in emergency scenarios.
In our experience, it should also underline that the COVID-19 era has contributed to limiting RS adoption.
Future shreds of evidence from randomized clinical trials with long-term follow-up are required to define the potential role of RS in AES. Nevertheless, the unavailable data on the cost-effectiveness of RS in AES are linked to lower use of robotic devices if compared to laparoscopic approaches. Our experience suggested that RS costs are superimposable to laparoscopic surgery if we analyse LOS and conversion rate data. To optimize the delivery of robotic technology in AES, a well-coordinated effort among health systems, clinicians, payers, and policymakers and dedicated training program for robotic teams are imperative.
Our study demonstrates that RS may be an useful and reliable approach also to emergency surgical procedures, especially when performed in selected patients in very well trained robotic centers allowing a safe managing of surgical challenging procedures as main indications for this technology, reducing the conversion rate when compared to laparoscopy.
As for laparoscopy the patient selection for robotic approach need hemodinamically stable condition and require a sharing of the surgical strategy by all the team: surgeons, nurses and anaesthesiologists. All the staff need to be trained in laparoscopic and robotic elective surgery, including technology functioning. The hybrid use of robotic/laparoscopic technology may be taken into consideration (a laparoscopic exploration may be the first step) to decide the following approach. The robotic approach may be reserved to challenging steps of the operation (suture/microsuture/dissections).
The availability of the device is the sine qua non condition for emergent and of course urgent use. The current organization in which the platform is shared by different teams, represent for the diffusion of its use in the emergent setting.
The cost reduction of platforms and instruments, together with new robotic devices in the health market, may represent a future perspective for emergencies use of robotic technology. So, the robotic technology may be one of the tools available in every operating theatre, to use in selected cases according to patient condition and surgical team experience.
Data availability
No datasets were generated or analysed during the current study.
Surlin V. Emergency and trauma surgery. Chirurgia (Bucur). 2021;116:643–4.
Article PubMed Google Scholar
E. U. o. M. S. S. o. S. a. E. B. o. Surgery. (2024), vol. 2024.
De Simone B, et al. The new timing in acute care surgery (new TACS) classification: a WSES Delphi consensus study. World J Emerg Surg. 2023;18:32.
Article PubMed PubMed Central Google Scholar
Crepaz L, et al. Minimally invasive approach to incisional hernia in elective and emergency surgery: a SICE (Italian Society of endoscopic surgery and new technologies) and ISHAWS (Italian society of hernia and abdominal wall surgery) online survey. Updates Surg. 2023;75:1671–80.
Coletta D, Patriti A. in Updates Surg . Italy. 2023;75:2047–2048.
Wakabayashi G, et al. Tokyo guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:73–86.
Gorter RR, et al. Diagnosis and management of acute appendicitis. Surg Endosc. 2016;30:4668–90. EAES consensus development conference 2015.
Jara RD, Guerrón AD, Portenier D. Complications of robotic surgery. Surg Clin North Am. 2020;100:461–8.
Rocca A, et al. Robotic surgery for colorectal liver metastases resection: a systematic review. Int J Med Robot. 2021;17:e2330.
Martins RS, et al. Quality of life after robotic versus conventional minimally invasive cancer surgery: a systematic review and meta-analysis. J Robot Surg. 2024;18:171.
Gomez Ruiz M, et al. Robotic surgery for locally advanced T4 rectal cancer: feasibility and oncological quality. Updates Surg. 2023;75:589–97.
Chatterjee S, Das S, Ganguly K, Mandal D. Advancements in robotic surgery: innovations, challenges and future prospects. J Robot Surg. 2024;18:28.
Minamimura K, et al. Current status of robotic gastrointestinal surgery. J Nippon Med Sch. 2023;90:308–15.
Rocca A, et al. Robotic versus open resection for colorectal liver metastases in a referral centre Hub&Spoke learning program. A multicenter propensity score matching analysis of perioperative outcomes. Heliyon. 2024;10:e24800.
Ceccarelli G, et al. Robotic revision surgery after failed Nissen anti-reflux surgery: a single center experience and a literature review. J Robot Surg. 2023;17:1517–24.
Knitter S, et al. Robotic versus laparoscopic versus open major hepatectomy - an analysis of costs and postoperative outcomes in a single-center setting. Langenbecks Arch Surg. 2023;408:214.
Liu R, Liu Q, Wang Z. Worldwide diffusion of robotic approach in general surgery. Updates Surg. 2021;73:795–7.
Daskalaki D, et al. Financial impact of the robotic approach in liver surgery: a comparative study of clinical outcomes and costs between the robotic and open technique in a single institution. J Laparoendosc Adv Surg Tech A. 2017;27:375–82.
de’Angelis N, et al. Robotic versus laparoscopic gastric resection for primary gastrointestinal stromal tumors > 5 cm: a size-matched and location-matched comparison. Surg Laparosc Endosc Percutan Tech. 2017;27:65–71.
van Dam P et al. Are costs of robot-assisted surgery warranted for gynecological procedures? Obstet Gynecol Int. 2011;2011:973830.
Vicente E, Quijano Y, Ferri V, Caruso R. Robot-assisted cholecystectomy with the new HUGO™ robotic-assisted system: first worldwide report with system description, docking settings, and video. Updates Surg. 2023;75:2039–42.
Leal Ghezzi T, Campos Corleta O. 30 years of robotic surgery. World J Surg. 2016;40:2550–7.
Sarkaria IS et al. Early operative outcomes and learning curve of robotic assisted giant paraesophageal hernia repair. Int J Med Robot. 2017;13.
Felder SI, et al. Robotic gastrointestinal surgery. Curr Probl Surg. 2018;55:198–246.
Ceccarelli G et al. Minimally invasive approach to gastric GISTs: analysis of a multicenter robotic and laparoscopic experience with literature review. Cancers (Basel). 2021;13.
Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Robot-assisted laparoscopic surgery of the colon and rectum. Surg Endosc. 2012;26:1–11.
Yeo HL, Isaacs AJ, Abelson JS, Milsom JW, Sedrakyan A. Comparison of open, laparoscopic, and robotic colectomies using a large national database: outcomes and trends related to surgery center volume. Dis Colon Rectum. 2016;59:535–42.
de’Angelis N, et al. Robotic versus laparoscopic colorectal cancer surgery in elderly patients: a propensity score match analysis. J Laparoendosc Adv Surg Tech A. 2018;28:1334–45.
Cheung TT, et al. Robotic versus laparoscopic liver resection for huge (≥ 10 cm) liver tumors: an international multicenter propensity-score matched cohort study of 799 cases. Hepatobiliary Surg Nutr. 2023;12:205–15.
Cillo U, D’Amico FE, Furlanetto A, Perin L, Gringeri E. Robotic hepatectomy and biliary reconstruction for perihilar cholangiocarcinoma: a pioneer western case series. Updates Surg. 2021;73:999–1006.
Giulianotti PC, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–84.
de’Angelis N, et al. Robotic surgery in emergency setting: 2021 WSES position paper. World J Emerg Surg. 2022;17:4.
Solaini L, et al. Open versus laparoscopic versus robotic gastric gastrointestinal stromal tumour resections: a multicentre cohort study. Int J Med Robot. 2021;17:e2198.
Wei D, Johnston S, Goldstein L, Nagle D. Minimally invasive colectomy is associated with reduced risk of anastomotic leak and other major perioperative complications and reduced hospital resource utilization as compared with open surgery: a retrospective population-based study of comparative effectiveness and trends of surgical approach. Surg Endosc. 2020;34:610–21.
Giovannetti A, et al. Laparoendoscopic single-site (LESS) versus robotic redo hiatal hernia repair with fundoplication: which approach is better? Am Surg. 2019;85:978–84.
Colvin J, et al. A comparison of robotic versus laparoscopic adrenalectomy in patients with primary hyperaldosteronism. Surg Laparosc Endosc Percutan Tech. 2017;27:391–3.
Tolboom RC, Draaisma WA, Broeders IA. Evaluation of conventional laparoscopic versus robot-assisted laparoscopic redo hiatal hernia and antireflux surgery: a cohort study. J Robot Surg. 2016;10:33–9.
Kim HI, et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg. 2016;263:103–9.
Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24.
Ceccarelli G, et al. Intraoperative and postoperative outcome of robot-assisted and traditional laparoscopic Nissen fundoplication. Eur Surg Res. 2009;43:198–203.
Article CAS PubMed Google Scholar
Ceccarelli G, et al. Robot-assisted surgery in elderly and very elderly population: our experience in oncologic and general surgery with literature review. Aging Clin Exp Res. 2017;29:55–63.
Ceccarelli G, et al. A new robot-assisted Billroth-I reconstruction: details of the technique and early results. Surg Laparosc Endosc Percutan Tech. 2018;28:e33–9.
Ceccarelli G, et al. Robot-assisted liver surgery in a general surgery unit with a referral centre hub&spoke learning program. Early outcomes after our first 70 consecutive patients. Minerva Chir. 2018;73:460–8.
Ceccarelli G, et al. Robot-assisted Toupet fundoplication and associated cholecystectomy in symptomatic giant hiatal hernia with situs viscerum inversus-A case report and literature review. Int J Surg Case Rep. 2019;60:371–5.
Ceccarelli G, et al. Minimally invasive laparoscopic and robot-assisted emergency treatment of strangulated giant hiatal hernias: report of five cases and literature review. World J Emerg Surg. 2020;15:37.
Ceccarelli G, et al. Minimally invasive robotic-assisted combined colorectal and liver excision surgery: feasibility, safety and surgical technique in a pilot series. Updates Surg. 2021;73:1015–22.
Costa G, et al. Clinico-pathological features of colon cancer patients undergoing emergency surgery: a comparison between elderly and non-elderly patients. Open Med (Wars). 2019;14:726–34.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–43.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Sermonesi G, et al. Cesena guidelines: WSES consensus statement on laparoscopic-first approach to general surgery emergencies and abdominal trauma. World J Emerg Surg. 2023;18:57.
Reinisch A, Liese J, Padberg W, Ulrich F. Robotic operations in urgent general surgery: a systematic review. J Robot Surg. 2023;17:275–90.
