What Are Clinical Trials?

Talk with your doctor about clinical trials when you discuss treatment options.
Clinical trials are research studies that test how well new medical approaches work in people.

What is the purpose of a clinical trial?
Clinical trials test new ways to find, prevent, and treat cancer. They also help doctors improve the quality of life for people with cancer by testing ways to manage the side effects of cancer and its treatment.
Why are clinical trials important?
Today, people are living longer lives thanks to results of past cancer clinical trials. When you take part in a clinical trial, you add to our knowledge about cancer and help improve cancer care for people in the future.
People join clinical trials for many reasons. People living with cancer often join trials because they want to help future patients. People with certain risk factors want to help doctors learn how to prevent cancer. Healthy volunteers want to help doctors learn how to find cancer early.
People with cancer and healthy volunteers join trials to play a role in cancer research and move science forward to help others.
What are the types of clinical trials?

"I feel that as an African American we do not participate in programs that could BE and sometimes ARE beneficial to us. I hope by my participation this will encourage others African American women to be more open." —Marsha Dukes, NCI clinical trial participant
There are several types of cancer clinical trials. Each type of trial is designed to answer different research questions and will help researchers learn things that can help people in the future.
Treatment Trials
Most cancer clinical trials are treatment studies that involve people who have cancer. These trials test new treatments or new ways of using existing treatments, including new:
- approaches to surgery or radiation therapy
- combinations of treatments
As researchers learn more about the genetic changes that lead to cancer, doctors are testing treatments that target these specific changes. So in some treatment trials, your tumor may be tested to see if treatments targeting specific genetic changes might work better than standard treatments.
Treatment trials are designed to answers questions such as:
- What is a safe dose of the treatment under study?
- How should the treatment be given?
- Does the treatment help people with cancer live longer than current treatment?
- Can the study treatment shrink tumors or slow their growth and spread?
- What are the treatment's side effects?
- Does the study treatment allow for a better quality of life with fewer side effects?
- Does the treatment help delay the return of the cancer?

Participate in Cancer Prevention Studies
Learn about cancer prevention studies and how to participate.
Prevention Trials
Prevention trials are studies that look at ways to prevent cancer.
In most prevention trials, the people who take part do not have cancer but are at high risk for developing it. Or they have had cancer and are at high risk for developing a new cancer.
There are two kinds of prevention trials, action studies and agent studies. In action studies, you are asked to do something, such as exercise or follow a special diet. In agent studies, you are asked to take something, such as a drug or vitamin. Learn about participating in prevention studies .
Researchers who conduct these studies want to know:
- How safe is the drug or activity?
- Does the new approach reduce the chance that someone will get cancer?

Cancer Screening Studies
Learn about joining a screening trial to help find cancer early.
Screening Trials
The goal of cancer screening trials is to test ways to find cancer before it causes symptoms, when it may be easier to treat.
An effective screening test will reduce the number of people who die from the cancer that is being screened for. Learn about joining a cancer screening study .
But screening tests can have harms, which include bleeding or other physical damage. Other possible harms include a result that shows you might have cancer when you don’t. When this happens, it may lead to unnecessary tests and procedures. On the other hand, the results may show no signs of cancer when you have it. And sometimes screening can find cancers that would not have harmed you during your lifetime.
Researchers who conduct cancer screening studies want to know:
- Does finding disease earlier, before people have any symptoms, save lives?
- Is one screening test better than another?
- Do the benefits of the screening test outweigh the harms?
Supportive Care/Palliative Care Trials
These trials look at ways to improve the quality of life of people with cancer, especially those who have side effects from cancer and its treatment.
They might test drugs, such as those that help with depression or nausea. Or they might test activities, such as attending support groups, exercising, or talking with a counselor.
Some of these trials test ways to help families and caregivers cope with their own needs, as well as those of the person with cancer.
- How does cancer and its treatment affect patients and their loved ones?
- What can improve the comfort and quality of life of people who have cancer?
Masks Strongly Recommended but Not Required in Maryland, Starting Immediately
Due to the downward trend in respiratory viruses in Maryland, masking is no longer required but remains strongly recommended in Johns Hopkins Medicine clinical locations in Maryland. Read more .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Making Decisions and Managing Your Treatment
- Understanding Your Options and Making Treatment Decisions
- How Treatment Is Planned and Scheduled
- When Treatment Should Start
- Cancer During Pregnancy
- Questions to Ask Your Doctor When You Have Cancer
- What Is Informed Consent?
- When Is Informed Consent Needed?
- Informed Consent for a Clinical Trial
- What Is an Advance Directive?
- Types of Advance Directives
- Frequently Asked Questions About Advance Directives
- Making Decisions for Your Advance Directive
- If Cancer Treatments Stop Working
- Getting Cancer Treatment at Home
- Intravenous (IV) Lines, Catheters, and Ports Used in Cancer Treatment
- Tubes, Catheters, and Drains Used in Cancer Treatment and Care
- Coping with Cancer After a Natural Disaster
- Personal Health Manager
Types and Phases of Clinical Trials
- Deciding Whether to Be Part of a Clinical Trial
- Protecting People in Clinical Trials
- Being in a Clinical Trial
- Finding a Clinical Trial
- Clinical Trials Videos
- Compassionate Drug Use
- Cancer Surveillance Programs in the United States
- Why do we need clinical trials?
Pre-clinical (or laboratory) studies
The investigational new drug (ind) application, phases of clinical trials, phase 0 clinical trials: exploring if and how a new drug may work, phase i clinical trials: is the treatment safe, phase ii clinical trials: does the treatment work, phase iii clinical trials: is it better than what’s already available, submission for fda approval: new drug application (nda), phase iv clinical trials: what else do we need to know.
Clinical trials are studies to test new drugs, already approved drugs, devices, or other forms of treatments. Many clinical trials look at new ways to detect, diagnose, or measure the extent of disease. Some even look at ways to prevent diseases from happening. Researchers still use human volunteers to test these methods, and the same rules apply.
Doctors use clinical trials to learn whether a new drug, treatment, or combination works and is safe to use for people. Clinical trials are important in developing new treatments for serious diseases like cancer. All new treatments must go through clinical trials before being approved by the Food and Drug Administration (FDA). Cancer clinical trials can take years to complete. It can take months, if not years, to see if a cancer treatment does what it is meant to do.
Why do we need clinical trials?
Clinical trials show us what works (and what doesn’t) in medicine and health care. They are the best way to learn what works in treating diseases like cancer. Clinical trials are designed to answer some important questions:
- Does the new treatment work in people? If it does, doctors will also look at how well it works. Is it better than treatment now being used? If it’s not better, is it as good and cause fewer side effects? Or does it work in some people who aren’t helped by current treatments?
- Is the new treatment safe? No treatment or procedure – even one already in common use – is without risk. But do the benefits of the new treatment outweigh the risks?
- Is this treatment better than the standard treatment given for this disease? Clinical trials help show if a new drug or treatment, or a new treatment combination, works better than what is now used.
Answering these questions, while giving as few people as possible an unknown treatment, often requires several clinical trials in different “phases.” Each phase is designed to answer certain questions while keeping the people taking part as safe as possible. Results from these phases show if the new drug or treatment is reasonably safe and effective.
Clinical trials are done only after pre-clinical findings suggest that the new drug or treatment is likely to be safe and will work in people.
Pre-clinical studies, also called laboratory studies, include:
- Cell studies: These are often the first tests done on a new treatment. To see if it might work, researchers look for effects of the new treatment on cancer cells that are grown in a lab dish or a test tube. These studies may be done on human cancer cells or animal cancer cells.
- Animal studies: Treatments that look promising in cell studies are tested next on cancers in live animals. This gives researchers an idea of how safe the new treatment is in a living creature.
Pre-clinical studies give a lot of useful information, but not all that is needed. Humans and mice can be very different in the way they absorb, process, and get rid of drugs or treatments. A treatment that works against cancer in a mouse might or might not work in people. There could also be side effects and other problems that didn’t show up when the treatment was used in mice but could show up in people.