Capibaribe DM, Avilez ND, Sacomani CAR, Lucena A, Reis LO. Robotic approach to vesicourethral anastomotic stenosis and resection of remaining prostate after radical prostatectomy. Int Braz J Urol. 2023;49:158–60.
Huser AS, et al. Simulated life-threatening emergency during robot-assisted surgery. J Endourol. 2014;28:717–21.
Ballas DA, Cesta M, Gothard D, Ahmed R. Emergency undocking curriculum in robotic surgery. Cureus. 2019;11:e4321.
PubMed PubMed Central Google Scholar
van der Schans EM, Hiep MAJ, Consten ECJ, Broeders I. From Da Vinci Si to Da Vinci Xi: realistic times in draping and docking the robot. J Robot Surg. 2020;14:835–9.
Alfieri S, et al. Short-term and long-term outcomes after robot-assisted versus laparoscopic distal pancreatectomy for pancreatic neuroendocrine tumors (pNETs): a multicenter comparative study. Langenbecks Arch Surg. 2019;404:459–68.
Abdel Raheem A, et al. Da Vinci Xi and Si platforms have equivalent perioperative outcomes during robot-assisted partial nephrectomy: preliminary experience. J Robot Surg. 2017;11:53–61.
Yuh B, et al. Use of a mobile tower-based robot–the initial Xi robot experience in surgical oncology. J Surg Oncol. 2016;113:5–7.
Hollandsworth HM, et al. Multiquadrant surgery in the robotic era: a technical description and outcomes for Da Vinci Xi robotic subtotal colectomy and total proctocolectomy. Surg Endosc. 2020;34:5153–9.
Bianchi G, et al. Short-term outcomes of Da Vinci Xi versus Si robotic systems for minor hepatectomies. Acta Biomed. 2022;93:e2022223.
Hill A, McCormick J. In experienced hands, does the robotic platform impact operative efficiency? Comparison of the Da Vinci Si versus Xi robot in colorectal surgery. J Robot Surg. 2020;14:789–92.
Oldani A, Bellora P, Monni M, Amato B, Gentilli S. Colorectal surgery in elderly patients: our experience with DaVinci Xi ® system. Aging Clin Exp Res. 2017;29:91–9.
Gallotta V, et al. Robotic surgery in elderly and very elderly gynecologic cancer patients. J Minim Invasive Gynecol. 2018;25:872–7.
Buchs NC, et al. Safety of robotic general surgery in elderly patients. J Robot Surg. 2010;4:91–8.
Barberio M, et al. Quantitative fluorescence angiography versus hyperspectral imaging to assess bowel ischemia: a comparative study in enhanced reality. Surgery. 2020;168:178–84.
Liot E, et al. Does near-infrared (NIR) fluorescence angiography modify operative strategy during emergency procedures? Surg Endosc. 2018;32:4351–6.
Sudan R, Desai SS. Emergency and weekend robotic surgery are feasible. J Robot Surg. 2012;6:263–6.
Cubas R, Garcia M, Mukherjee K. Robotic repair of incarcerated morgagni hernia in an adult on the acute care surgery service. Rev Fac Cien Med Univ Nac Cordoba. 2021;78:91–4.
Kim JK, Desai A, Kunac A, Merchant AM, Lovoulos C. Robotic Transthoracic Repair of a Right-Sided Traumatic Diaphragmatic Rupture. Surg J (N Y). 2020;6(3):e164–e166.
Hosein S, Carlson T, Flores L, Armijo PR, Oleynikov D. Minimally invasive approach to hiatal hernia repair is superior to open, even in the emergent setting: a large national database analysis. Surg Endosc. 2021;35:423–8.
Robinson TD, et al. Emergent robotic versus laparoscopic surgery for perforated gastrojejunal ulcers: a retrospective cohort study of 44 patients. Surg Endosc. 2022;36:1573–7.
Sartelli M, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg. 2020;15:32.
de’Angelis N, et al. 2017 WSES guidelines for the management of iatrogenic colonoscopy perforation. World J Emerg Surg. 2018;13:5.
Marano A, Giuffrida MC, Giraudo G, Pellegrino L, Borghi F. Management of peritonitis after minimally invasive colorectal surgery: can we stick to laparoscopy? J Laparoendosc Adv Surg Tech A. 2017;27:342–7.
Wind J, et al. Laparoscopic reintervention for anastomotic leakage after primary laparoscopic colorectal surgery. Br J Surg. 2007;94:1562–6.
Vennix S, et al. Emergency laparoscopic sigmoidectomy for perforated diverticulitis with generalised peritonitis: a systematic review. Dig Surg. 2016;33:1–7.
Pedraza R, Ragupathi M, Martinez T, Haas EM. Robotic-assisted laparoscopic primary repair of acute iatrogenic colonic perforation: case report. Int J Med Robot. 2012;8:375–8.
Felli E, et al. Robotic right colectomy for hemorrhagic right colon cancer: a case report and review of the literature of minimally invasive urgent colectomy. World J Emerg Surg. 2014;9:32.
Chen ZL, Du QL, Zhu YB, Wang HF. A systematic review and meta-analysis of short-term outcomes comparing the efficacy of robotic versus laparoscopic colorectal surgery in obese patients. J Robot Surg. 2024;18:167.
Alkhamis A, et al. Outcomes in robotic-assisted compared to laparoscopic-assisted colorectal surgery in a newly established colorectal tertiary center: a retrospective comparative cohort study. J Robot Surg. 2024;18:152.
de Almeida Leite RM et al. Surgical and medical outcomes in robotic compared to laparoscopic colectomy global prospective cohort from the American college of surgeons national surgical quality improvement program. Surg Endosc. 2024.
Beltzer C, et al. Robotic versus laparoscopic sigmoid resection for diverticular disease: a single-center experience of 106 cases. J Laparoendosc Adv Surg Tech A. 2019;29:1451–5.
Kudsi OY, Gokcal F. Urgent robotic mesocolic excision for obstructing proximal transverse colon cancer - a video vignette. Colorectal Dis. 2019;21:1093–4.
Kudsi OY, Bou-Ayash N. Bleeding sigmoid diverticulosis - urgent stapleless totally robotic sigmoidectomy-a video vignette. Colorectal Dis. 2020;22:1205.
Kudsi OY, Bou-Ayash N. Caecal volvulus - urgent totally robotic right colectomy - a video vignette. Colorectal Dis. 2020;22:1448–9.
Anderson M, et al. Early experience with urgent robotic subtotal colectomy for severe acute ulcerative colitis has comparable perioperative outcomes to laparoscopic surgery. J Robot Surg. 2020;14:249–53.
Yang Y, et al. The global burden of appendicitis in 204 countries and territories from 1990 to 2019. Clin Epidemiol. 2022;14:1487–99.
Cadière GB, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg. 2001;25:1467–77.
Kelkar D, Borse MA, Godbole GP, Kurlekar U, Slack M. Interim safety analysis of the first-in-human clinical trial of the versius surgical system, a new robot-assisted device for use in minimal access surgery. Surg Endosc. 2021;35:5193–202.
Kibar Y, Yalcin S, Kopru B, Kaya E, Topuz B, Ebiloglu T. Robot-Assisted Laparoscopic Repair of Spontaneous Appendicovesical Fistula. J Endourol Case Rep. 2016;2(1):111–3.
Yi B, et al. The first clinical use of domestically produced Chinese minimally invasive surgical robot system micro hand S. Surg Endosc. 2016;30:2649–55.
Yi B, et al. Domestically produced Chinese minimally invasive surgical robot system micro hand S is applied to clinical surgery preliminarily in China. Surg Endosc. 2017;31:487–93.
Hüttenbrink C, et al. Incidental appendectomy during robotic laparoscopic prostatectomy-safe and worth to perform? Langenbecks Arch Surg. 2018;403:265–9.
PubMed Google Scholar
Lunardi N, et al. Robotic technology in emergency general surgery cases in the era of minimally invasive surgery. JAMA Surg. 2024;159:493–9.
Kubat E, Hansen N, Nguyen H, Wren SM, Eisenberg D. Urgent and elective robotic single-site cholecystectomy: analysis and learning curve of 150 consecutive cases. J Laparoendosc Adv Surg Tech A. 2016;26:185–91.
Lee KF, et al. A minimally invasive strategy for Mirizzi syndrome: the combined endoscopic and robotic approach. Surg Endosc. 2014;28:2690–4.
Magge D, et al. Performing the difficult cholecystectomy using combined endoscopic and robotic techniques: how I do it. J Gastrointest Surg. 2017;21:583–9.
Valderrama-Treviño AI, et al. Updates in Mirizzi syndrome. Hepatobiliary Surg Nutr. 2017;6:170–8.
Gangemi A, Danilkowicz R, Bianco F, Masrur M, Giulianotti PC. Risk factors for open conversion in minimally invasive cholecystectomy. Jsls. 2017;21.
Milone M, et al. Robotic cholecystectomy for acute cholecystitis: three case reports. Med (Baltim). 2019;98:e16010.
Article CAS Google Scholar
Giuliante F, et al. Bile duct injury after cholecystectomy: timing of surgical repair should be based on clinical presentation. The experience of a tertiary referral center with Hepp-Couinaud hepatico-jejunostomy. Updates Surg. 2023;75:1509–17.
Cubisino A, Dreifuss NH, Cassese G, Bianco FM, Panaro F. Minimally invasive biliary anastomosis after iatrogenic bile duct injury: a systematic review. Updates Surg. 2023;75:31–9.
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–25.
CAS PubMed Google Scholar
Bou-Ayash N, Gokcal F, Kudsi OY. Robotic inguinal hernia repair for incarcerated hernias. J Laparoendosc Adv Surg Tech A. 2021;31:926–30.
Kudsi OY, Bou-Ayash N, Chang K, Gokcal F. Perioperative and midterm outcomes of emergent robotic repair of incarcerated ventral and incisional hernia. J Robot Surg. 2021;15:473–81.
Muysoms F et al. Economic assessment of starting robot-assisted laparoscopic inguinal hernia repair in a single-centre retrospective comparative study: the EASTER study. BJS Open. 2021;5.