If the pre-clinical studies are completed and the treatment still seems promising, the US Food and Drug Administration (FDA) must give permission before the treatment can be tested people.
Before a clinical trial can be started, the research must be approved. An investigational new drug or IND application or request must be filed with the FDA when researchers want to study a drug in humans. The IND application must contain certain information, such as:
- Results from studies so that the FDA can decide whether the treatment is safe for testing in people.
- How the drug is made, who makes it, what’s in it, how stable it is, and more.
- Detailed outlines for the planned clinical studies, called study protocols, are reviewed to see if people might be exposed to needless risks.
- Details about the clinical trial team to see if they have the knowledge and skill to run clinical trials.
The research sponsor must commit to getting informed consent from everyone on the clinical trial. They must also commit to having the study reviewed by an institutional review board (IRB) and following all the rules required for studying investigational new drugs
Clinical trials are usually conducted in phases that build on one another. Each phase is designed to answer certain questions. Knowing the phase of the clinical trial is important because it can give you some idea about how much is known about the treatment being studied. There are benefits and risks to taking part in each phase of a clinical trial.
Although there are clinical trials for devices as well as other diseases and treatments, drugs for cancer patients are used in the examples of clinical trial phases described here.
Even though phase 0 studies are done in humans, this type of study isn’t like the other phases of clinical trials. The purpose of this phase is to help speed up and streamline the drug approval process. Phase 0 studies may help researchers find out if the drugs do what they’re expected to do. This may help save time and money that would have been spent on later phase trials.
Phase 0 studies use only a few small doses of a new drug in a few people. They might test whether the drug reaches the tumor, how the drug acts in the human body, and how cancer cells in the human body respond to the drug. People in these studies might need extra tests such as biopsies, scans, and blood samples as part of the process.
Unlike other phases of clinical trials, there’s almost no chance the people in phase 0 trials will benefit. The benefit will be for other people in the future. And because drug doses are low, there’s also less risk to those in the trial.
Phase 0 studies aren’t widely used, and there are some drugs for which they wouldn’t be helpful. Phase 0 studies are very small, often with fewer than 15 people, and the drug is given only for a short time. They’re not a required part of testing a new drug.
Phase I studies of a new drug are usually the first that involve people. Phase I studies are done to find the highest dose of the new treatment that can be given safely without causing severe side effects. Although the treatment has been tested in lab and animal studies, the side effects in people can’t be known for sure. These studies also help to decide on the best way to give the new treatment.
Key points of phase I clinical trials
- The first few people in the study get a very low dose of the treatment and are watched very closely. If there are only minor side effects, the next few participants get a higher dose. This process continues until doctors find a dose that’s most likely to work while having an acceptable level of side effects.
- Phase I trials are also looking at what the drug does to the body and what the body does with the drug.
- Safety is the main concern. The research team keeps a close eye on the people and watches for any severe side effects. Because of the small numbers of people in phase I studies, rare side effects may not be seen until later phases of trials when more people receive the treatment.
- While some people may benefit from being on one, disease response is not the main purpose of a phase I trial,
- Placebos (inactive treatments) are not used in phase I trials.
- Phase I trials usually include a small number of people (up to a few dozen).
- Phase I trials most often include people with different types of cancer.
- These studies are usually done in major cancer centers.
Phase I trials carry the most potential risk. But phase I studies do help some patients. For those with life-threatening illnesses, weighing the potential risks and benefits carefully is key. Sometimes people choose to join phase I trials when all other treatment options have already been tried.
If a new treatment is found to be safe in phase I clinical trials, a phase II clinical trial is done to see if it works in certain types of cancer. The benefit the doctors look for depends on the goal of the treatment. It may mean the cancer shrinks or disappears. Or it might mean there’s a long period of time where the cancer doesn’t get any bigger, or there’s a longer time before the cancer comes back. In some studies, the benefit may be an improved quality of life. Many clinical trials look to see if people getting the new treatment live longer than most people do without the treatment.