Giulianotti PC, et al. Robot-assisted treatment of splenic artery aneurysms. Ann Vasc Surg. 2011;25:377–83.
Smith AL, et al. Dual-console robotic surgery: a new teaching paradigm. J Robot Surg. 2013;7:113–8.
Thomas A, et al. Effective implementation and adaptation of structured robotic colorectal programme in a busy tertiary unit. J Robot Surg. 2021;15:731–9.
Panteleimonitis S, et al. Implementation of robotic rectal surgery training programme: importance of standardisation and structured training. Langenbecks Arch Surg. 2018;403:749–60.
Zheng J, et al. 5G ultra-remote robot-assisted laparoscopic surgery in China. Surg Endosc. 2020;34:5172–80.
Mohan A, Wara UU, Arshad Shaikh MT, Rahman RM, Zaidi ZA. Telesurgery and robotics: an improved and efficient era. Cureus. 2021;13:e14124.
Anvari M, Manoharan B, Barlow K. From telementorship to automation. J Surg Oncol. 2021;124:246–9.
Giulianotti PC, Quadri P, Durgam S, Bianco FM. Reconstruction/repair of iatrogenic biliary injuries: is the robot offering a new option? Short clinical report. Ann Surg. 2018;267:e7–9.
Cuendis-Velázquez A, et al. A new era of bile duct repair: robotic-assisted versus laparoscopic hepaticojejunostomy. J Gastrointest Surg. 2019;23:451–9.
Marino MV, Mirabella A, Guarrasi D, Lupo M, Komorowski AL. Robotic-assisted repair of iatrogenic common bile duct injury after laparoscopic cholecystectomy: surgical technique and outcomes. Int J Med Robot. 2019;15:e1992.
Sucandy I, et al. Robotic versus open extrahepatic biliary reconstruction for iatrogenic bile duct injury. Am Surg. 2022;88:345–7.
D’Hondt M, Wicherts DA. Robotic biliary surgery for benign and malignant bile duct obstruction: a case series. J Robot Surg. 2023;17:55–62.
Download references
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Department of General Surgery, “San Giovanni Battista” Hospital, USL Umbria 2, Foligno, Perugia, Italy
Graziano Ceccarelli, Fabio Rondelli & Michele De Rosa
Division of General Surgery, Bufalini Hospital, Cesena, Italy
Fausto Catena
Department of General Surgery and Hepatobiliary and Pancreatic Surgery Unit, Pineta Grande Hospital, Castel Volturno, Caserta, Italy
Pasquale Avella & Aldo Rocca
Department of Medicine and Health Science “V. Tiberio”, University of Molise, Campobasso, Italy
Pasquale Avella, Germano Guerra & Aldo Rocca
Department of General Surgery, Singapore General Hospital, Singapore, Singapore
Brian WCA Tian
You can also search for this author in PubMed Google Scholar
Contributions
G.C.: study conception and design, literature search, data acquisition, interpretation and analysis; drafting and critically revising the article for important intellectual content; final approval of the version to be published. P.A.: literature search, data acquisition, interpretation and analysis; drafting and critically revising the article for important intellectual content; final approval of the version to be published. F.C.: drafting and critically revising the article for important intellectual content; final approval of the version to be published. B.W.T.: data acquisition, interpretation and analysis; final approval of the version to be published. F.R.: study conception and design, literature search, data acquisition, interpretation and analysis; final approval of the version to be published. All authors: data acquisition, interpretation, and analysis; final approval of the version to be published. A.R.: supervision, drafted and critically revised the article for important intellectual content; final approval of the version to be published.
Corresponding author
Correspondence to Pasquale Avella .
Ethics declarations
Human ethics and consent to participate.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. An informed consent for treating personal and sensible data was obtained from all patients before the data collection and evaluation. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approved date: May 12, 2021).
Consent to publish
Patients signed informed consent regarding publishing their data and photographs.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ceccarelli, G., Catena, F., Avella, P. et al. Emergency robotic surgery: the experience of a single center and review of the literature. World J Emerg Surg 19 , 28 (2024). https://doi.org/10.1186/s13017-024-00555-6
Download citation
Received : 14 May 2024
Accepted : 22 July 2024
Published : 17 August 2024
DOI : https://doi.org/10.1186/s13017-024-00555-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Abdominal emergency surgery
- Urgent robotic surgery
- Minimally invasive surgery
- Learning curve
- Complicated diverticulitis
- Emergency setting
World Journal of Emergency Surgery
ISSN: 1749-7922
- Submission enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Analysing near-miss incidents in construction: a systematic literature review.

1. Introduction
- Q 1 —Are near-miss events in construction industry the subject of scientific research?
- Q 2 —What methods have been employed thus far to obtain information on near misses and systems for recording incidents in construction companies?
- Q 3 —What methods have been used to analyse the information and figures obtained?
- Q 4 —What are the key aspects of near misses in the construction industry that have been of interest to the researchers?
2. Definition of Near-Miss Events
3. research methodology, 4.1. a statistical analysis of publications, 4.2. methods used to obtain information about near misses, 4.2.1. traditional methods.
- Traditional registration forms
- Computerized systems for the recording of events
- Surveys and interviews
4.2.2. Real-Time Monitoring Systems
- Employee-tracking systems
- Video surveillance systems
- Wearable technology
- Motion sensors
4.3. Methods Used to Analyse the Information and Figures That Have Been Obtained
4.3.1. quantitative and qualitative statistical methods, 4.3.2. analysis using artificial intelligence (ai), 4.3.3. building information modelling, 4.4. key aspects of near-miss investigations in the construction industry, 4.4.1. occupational risk assessment, 4.4.2. causes of hazards in construction, 4.4.3. time series of near misses, 4.4.4. material factors of construction processes, 4.5. a comprehensive overview of the research questions and references on near misses in the construction industry, 5. discussion, 5.1. interest of researchers in near misses in construction (question 1), 5.2. methods used to obtain near-miss information (question 2), 5.3. methods used to analyse the information and data sets (question 3), 5.4. key aspects of near-miss investigations in the construction industry (question 4), 6. conclusions.
- A quantitative analysis of the Q 1 question has revealed a positive trend, namely that there is a growing interest among researchers in studying near misses in construction. The greatest interest in NM topics is observed in the United States of America, China, the United Kingdom, Australia, Hong Kong, and Germany. Additionally, there has been a recent emergence of interest in Poland. The majority of articles are mainly published in journals such as Safety Science (10), Journal of Construction Engineering and Management (8), and Automation in Construction (5);
- The analysis of question Q 2 illustrates that traditional paper-based event registration systems are currently being superseded by advanced IT systems. However, both traditional and advanced systems are subject to the disadvantage of relying on employee-reported data, which introduces a significant degree of uncertainty regarding in the quality of the information provided. A substantial proportion of the data and findings presented in the studies was obtained through surveys and interviews. The implementation of real-time monitoring systems is becoming increasingly prevalent in construction sites. The objective of such systems is to provide immediate alerts in the event of potential hazards, thereby preventing a significant number of near misses. Real-time monitoring systems employ a range of technologies, including ultrasonic technology, radio frequency identification (RFID), inertial measurement units (IMUs), real-time location systems (RTLSs), industrial cameras, wearable technology, motion sensors, and advanced IT technologies, among others;
- The analysis of acquired near-miss data is primarily conducted through the utilisation of quantitative and qualitative statistical methods, as evidenced by the examination of the Q 3 question. In recent years, research utilising artificial intelligence (AI) has made significant advances. The most commonly employed artificial intelligence techniques include text mining, machine learning, and artificial neural networks. The growing deployment of Building Information Modelling (BIM) technology has precipitated a profound transformation in the safety management of construction sites, with the advent of sophisticated tools for the identification and management of hazardous occurrences;
- In response to question Q 4 , the study of near misses in the construction industry has identified several key aspects that have attracted the attention of researchers. These include the utilisation of both quantitative and qualitative methodologies for risk assessment, the analysis of the causes of hazards, the identification of accident precursors through the creation of time series, and the examination of material factors pertaining to construction processes. Researchers are focusing on the utilisation of both databases and advanced technologies, such as real-time location tracking, for the assessment and analysis of occupational risks. Techniques such as Analytic Hierarchy Process (AHP) and clustering facilitate a comprehensive assessment and categorisation of incidents, thereby enabling the identification of patterns and susceptibility to specific types of accidents. Moreover, the impact of a company’s safety climate and organisational culture on the frequency and characteristics of near misses represents a pivotal area of investigation. The findings of this research indicate that effective safety management requires a holistic approach that integrates technology, risk management and safety culture, with the objective of reducing accidents and enhancing overall working conditions on construction sites.
7. Gaps and Future Research Directions, Limitations
- Given the diversity and variability of construction sites and the changing conditions and circumstances of work, it is essential to create homogeneous clusters of near misses and to analyse the phenomena within these clusters. The formation of such clusters may be contingent upon the direct causes of the events in question;
- Given the inherently dynamic nature of construction, it is essential to analyse time series of events that indicate trends in development and safety levels. The numerical characteristics of these trends may be used to construct predictive models for future accidents and near misses;
- The authors have identified potential avenues for future research, which could involve the development of mathematical models using techniques such as linear regression, artificial intelligence, and machine learning. The objective of these models is to predict the probable timing of occupational accidents within defined incident categories, utilising data from near misses. Moreover, efforts are being made to gain access to the hazardous incident recording systems of different construction companies, with a view to facilitating comparison of the resulting data;
- One significant limitation of near-miss research is the lack of an integrated database that encompasses a diverse range of construction sites and construction work. A data resource of this nature would be of immense value for the purpose of conducting comprehensive analyses and formulating effective risk management strategies. This issue can be attributed to two factors: firstly, the reluctance of company managers to share their databases with researchers specialising in risk assessment, and secondly, the reluctance of employees to report near-miss incidents. Such actions may result in adverse consequences for employees, including disciplinary action or negative perceptions from managers. This consequently results in the recording of only a subset of incidents, thereby distorting the true picture of safety on the site.
Author Contributions
Institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
| Year | Source Title | DOI/ISBN/ISSN | Reference |
|---|---|---|---|
| 1999 | Construction Management and Economics | 10.1080/014461999371691 | [ ] |
| 2002 | Structural Engineer | 14665123 | [ ] |
| 2009 | Building a Sustainable Future—Proceedings of the 2009 Construction Research Congress | 10.1061/41020(339)4 | [ ] |
| 2010 | Safety Science | 10.1016/j.ssci.2010.04.009 | [ ] |
| 2010 | Automation in Construction | 10.1016/j.autcon.2009.11.017 | [ ] |
| 2010 | Safety Science | 10.1016/j.ssci.2009.06.006 | [ ] |
| 2012 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0000518 | [ ] |
| 2013 | ISARC 2013—30th International Symposium on Automation and Robotics in Construction and Mining, Held in Conjunction with the 23rd World Mining Congress | 10.22260/isarc2013/0113 | [ ] |
| 2014 | Proceedings of the Institution of Civil Engineers: Civil Engineering | 10.1680/cien.14.00010 | [ ] |
| 2014 | Safety Science | 10.1016/j.ssci.2013.12.012 | [ ] |
| 2014 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0000795 | [ ] |
| 2014 | 31st International Symposium on Automation and Robotics in Construction and Mining, ISARC 2014—Proceedings | 10.22260/isarc2014/0115 | [ ] |
| 2014 | Construction Research Congress 2014: Construction in a Global Network—Proceedings of the 2014 Construction Research Congress | 10.1061/9780784413517.0181 | [ ] |
| 2014 | Construction Research Congress 2014: Construction in a Global Network—Proceedings of the 2014 Construction Research Congress | 10.1061/9780784413517.0235 | [ ] |
| 2014 | Construction Research Congress 2014: Construction in a Global Network—Proceedings of the 2014 Construction Research Congress | 10.1061/9780784413517.0096 | [ ] |
| 2015 | Automation in Construction | 10.1016/j.autcon.2015.09.003 | [ ] |
| 2015 | 32nd International Symposium on Automation and Robotics in Construction and Mining: Connected to the Future, Proceedings | 10.22260/isarc2015/0062 | [ ] |
| 2015 | ASSE Professional Development Conference and Exposition 2015 | - | [ ] |
| 2015 | Congress on Computing in Civil Engineering, Proceedings | 10.1061/9780784479247.019 | [ ] |
| 2016 | Automation in Construction | 10.1016/j.autcon.2016.03.008 | [ ] |
| 2016 | Automation in Construction | 10.1016/j.autcon.2016.04.007 | [ ] |
| 2016 | IEEE IAS Electrical Safety Workshop | 10.1109/ESW.2016.7499701 | [ ] |
| 2016 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0001100 | [ ] |
| 2016 | Safety Science | 10.1016/j.ssci.2015.11.025 | [ ] |
| 2016 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0001049 | [ ] |
| 2016 | IEEE Transactions on Industry Applications | 10.1109/TIA.2015.2461180 | [ ] |
| 2017 | Safety Science | 10.1016/j.ssci.2017.06.012 | [ ] |
| 2017 | ENR (Engineering News-Record) | 8919526 | [ ] |
| 2017 | 6th CSCE-CRC International Construction Specialty Conference 2017—Held as Part of the Canadian Society for Civil Engineering Annual Conference and General Meeting 2017 | 978-151087841-9 | [ ] |
| 2017 | Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) | 10.1007/978-3-319-72323-5_12 | [ ] |
| 2017 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0001209 | [ ] |
| 2017 | Safety Science | 10.1016/j.ssci.2016.08.027 | [ ] |
| 2017 | Safety Science | 10.1016/j.ssci.2016.08.022 | [ ] |
| 2018 | Safety Science | 10.1016/j.ssci.2018.04.004 | [ ] |
| 2018 | International Journal of Construction Management | 10.1080/15623599.2017.1382067 | [ ] |
| 2018 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0001420 | [ ] |
| 2018 | Proceedings of SPIE—The International Society for Optical Engineering | 10.1117/12.2296548 | [ ] |
| 2019 | Automation in Construction | 10.1016/j.autcon.2019.102854 | [ ] |
| 2019 | Physica A: Statistical Mechanics and its Applications | 10.1016/j.physa.2019.121495 | [ ] |
| 2019 | Sustainability (Switzerland) | 10.3390/su11051264 | [ ] |
| 2019 | Computing in Civil Engineering 2019: Data, Sensing, and Analytics—Selected Papers from the ASCE International Conference on Computing in Civil Engineering 2019 | 978-078448243-8 | [ ] |
| 2019 | Journal of Health, Safety and Environment | 18379362 | [ ] |
| 2019 | Computing in Civil Engineering 2019: Data, Sensing, and Analytics—Selected Papers from the ASCE International Conference on Computing in Civil Engineering 2019 | 978-078448243-8 | [ ] |
| 2019 | Computing in Civil Engineering 2019: Smart Cities, Sustainability, and Resilience—Selected Papers from the ASCE International Conference on Computing in Civil Engineering 2019 | 10.1061/9780784482445.026 | [ ] |
| 2019 | Journal of Construction Engineering and Management | 10.1061/(ASCE)CO.1943-7862.0001582 | [ ] |
| 2019 | Advances in Intelligent Systems and Computing | 10.1007/978-3-030-02053-8_107 | [ ] |
| 2020 | Accident Analysis and Prevention | 10.1016/j.aap.2020.105496 | [ ] |
| 2020 | Advanced Engineering Informatics | 10.1016/j.aei.2020.101062 | [ ] |
| 2020 | Advanced Engineering Informatics | 10.1016/j.aei.2020.101060 | [ ] |
| 2020 | ARCOM 2020—Association of Researchers in Construction Management, 36th Annual Conference 2020—Proceedings | 978-099554633-2 | [ ] |
| 2020 | International Journal of Building Pathology and Adaptation | 10.1108/IJBPA-03-2020-0018 | [ ] |
| 2020 | Communications in Computer and Information Science | 10.1007/978-3-030-42852-5_8 | [ ] |
| 2021 | Journal of Architectural Engineering | 10.1061/(ASCE)AE.1943-5568.0000501 | [ ] |
| 2021 | Safety Science | 10.1016/j.ssci.2021.105368 | [ ] |
| 2021 | ACM International Conference Proceeding Series | 10.1145/3482632.3487473 | [ ] |
| 2021 | Reliability Engineering and System Safety | 10.1016/j.ress.2021.107687 | [ ] |
| 2021 | Proceedings of the 37th Annual ARCOM Conference, ARCOM 2021 | - | [ ] |
| 2022 | Buildings | 10.3390/buildings12111855 | [ ] |
| 2022 | Safety Science | 10.1016/j.ssci.2022.105704 | [ ] |
| 2022 | Sensors | 10.3390/s22093482 | [ ] |
| 2022 | Proceedings of International Structural Engineering and Construction | 10.14455/ISEC.2022.9(2).CSA-03 | [ ] |
| 2022 | Journal of Information Technology in Construction | 10.36680/j.itcon.2022.045 | [ ] |
| 2022 | Forensic Engineering 2022: Elevating Forensic Engineering—Selected Papers from the 9th Congress on Forensic Engineering | 10.1061/9780784484555.005 | [ ] |
| 2022 | Computational Intelligence and Neuroscience | 10.1155/2022/4851615 | [ ] |
| 2022 | International Journal of Construction Management | 10.1080/15623599.2020.1839704 | [ ] |
| 2023 | Journal of Construction Engineering and Management | 10.1061/JCEMD4.COENG-13979 | [ ] |
| 2023 | Heliyon | 10.1016/j.heliyon.2023.e21607 | [ ] |
| 2023 | Accident Analysis and Prevention | 10.1016/j.aap.2023.107224 | [ ] |
| 2023 | Safety | 10.3390/safety9030047 | [ ] |
| 2023 | Engineering, Construction and Architectural Management | 10.1108/ECAM-09-2021-0797 | [ ] |
| 2023 | Advanced Engineering Informatics | 10.1016/j.aei.2023.101929 | [ ] |
| 2023 | Engineering, Construction and Architectural Management | 10.1108/ECAM-05-2023-0458 | [ ] |
| 2023 | Intelligent Automation and Soft Computing | 10.32604/iasc.2023.031359 | [ ] |
| 2023 | International Journal of Construction Management | 10.1080/15623599.2020.1847405 | [ ] |
| 2024 | Heliyon | 10.1016/j.heliyon.2024.e26410 | [ ] |
- Occupational Risk|Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/en/tools-and-resources/eu-osha-thesaurus/term/70194i (accessed on 28 June 2023).
- Guo, S.; Zhou, X.; Tang, B.; Gong, P. Exploring the Behavioral Risk Chains of Accidents Using Complex Network Theory in the Construction Industry. Phys. A Stat. Mech. Its Appl. 2020 , 560 , 125012. [ Google Scholar ] [ CrossRef ]
- Woźniak, Z.; Hoła, B. The Structure of near Misses and Occupational Accidents in the Polish Construction Industry. Heliyon 2024 , 10 , e26410. [ Google Scholar ] [ CrossRef ]
- Li, X.; Sun, W.; Fu, H.; Bu, Q.; Zhang, Z.; Huang, J.; Zang, D.; Sun, Y.; Ma, Y.; Wang, R.; et al. Schedule Risk Model of Water Intake Tunnel Construction Considering Mood Factors and Its Application. Sci. Rep. 2024 , 14 , 3857. [ Google Scholar ] [ CrossRef ]
- Li, X.; Huang, J.; Li, C.; Luo, N.; Lei, W.; Fan, H.; Sun, Y.; Chen, W. Study on Construction Resource Optimization and Uncertain Risk of Urban Sewage Pipe Network. Period. Polytech. Civ. Eng. 2022 , 66 , 335–343. [ Google Scholar ] [ CrossRef ]
- Central Statistical Office Central Statistical Office/Thematic Areas/Labor Market/Working Conditions/Accidents at Work/Accidents at Work in the 1st Quarter of 2024. Available online: https://stat.gov.pl/obszary-tematyczne/rynek-pracy/warunki-pracy-wypadki-przy-pracy/wypadki-przy-pracy-w-1-kwartale-2024-roku,3,55.html (accessed on 17 July 2024).
- Manzo, J. The $ 5 Billion Cost of Construction Fatalities in the United States: A 50 State Comparison ; The Midwest Economic Policy Institute (MEPI): Saint Paul, MN, USA, 2017. [ Google Scholar ]
- Sousa, V.; Almeida, N.M.; Dias, L.A. Risk-Based Management of Occupational Safety and Health in the Construction Industry—Part 1: Background Knowledge. Saf. Sci. 2014 , 66 , 75–86. [ Google Scholar ] [ CrossRef ]
- Amirah, N.A.; Him, N.F.N.; Rashid, A.; Rasheed, R.; Zaliha, T.N.; Afthanorhan, A. Fostering a Safety Culture in Manufacturing through Safety Behavior: A Structural Equation Modelling Approach. J. Saf. Sustain. 2024; in press . [ Google Scholar ] [ CrossRef ]
- Heinrich, H.W. Industrial Accident Prevention ; A Scientific Approach; McGraw-Hill: New York, NY, USA, 1931. [ Google Scholar ]
- Near Miss Definition Per OSHA—What Is a Near Miss? Available online: https://safetystage.com/osha-compliance/near-miss-definition-osha/ (accessed on 17 August 2024).
- Cambraia, F.B.; Saurin, T.A.; Formoso, C.T. Identification, Analysis and Dissemination of Information on near Misses: A Case Study in the Construction Industry. Saf. Sci. 2010 , 48 , 91–99. [ Google Scholar ] [ CrossRef ]
- Tan, J.; Li, M. How to Achieve Accurate Accountability under Current Administrative Accountability System for Work Safety Accidents in Chemical Industry in China: A Case Study on Major Work Safety Accidents during 2010–2020. J. Chin. Hum. Resour. Manag. 2022 , 13 , 26–40. [ Google Scholar ] [ CrossRef ]
- Wu, W.; Gibb, A.G.F.; Li, Q. Accident Precursors and near Misses on Construction Sites: An Investigative Tool to Derive Information from Accident Databases. Saf. Sci. 2010 , 48 , 845–858. [ Google Scholar ] [ CrossRef ]
- Janicak, C.A. Fall-Related Deaths in the Construction Industry. J. Saf. Res. 1998 , 29 , 35–42. [ Google Scholar ] [ CrossRef ]
- Li, H.; Yang, X.; Wang, F.; Rose, T.; Chan, G.; Dong, S. Stochastic State Sequence Model to Predict Construction Site Safety States through Real-Time Location Systems. Saf. Sci. 2016 , 84 , 78–87. [ Google Scholar ] [ CrossRef ]
- Yang, K.; Aria, S.; Ahn, C.R.; Stentz, T.L. Automated Detection of Near-Miss Fall Incidents in Iron Workers Using Inertial Measurement Units. In Proceedings of the Construction Research Congress 2014: Construction in a Global Network, Atlanta, GA, USA, 19–21 May 2014; pp. 935–944. [ Google Scholar ] [ CrossRef ]
- Raviv, G.; Fishbain, B.; Shapira, A. Analyzing Risk Factors in Crane-Related near-Miss and Accident Reports. Saf. Sci. 2017 , 91 , 192–205. [ Google Scholar ] [ CrossRef ]
- Zhao, X.; Zhang, M.; Cao, T. A Study of Using Smartphone to Detect and Identify Construction Workers’ near-Miss Falls Based on ANN. In Proceedings of the Nondestructive Characterization and Monitoring of Advanced Materials, Aerospace, Civil Infrastructure, and Transportation XII, Denver, CO, USA, 4–8 March 2018; p. 80. [ Google Scholar ] [ CrossRef ]
- Santiago, K.; Yang, X.; Ruano-Herreria, E.C.; Chalmers, J.; Cavicchia, P.; Caban-Martinez, A.J. Characterising near Misses and Injuries in the Temporary Agency Construction Workforce: Qualitative Study Approach. Occup. Environ. Med. 2020 , 77 , 94–99. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- What Is OSHA’s Definition of a Near Miss. Available online: https://www.osha.com/blog/near-miss-definition (accessed on 4 August 2023).
- Martins, I. Investigation of Occupational Accidents and Diseases a Practical Guide for Labour Inspectors ; International Labour Office: Geneva, Switzerland, 2015. [ Google Scholar ]
- National Safety Council. Near Miss Reporting Systems ; National Safety Council: Singapore, 2013. [ Google Scholar ]
- PKN PN-ISO 45001:2018-06 ; Occupational Health and Safety Management Systems—Requirements with Guidance for Use. CRC Press: Boca Raton, FL, USA, 2019.
- PKN PN-N-18001:2004 ; Occupational Health and Safety Management Systems—Requirements. CRC Press: Boca Raton, FL, USA, 2004.
- World Health Organisation. WHO Draft GuiDelines for Adverse Event Reporting and Learning Systems ; World Health Organisation: Geneva, Switzerland, 2005. [ Google Scholar ]
- International Atomic Energy Agency IAEA Satety Glossary. Terminology Used in Nuclear Safety and Radiation Protection: 2007 Edition ; International Atomic Energy Agency: Vienna, Austria, 2007. [ Google Scholar ]
- Marks, E.; Teizer, J.; Hinze, J. Near Miss Reporting Program to Enhance Construction Worker Safety Performance. In Proceedings of the Construction Research Congress 2014: Construction in a Global Network, Atlanta, GA, USA, 19 May 2014; pp. 2315–2324. [ Google Scholar ] [ CrossRef ]
- Gnoni, M.G.; Saleh, J.H. Near-Miss Management Systems and Observability-in-Depth: Handling Safety Incidents and Accident Precursors in Light of Safety Principles. Saf. Sci. 2017 , 91 , 154–167. [ Google Scholar ] [ CrossRef ]
- Thoroman, B.; Goode, N.; Salmon, P. System Thinking Applied to near Misses: A Review of Industry-Wide near Miss Reporting Systems. Theor. Issues Ergon. Sci. 2018 , 19 , 712–737. [ Google Scholar ] [ CrossRef ]
- Gnoni, M.G.; Tornese, F.; Guglielmi, A.; Pellicci, M.; Campo, G.; De Merich, D. Near Miss Management Systems in the Industrial Sector: A Literature Review. Saf. Sci. 2022 , 150 , 105704. [ Google Scholar ] [ CrossRef ]
- Bird, F. Management Guide to Loss Control ; Loss Control Publications: Houston, TX, USA, 1975. [ Google Scholar ]
- Zimmermann. Bauer International Norms and Identity ; Zimmermann: Sydney, NSW, Australia, 2006; pp. 5–21. [ Google Scholar ]
- Arslan, M.; Cruz, C.; Ginhac, D. Semantic Trajectory Insights for Worker Safety in Dynamic Environments. Autom. Constr. 2019 , 106 , 102854. [ Google Scholar ] [ CrossRef ]
- Arslan, M.; Cruz, C.; Ginhac, D. Visualizing Intrusions in Dynamic Building Environments for Worker Safety. Saf. Sci. 2019 , 120 , 428–446. [ Google Scholar ] [ CrossRef ]
- Zhou, C.; Chen, R.; Jiang, S.; Zhou, Y.; Ding, L.; Skibniewski, M.J.; Lin, X. Human Dynamics in Near-Miss Accidents Resulting from Unsafe Behavior of Construction Workers. Phys. A Stat. Mech. Its Appl. 2019 , 530 , 121495. [ Google Scholar ] [ CrossRef ]
- Chen, F.; Wang, C.; Wang, J.; Zhi, Y.; Wang, Z. Risk Assessment of Chemical Process Considering Dynamic Probability of near Misses Based on Bayesian Theory and Event Tree Analysis. J. Loss Prev. Process Ind. 2020 , 68 , 104280. [ Google Scholar ] [ CrossRef ]
- Wright, L.; Van Der Schaaf, T. Accident versus near Miss Causation: A Critical Review of the Literature, an Empirical Test in the UK Railway Domain, and Their Implications for Other Sectors. J. Hazard. Mater. 2004 , 111 , 105–110. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saleh, J.H.; Saltmarsh, E.A.; Favar, F.M.; Loı¨c Brevault, L. Accident Precursors, near Misses, and Warning Signs: Critical Review and Formal Definitions within the Framework of Discrete Event Systems. Reliab. Eng. Syst. Saf. 2013 , 114 , 148–154. [ Google Scholar ] [ CrossRef ]
- Fred, A. Manuele Reviewing Heinrich. Am. Soc. Saf. Prof. 2011 , 56 , 52–61. [ Google Scholar ]
- Love, P.E.D.; Tenekedjiev, K. Understanding Near-Miss Count Data on Construction Sites Using Greedy D-Vine Copula Marginal Regression: A Comment. Reliab. Eng. Syst. Saf. 2022 , 217 , 108021. [ Google Scholar ] [ CrossRef ]
- Jan van Eck, N.; Waltman, L. VOSviewer Manual ; Universiteit Leiden: Leiden, The Netherlands, 2015. [ Google Scholar ]
- Scopus. Content Coverage Guide ; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–24. [ Google Scholar ]
- Lukic, D.; Littlejohn, A.; Margaryan, A. A Framework for Learning from Incidents in the Workplace. Saf. Sci. 2012 , 50 , 950–957. [ Google Scholar ] [ CrossRef ]
- Teizer, J.; Cheng, T. Proximity Hazard Indicator for Workers-on-Foot near Miss Interactions with Construction Equipment and Geo-Referenced Hazard Area. Autom. Constr. 2015 , 60 , 58–73. [ Google Scholar ] [ CrossRef ]
- Zong, L.; Fu, G. A Study on Designing No-Penalty Reporting System about Enterprise Staff’s near Miss. Adv. Mater. Res. 2011 , 255–260 , 3846–3851. [ Google Scholar ] [ CrossRef ]
- Golovina, O.; Teizer, J.; Pradhananga, N. Heat Map Generation for Predictive Safety Planning: Preventing Struck-by and near Miss Interactions between Workers-on-Foot and Construction Equipment. Autom. Constr. 2016 , 71 , 99–115. [ Google Scholar ] [ CrossRef ]
- Zou, P.X.W.; Lun, P.; Cipolla, D.; Mohamed, S. Cloud-Based Safety Information and Communication System in Infrastructure Construction. Saf. Sci. 2017 , 98 , 50–69. [ Google Scholar ] [ CrossRef ]
- Hinze, J.; Godfrey, R. An Evaluation of Safety Performance Measures for Construction Projects. J. Constr. Res. 2011 , 4 , 5–15. [ Google Scholar ] [ CrossRef ]
- Construction Inspection Software|IAuditor by SafetyCulture. Available online: https://safetyculture.com/construction/ (accessed on 25 August 2023).
- Incident Reporting Made Easy|Safety Compliance|Mobile EHS Solutions. Available online: https://www.safety-reports.com/lp/safety/incident/ (accessed on 25 August 2023).
- Wu, F.; Wu, T.; Yuce, M.R. An Internet-of-Things (IoT) Network System for Connected Safety and Health Monitoring Applications. Sensors 2019 , 19 , 21. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fang, W.; Luo, H.; Xu, S.; Love, P.E.D.; Lu, Z.; Ye, C. Automated Text Classification of Near-Misses from Safety Reports: An Improved Deep Learning Approach. Adv. Eng. Inform. 2020 , 44 , 101060. [ Google Scholar ] [ CrossRef ]
- Gatti, U.C.; Lin, K.-Y.; Caldera, C.; Chiang, R. Exploring the Relationship between Chronic Sleep Deprivation and Safety on Construction Sites. In Proceedings of the Construction Research Congress 2014: Construction in a Global Network, Atlanta, GA, USA, 19–24 May 2014; pp. 1772–1781. [ Google Scholar ] [ CrossRef ]
- Hon, C.K.H.; Chan, A.P.C.; Yam, M.C.H. Relationships between Safety Climate and Safety Performance of Building Repair, Maintenance, Minor Alteration, and Addition (RMAA) Works. Saf. Sci. 2014 , 65 , 10–19. [ Google Scholar ] [ CrossRef ]
- Oni, O.; Olanrewaju, A.; Cheen, K.S. Accidents at construction sites and near-misses: A constant problem. Int. Struct. Eng. Constr. 2022 , 9 , 2022. [ Google Scholar ] [ CrossRef ]
- Wu, W.; Yang, H.; Chew, D.A.S.; Yang, S.-H.; Gibb, A.G.F.; Li, Q. Towards an Autonomous Real-Time Tracking System of near-Miss Accidents on Construction Sites. Autom. Constr. 2010 , 19 , 134–141. [ Google Scholar ] [ CrossRef ]
- Aria, S.S.; Yang, K.; Ahn, C.R.; Vuran, M.C. Near-Miss Accident Detection for Ironworkers Using Inertial Measurement Unit Sensors. In Proceedings of the International Symposium on Automation and Robotics in Construction, ISARC 2014, Sydney, Australia, 9–11 July 2014; Volume 31, pp. 854–859. [ Google Scholar ] [ CrossRef ]
- Hasanzadeh, S.; Garza, J.M. de la Productivity-Safety Model: Debunking the Myth of the Productivity-Safety Divide through a Mixed-Reality Residential Roofing Task. J. Constr. Eng. Manag. 2020 , 146 , 04020124. [ Google Scholar ] [ CrossRef ]
- Teizer, J. Magnetic Field Proximity Detection and Alert Technology for Safe Heavy Construction Equipment Operation. In Proceedings of the 32nd International Symposium on Automation and Robotics in Construction, Oulu, Finland, 15–18 June 2015. [ Google Scholar ] [ CrossRef ]
- Mohajeri, M.; Ardeshir, A.; Banki, M.T.; Malekitabar, H. Discovering Causality Patterns of Unsafe Behavior Leading to Fall Hazards on Construction Sites. Int. J. Constr. Manag. 2022 , 22 , 3034–3044. [ Google Scholar ] [ CrossRef ]
- Kisaezehra; Farooq, M.U.; Bhutto, M.A.; Kazi, A.K. Real-Time Safety Helmet Detection Using Yolov5 at Construction Sites. Intell. Autom. Soft Comput. 2023 , 36 , 911–927. [ Google Scholar ] [ CrossRef ]
- Li, C.; Ding, L. Falling Objects Detection for near Miss Incidents Identification on Construction Site. In Proceedings of the ASCE International Conference on Computing in Civil Engineering, Atlanta, GA, USA, 17–19 June 2019; pp. 138–145. [ Google Scholar ] [ CrossRef ]
- Jeelani, I.; Ramshankar, H.; Han, K.; Albert, A.; Asadi, K. Real-Time Hazard Proximity Detection—Localization of Workers Using Visual Data. In Proceedings of the ASCE International Conference on Computing in Civil Engineering, Atlanta, GA, USA, 17–19 June 2019; pp. 281–289. [ Google Scholar ] [ CrossRef ]
- Lim, T.-K.; Park, S.-M.; Lee, H.-C.; Lee, D.-E. Artificial Neural Network–Based Slip-Trip Classifier Using Smart Sensor for Construction Workplace. J. Constr. Eng. Manag. 2015 , 142 , 04015065. [ Google Scholar ] [ CrossRef ]
- Yang, K.; Jebelli, H.; Ahn, C.R.; Vuran, M.C. Threshold-Based Approach to Detect Near-Miss Falls of Iron Workers Using Inertial Measurement Units. In Proceedings of the 2015 International Workshop on Computing in Civil Engineering, Austin, TX, USA, 21–23 June 2015; 2015; 2015, pp. 148–155. [ Google Scholar ] [ CrossRef ]
- Yang, K.; Ahn, C.R.; Vuran, M.C.; Aria, S.S. Semi-Supervised near-Miss Fall Detection for Ironworkers with a Wearable Inertial Measurement Unit. Autom. Constr. 2016 , 68 , 194–202. [ Google Scholar ] [ CrossRef ]
- Raviv, G.; Shapira, A.; Fishbain, B. AHP-Based Analysis of the Risk Potential of Safety Incidents: Case Study of Cranes in the Construction Industry. Saf. Sci. 2017 , 91 , 298–309. [ Google Scholar ] [ CrossRef ]
- Saurin, T.A.; Formoso, C.T.; Reck, R.; Beck da Silva Etges, B.M.; Ribeiro JL, D. Findings from the Analysis of Incident-Reporting Systems of Construction Companies. J. Constr. Eng. Manag. 2015 , 141 , 05015007. [ Google Scholar ] [ CrossRef ]
- Williams, E.; Sherratt, F.; Norton, E. Exploring the Value in near Miss Reporting for Construction Safety. In Proceedings of the 37th Annual Conference, Virtual Event, 6–10 December 2021; pp. 319–328. [ Google Scholar ]
- Baker, H.; Smith, S.; Masterton, G.; Hewlett, B. Data-Led Learning: Using Natural Language Processing (NLP) and Machine Learning to Learn from Construction Site Safety Failures. In Proceedings of the 36th Annual ARCOM Conference, Online, 7–8 September 2020; pp. 356–365. [ Google Scholar ]
- Jin, R.; Wang, F.; Liu, D. Dynamic Probabilistic Analysis of Accidents in Construction Projects by Combining Precursor Data and Expert Judgments. Adv. Eng. Inform. 2020 , 44 , 101062. [ Google Scholar ] [ CrossRef ]
- Zhou, Z.; Li, C.; Mi, C.; Qian, L. Exploring the Potential Use of Near-Miss Information to Improve Construction Safety Performance. Sustainability 2019 , 11 , 1264. [ Google Scholar ] [ CrossRef ]
- Boateng, E.B.; Pillay, M.; Davis, P. Predicting the Level of Safety Performance Using an Artificial Neural Network. Adv. Intell. Syst. Comput. 2019 , 876 , 705–710. [ Google Scholar ] [ CrossRef ]
- Zhang, M.; Cao, T.; Zhao, X. Using Smartphones to Detect and Identify Construction Workers’ Near-Miss Falls Based on ANN. J. Constr. Eng. Manag. 2018 , 145 , 04018120. [ Google Scholar ] [ CrossRef ]
- Gadekar, H.; Bugalia, N. Automatic Classification of Construction Safety Reports Using Semi-Supervised YAKE-Guided LDA Approach. Adv. Eng. Inform. 2023 , 56 , 101929. [ Google Scholar ] [ CrossRef ]
- Zhu, Y.; Liao, H.; Huang, D. Using Text Mining and Multilevel Association Rules to Process and Analyze Incident Reports in China. Accid. Anal. Prev. 2023 , 191 , 107224. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, M.; Lin, Q.; Jin, H. Research on Near-Miss Incidents Monitoring and Early Warning System for Building Construction Sites Based on Blockchain Technology. J. Constr. Eng. Manag. 2023 , 149 , 04023124. [ Google Scholar ] [ CrossRef ]
- Chung, W.W.S.; Tariq, S.; Mohandes, S.R.; Zayed, T. IoT-Based Application for Construction Site Safety Monitoring. Int. J. Constr. Manag. 2020 , 23 , 58–74. [ Google Scholar ] [ CrossRef ]
- Liu, X.; Xu, F.; Zhang, Z.; Sun, K. Fall-Portent Detection for Construction Sites Based on Computer Vision and Machine Learning. Eng. Constr. Archit. Manag. 2023; ahead-of-print . [ Google Scholar ] [ CrossRef ]
- Abbasi, H.; Guerrieri, A.; Lee, J.; Yang, K. Mobile Device-Based Struck-By Hazard Recognition in Construction Using a High-Frequency Sound. Sensors 2022 , 22 , 3482. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, F.; Li, H.; Dong, C. Understanding Near-Miss Count Data on Construction Sites Using Greedy D-Vine Copula Marginal Regression. Reliab. Eng. Syst. Saf. 2021 , 213 , 107687. [ Google Scholar ] [ CrossRef ]
- Bugalia, N.; Tarani, V.; Student, G.; Kedia, J.; Gadekar, H. Machine Learning-Based Automated Classification Of Worker-Reported Safety Reports In Construction. J. Inf. Technol. Constr. 2022 , 27 , 926–950. [ Google Scholar ] [ CrossRef ]
- Chen, S.; Xi, J.; Chen, Y.; Zhao, J. Association Mining of Near Misses in Hydropower Engineering Construction Based on Convolutional Neural Network Text Classification. Comput. Intell. Neurosci. 2022 , 2022 , 4851615. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tang, S.; Golparvar-Fard, M.; Naphade, M.; Gopalakrishna, M.M. Video-Based Activity Forecasting for Construction Safety Monitoring Use Cases. In Proceedings of the ASCE International Conference on Computing in Civil Engineering, Atlanta, GA, USA, 17–19 June 2019; pp. 204–210. [ Google Scholar ] [ CrossRef ]
- Rashid, K.M.; Behzadan, A.H. Risk Behavior-Based Trajectory Prediction for Construction Site Safety Monitoring. J. Constr. Eng. Manag. 2018 , 144 , 04017106. [ Google Scholar ] [ CrossRef ]
- Shen, X.; Marks, E. Near-Miss Information Visualization Tool in BIM for Construction Safety. J. Constr. Eng. Manag. 2016 , 142 , 04015100. [ Google Scholar ] [ CrossRef ]
- Erusta, N.E.; Sertyesilisik, B. An Investigation into Improving Occupational Health and Safety Performance of Construction Projects through Usage of BIM for Lean Management. In Communications in Computer and Information Science (CCIS) ; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1188, pp. 91–100. [ Google Scholar ] [ CrossRef ]
- Coffland, M.M.; Kim, A.; Sadatsafavi, H.; Uber, M.M. Improved Data Storage for Better Safety Analysis and Decision Making in Large Construction Management Firms. Available online: https://www.researchgate.net/publication/320474383_Improved_Data_Storage_for_Better_Safety_Analysis_and_Decision_Making_in_Large_Construction_Management_Firms (accessed on 12 June 2024).
- Zhou, Z.; Li, Q.; Wu, W. Developing a Versatile Subway Construction Incident Database for Safety Management. J. Constr. Eng. Manag. 2011 , 138 , 1169–1180. [ Google Scholar ] [ CrossRef ]
- Wu, W.; Yang, H.; Li, Q.; Chew, D. An Integrated Information Management Model for Proactive Prevention of Struck-by-Falling-Object Accidents on Construction Sites. Autom. Constr. 2013 , 34 , 67–74. [ Google Scholar ] [ CrossRef ]
- Hoła, B. Identification and Evaluation of Processes in a Construction Enterprise. Arch. Civ. Mech. Eng. 2015 , 15 , 419–426. [ Google Scholar ] [ CrossRef ]
- Zhou, C.; Ding, L.; Skibniewski, M.J.; Luo, H.; Jiang, S. Characterizing Time Series of Near-Miss Accidents in Metro Construction via Complex Network Theory. Saf. Sci. 2017 , 98 , 145–158. [ Google Scholar ] [ CrossRef ]
- Woźniak, Z.; Hoła, B. Time Series Analysis of Hazardous Events Based on Data Recorded in a Polish Construction Company. Arch. Civ. Eng. 2024; in process . [ Google Scholar ]
- Drozd, W. Characteristics of Construction Site in Terms of Occupational Safety. J. Civ. Eng. Environ. Archit. 2016 , 63 , 165–172. [ Google Scholar ]
- Meliá, J.L.; Mearns, K.; Silva, S.A.; Lima, M.L. Safety Climate Responses and the Perceived Risk of Accidents in the Construction Industry. Saf. Sci. 2008 , 46 , 949–958. [ Google Scholar ] [ CrossRef ]
- Bugalia, N.; Maemura, Y.; Ozawa, K. A System Dynamics Model for Near-Miss Reporting in Complex Systems. Saf. Sci. 2021 , 142 , 105368. [ Google Scholar ] [ CrossRef ]
- Gyi, D.E.; Gibb, A.G.F.; Haslam, R.A. The Quality of Accident and Health Data in the Construction Industry: Interviews with Senior Managers. Constr. Manag. Econ. 1999 , 17 , 197–204. [ Google Scholar ] [ CrossRef ]
- Menzies, J. Structural Safety: Learning and Warnings. Struct. Eng. 2002 , 80 , 15–16. [ Google Scholar ]
- Fullerton, C.E.; Allread, B.S.; Teizer, J. Pro-Active-Real-Time Personnel Warning System. In Proceedings of the Construction Research Congress 2009: Building a Sustainable Future, Seattle, WA, USA, 5–7 April 2009; pp. 31–40. [ Google Scholar ] [ CrossRef ]
- Marks, E.D.; Wetherford, J.E.; Teizer, J.; Yabuki, N. Potential of Leading Indicator Data Collection and Analysis for Proximity Detection and Alert Technology in Construction. In Proceedings of the 30th ISARC—International Symposium on Automation and Robotics in Construction Conference, Montreal, QC, Canada, 11–15 August 2013; pp. 1029–1036. [ Google Scholar ] [ CrossRef ]
- Martin, H.; Lewis, T.M. Pinpointing Safety Leadership Factors for Safe Construction Sites in Trinidad and Tobago. J. Constr. Eng. Manag. 2014 , 140 , 04013046. [ Google Scholar ] [ CrossRef ]
- Hobson, P.; Emery, D.; Brown, L.; Bashford, R.; Gill, J. People–Plant Interface Training: Targeting an Industry Fatal Risk. Proc. Inst. Civ. Eng. Civ. Eng. 2014 , 167 , 138–144. [ Google Scholar ] [ CrossRef ]
- Marks, E.; Mckay, B.; Awolusi, I. Using near Misses to Enhance Safety Performance in Construction. In Proceedings of the ASSE Professional Development Conference and Exposition, Dallas, TX, USA, 7–10 June 2015. [ Google Scholar ]
- Popp, J.D.; Scarborough, M.S. Investigations of near Miss Incidents—New Facility Construction and Commissioning Activities. IEEE Trans. Ind. Appl. 2016 , 53 , 615–621. [ Google Scholar ] [ CrossRef ]
- Nickel, P.; Lungfiel, A.; Trabold, R.J. Reconstruction of near Misses and Accidents for Analyses from Virtual Reality Usability Study. In Lecture Notes in Computer Science ; Springer: Berlin/Heidelberg, Germany, 2017; Volume 10700, pp. 182–191. [ Google Scholar ] [ CrossRef ]
- Gambatese, J.A.; Pestana, C.; Lee, H.W. Alignment between Lean Principles and Practices and Worker Safety Behavior. J. Constr. Eng. Manag. 2017 , 143 , 04016083. [ Google Scholar ] [ CrossRef ]
- Van Voorhis, S.; Korman, R. Reading Signs of Trouble. Eng. News-Rec. 2017 , 278 , 14–17. [ Google Scholar ]
- Doan, D.R. Investigation of a near-miss shock incident. IEEE Trans. Ind. Appl. 2016 , 52 , 560–561. [ Google Scholar ] [ CrossRef ]
- Oswald, D.; Sherratt, F.; Smith, S. Problems with safety observation reporting: A construction industry case study. Saf. Sci. 2018 , 107 , 35–45. [ Google Scholar ] [ CrossRef ]
- Raviv, G.; Shapira, A. Systematic approach to crane-related near-miss analysis in the construction industry. Int. J. Constr. Manag. 2018 , 18 , 310–320. [ Google Scholar ] [ CrossRef ]
- Whiteoak, J.; Appleby, J. Mate, that was bloody close! A case history of a nearmiss program in the Australian construction industry. J. Health Saf. Environ. 2019 , 35 , 31–43. [ Google Scholar ]
- Duryan, M.; Smyth, H.; Roberts, A.; Rowlinson, S.; Sherratt, F. Knowledge transfer for occupational health and safety: Cultivating health and safety learning culture in construction firms. Accid. Anal. Prev. 2020 , 139 , 105496. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Shaikh, A.Y.; Osei-Kyei, R.; Hardie, M. A critical analysis of safety performance indicators in construction. Int. J. Build. Pathol. Adapt. 2020 , 39 , 547–580. [ Google Scholar ] [ CrossRef ]
- Martin, H.; Mohan, N.; Ellis, L.; Dunne, S. Exploring the Role of PPE Knowledge, Attitude, and Correct Practices in Safety Outcomes on Construction Sites. J. Archit. Eng. 2021 , 27 , 05021011. [ Google Scholar ] [ CrossRef ]
- Qin, Z.; Wu, S. A simulation model of engineering construction near-miss event disclosure strategy based on evolutionary game theory. In Proceedings of the 2021 4th International Conference on Information Systems and Computer Aided Education, Dalian, China, 24–26 September 2021; pp. 2572–2577. [ Google Scholar ] [ CrossRef ]
- Alamoudi, M. The Integration of NOSACQ-50 with Importance-Performance Analysis Technique to Evaluate and Analyze Safety Climate Dimensions in the Construction Sector in Saudi Arabia. Buildings 2022 , 12 , 1855. [ Google Scholar ] [ CrossRef ]
- Herrmann, A.W. Development of CROSS in the United States. In Proceedings of the Forensic Engineering 2022: Elevating Forensic Engineering—Selected Papers from the 9th Congress on Forensic Engineering, Denver, Colorado, 4–7 November 2022; Volume 2, pp. 40–43. [ Google Scholar ] [ CrossRef ]
- Al Shaaili, M.; Al Alawi, M.; Ekyalimpa, R.; Al Mawli, B.; Al-Mamun, A.; Al Shahri, M. Near-miss accidents data analysis and knowledge dissemination in water construction projects in Oman. Heliyon 2023 , 9 , e21607. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Agnusdei, G.P.; Gnoni, M.G.; Tornese, F.; De Merich, D.; Guglielmi, A.; Pellicci, M. Application of Near-Miss Management Systems: An Exploratory Field Analysis in the Italian Industrial Sector. Safety 2023 , 9 , 47. [ Google Scholar ] [ CrossRef ]
- Duan, P.; Zhou, J. A science mapping approach-based review of near-miss research in construction. Eng. Constr. Archit. Manag. 2023 , 30 , 2582–2601. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| No. | Name of Institution/Organization | Definition |
|---|---|---|
| 1 | Occupational Safety and Health Administration (OSHA) [ ] | “A near-miss is a potential hazard or incident in which no property was damaged and no personal injury was sustained, but where, given a slight shift in time or position, damage or injury easily could have occurred. Near misses also may be referred to as close calls, near accidents, or injury-free events.” |
| 2 | International Labour Organization (ILO) [ ] | “An event, not necessarily defined under national laws and regulations, that could have caused harm to persons at work or to the public, e.g., a brick that falls off scaffolding but does not hit anyone” |
| 3 | American National Safety Council (NSC) [ ] | “A Near Miss is an unplanned event that did not result in injury, illness, or damage—but had the potential to do so” |
| 4 | PN-ISO 45001:2018-06 [ ] | A near-miss incident is described as an event that does not result in injury or health issues. |
| 5 | PN-N-18001:2004 [ ] | A near-miss incident is an accident event without injury. |
| 6 | World Health Organization (WHO) [ ] | Near misses have been defined as a serious error that has the potential to cause harm but are not due to chance or interception. |
| 7 | International Atomic Energy Agency (IAEA) [ ] | Near misses have been defined as potentially significant events that could have consequences but did not due to the conditions at the time. |
| No. | Journal | Number of Publications |
|---|---|---|
| 1 | Safety Science | 10 |
| 2 | Journal of Construction Engineering and Management | 8 |
| 3 | Automation in Construction | 5 |
| 4 | Advanced Engineering Informatics | 3 |
| 5 | Construction Research Congress 2014 Construction in a Global Network Proceedings of the 2014 Construction Research Congress | 3 |
| 6 | International Journal of Construction Management | 3 |
| 7 | Accident Analysis and Prevention | 2 |
| 8 | Computing in Civil Engineering 2019 Data Sensing and Analytics Selected Papers From The ASCE International Conference | 2 |
| 9 | Engineering Construction and Architectural Management | 2 |
| 10 | Heliyon | 2 |
| Cluster Number | Colour | Basic Keywords |
|---|---|---|
| 1 | blue | construction, construction sites, decision making, machine learning, near misses, neural networks, project management, safety, workers |
| 2 | green | building industry, construction industry, construction projects, construction work, human, near miss, near misses, occupational accident, occupational safety, safety, management, safety performance |
| 3 | red | accident prevention, construction equipment, construction, safety, construction workers, hazards, human resource management, leading indicators, machinery, occupational risks, risk management, safety engineering |
| 4 | yellow | accidents, risk assessment, civil engineering, near miss, surveys |
| Number of Question | Question | References |
|---|---|---|
| Q | Are near misses in the construction industry studied scientifically? | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Q | What methods have been used to obtain information on near misses and systems for recording incidents in construction companies? | [ , , , , , , , , , , , , , , , , , , , , ] |
| Q | What methods have been used to analyse the information and figures that have been obtained? | [ , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] |
| Q | What are the key aspects of near misses in the construction industry that have been of interest to the researchers? | [ , , , , , , , , , , , , ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Woźniak, Z.; Hoła, B. Analysing Near-Miss Incidents in Construction: A Systematic Literature Review. Appl. Sci. 2024 , 14 , 7260. https://doi.org/10.3390/app14167260
Woźniak Z, Hoła B. Analysing Near-Miss Incidents in Construction: A Systematic Literature Review. Applied Sciences . 2024; 14(16):7260. https://doi.org/10.3390/app14167260
Woźniak, Zuzanna, and Bożena Hoła. 2024. "Analysing Near-Miss Incidents in Construction: A Systematic Literature Review" Applied Sciences 14, no. 16: 7260. https://doi.org/10.3390/app14167260
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
COMMENTS
The review also gives a brief idea about different strategies employed for xylanase purification and characterization, biotechnological approach for enhanced xylanase production with desired properties which are further used for different industrial applications.
Microbial xylanase: A review July 2016 Authors: Aglaia Popa University of Agricultural Sciences and Veterinary Medicine of Bucharest Florentina Israel-Roming
The present review gives an insight of into microbial xylanases, its source, production, purification and biotechnological applications. This review also provides some important aspects on advancement of xylanase production through recombinant DNA technology, mutation and cloning.
Optimization, purification, and characterization of xylanase production by a newly isolated Trichoderma harzianum strain by a two-step statistical experimental design strategy Priyashini Dhaver ...
A detailed overview of xylanases: an emerging biomolecule for current and future prospective December 2019 Bioresources and Bioprocessing 6 (1) DOI: 10.1186/s40643-019-0276-2 License CC BY Authors ...
Therefore, the present review provides a synopsis of present scenario of xylanase as a major participant in biofuel production, along with its various applications and factors affecting xylanase production. This present review provides evidence that narrates its presence in the production of biofuels such as bioethanol and biobutanol.
In this review, a comprehensive discussion exclusively on bacterial xylanases; their gene organization; different factors and conditions affecting enzyme yield and activity; and their commercial application have been deliberated in the light of recent ...
This review gives an overview of the recent advancements made in regards to xylanase enzyme being used commercially with main focus on its role in fruit juice clarification.
This review will focus on complex xylan structure and the microbial enzyme complex involved in its complete breakdown, studies on xylanase regulation and production and their potential industrial applications, with special reference to biobleaching.
This review has focused on the microbial enzyme complex involved in the complete breakdown of xylan and the studies on xylanase regulation and their potential industrial applications with special reference to pulp biobleaching, which is directly related to increasing pulp brightness and reduction in environmental pollution.
Various strategies such as process optimization using statistical analysis, overexpression of xylanase encoding gene, etc., have been applied to enhance xylanase production to fulfil its requirement in different industries.
Besides this, review has focused on the microbial enzyme complex involved in the complete breakdown of xylan and the studies on xylanase regulation and their potential industrial applications with special reference to pulp biobleaching, which is directly related to increasing pulp brightness and reduction in environmental pollution.
Xylanase is a hydrolytic enzyme capable of hydrolyzing xylan, a major constituent of hemicellulose and the second most abundant linear polysaccharide molecule consisting of monosaccharides such as galactose, mannose, and arabinose (Bhardwaj et al., 2019 ). Most of the microorganisms serve as readily available sources for xylanase.
Therefore, as shown in Fig. 3, in view of the knowledge and literature reviewed here, we propose perspectives for applications of actinobacterial xylanase in the food industry, although more studies in the area should be carried out.
A review on xylanase production by the fermentation of xylan: classification, characterization, and applications. In: Sustainable degradation of lignocellulosic biomass - techniques, applications and commercialization.
In conclusion, dietary supplementation of xylanase 45,000 U/kg significantly improved laying hen performance and digestibility. Furthermore, microbiome data suggest that xylanase modulates the laying hen bacterial population beneficially, thus potentially exerting a prebiotic effect.
Considering the future prospects of xylanases in biotechnological applications, the goal of this review chapter is to present an overview of xylanase production via fermentation and to describe some of the characteristics of these enzymes and their primary substrate, xylan.
Information is minimal and while we have demonstrated xylanase activity by these enzymes [79], little literature has been published in direct relation to these two xylanases.
ABSTRACT Xylanase (EC 3.2.1.8, endo-(1-4)-β-xylan 4-xylanohydrolase, endo-1,4-xylanase, endo-1,4- β-xylanase, β-1,4-xylanase, endo-1,4-β-D-xylanase, 1,4- β-xylan xylanohydrolase, β-xylanase, β-1,4-xylan xylanohydrolase, β-D-xylanase) is the name given to a class of enzymes which degrade the linear polysaccharide beta-1,4-xylan into xylose, thus breaking down hemicellulose, one of the ...
ABSTRACT Endo-1, 4-β-xylanase (Endo-β-1, 4-xylan, xylanohydrolase; EC. 3.2.1.8, commonly called xylanase) is an industrially important enzyme which degrades xylan randomly and produces xylooligosaccharides, xylobiose and xylose. It is mainly present in microbes and plants but not in animals. Xylanases from fungal and bacterial sources have been extensively studied and produced commercially ...
This review will focus on complex xylan structure and the microbial enzyme complex involved in its complete breakdown, studies on xylanase regulation and production and their potential industrial ...
Delayed diagnosis and treatment, due to a paucity of literature on optimal treatment strategies, can increase morbidity. This case report describes a 19-year-old male patient with aggressive thoracic hemangioma who presented with upper back pain and progressive weakness of the lower extremities.
The present literature review was based on the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) method, using a checklist, which is linked to a flow diagram of three phases: identification, screening and inclusion. PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses.
Traumatic pseudoaneurysm of the occipital artery: case report and review of the literature. Ear Nose Throat J 2008 Nov; 87: E7-12. Google Scholar. 9. Changa AR, Meleis AM, Bassani L. Delayed occipital artery pseudoaneurysm following blunt force trauma. World Neurosurg 2016 May; 89: 732.e1-732.e6. Crossref. PubMed. Google Scholar. 10.
A thermoalkalophilic new species of Bacillus, similar to Bacillus arseniciselenatis DSM 15340, produced extracellular xylanase under solid state fermentation when wheat bran is used as carbon source. The extracellular xylanase was isolated by ammonium ...
The review by Woo et al. reports on factors influencing behaviour in the care of the diabetic foot, wich are common in diabetic patients and have a high risk of infection and amputation. To improve patient's knowledge and education on foot care, this commentary proposes the Barrows cards as an innovative user-friendly educational method.
The Vicar of Christ wants you to read more fiction and poetry. Who are you to judge?
Our data were also compared to other experiences though an extensive literature review. We retrospectively analysed a single surgeon series of the last 10 years. From January 2014 to December 2023, 36 patients underwent urgent or emergency RS. The robotic devices used were Da Vinci Si (15 cases) and Xi (21 cases). 36 (4.3%) out of 834 robotic ...
The construction sector is notorious for its high rate of fatalities globally. Previous research has established that near-miss incidents act as precursors to accidents. This study aims to identify research gaps in the literature on near-miss events in construction and to define potential directions for future research. The Scopus database serves as the knowledge source for this study. To ...