Key points of phase II clinical trials
- A group of 25 to 100 patients with the same type of cancer get the new treatment in a phase II study. They’re treated using the dose and method found to be the safest and most effective in phase I studies.
- Usually in a phase II clinical trials, everyone gets the same dose. But some phase II studies randomly assign people to different treatment groups. These groups may get different doses or get the treatment in different ways to see which provides the best balance of safety and response.
- Placebos (inactive treatments) are not used in phase II trials.
- Phase II studies may be done at major cancer centers, community hospitals or even doctors’ offices.
Larger numbers of patients get the treatment in phase II trials, so less common side effects may be seen. If enough patients benefit from the treatment, and the side effects aren’t too bad, phase III clinical trials are begun.
Treatments that have been shown to work in phase II clinical trials must succeed in one more phase before they’re approved for general use. Phase III clinical trials compare the safety and effectiveness of the new treatment against the current standard treatment.
Because doctors do not yet know which treatment is better, study participants are often picked at random (called randomized ) to get either the standard treatment or the new treatment. When possible, neither the doctor nor the patient knows which of the treatments the patient is getting. This type of study is called a double-blind study . Randomization and blinding are discussed in more detail later.
Key points of phase III clinical trials
- Most phase III clinical trials include a large number of patients, at least several hundred.
- These studies are often done in many places across the country (or even around the world) at the same time.
- Phase III clinical trials are more likely to be offered in local community hospitals and doctor's offices.
- These studies tend to last longer than phase I and II studies.
- Placebos may be used in some phase III studies, but they’re never used alone if there’s a treatment available that works. Sometimes, a patient who is randomly assigned to the placebo for part of the study will at some point be offered the standard treatment as well.
As with other trials, patients in phase III clinical trials are watched closely for side effects, and treatment is stopped if they’re too hard to manage.
In the United States, when phase III clinical trials (or sometimes phase II trials) show a new drug is more effective or safer than the current treatment, a new drug application (NDA) is submitted to the Food and Drug Administration (FDA) for approval. The FDA reviews the results from the clinical trials and other relevant information.
Based on the review, the FDA decides whether to approve the treatment for use in patients with the illness the drug was tested on. If approved, the new treatment often becomes a standard of care, and newer drugs may be tested against it before they can be approved.
If the FDA feels that more evidence is needed to show that the new treatment's benefits outweigh its risks, it may ask for more information or even require that more studies be done.
Drugs approved by the FDA are often watched over a long period of time in phase IV studies. Even after testing a new medicine on thousands of people, all the effects of the treatment may not be known. Some questions may still need to be answered. For example, a drug may get FDA approval because it was shown to reduce the risk of cancer coming back after treatment. But does this mean that those who get it are more likely to live longer? Are there rare side effects that haven’t been seen yet, or side effects that only show up after a person has taken the drug for a long time? These types of questions may take many more years to answer, and are often addressed in phase IV clinical trials.
Key points of phase IV clinical trials
- Phase IV studies look at drugs that have already been approved by the FDA. The drugs are available for doctors to prescribe for patients, but phase IV studies might still be needed to answer important questions.
- These studies may involve thousands of people.
- This is often the safest type of clinical trial because the treatment has already been studied a lot and has likely been given to many people. Phase IV studies look at safety over time.
- These studies may also look at other aspects of the treatment, such as quality of life or cost effectiveness.
You can get the drugs used in a phase IV trial without being in a study. And the care you would get in a phase IV study is very much like the care you could expect if you were to get the treatment outside of a trial. But in phase IV studies you’re helping researchers learn more about the treatment and doing a service to future patients.

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
Children’s Oncology Group. Research. https://childrensoncologygroup.org/index.php/research. Accessed July 29, 2020.
Gooding K, Phiri M, Peterson I, Parker M, Desmond N. Six dimensions of research trial acceptability: how much, what, when, in what circumstances, to whom, and why? Soc Sci Med . 2018; 213:190-198.
National Cancer Institute. Clinical Trials Information for Patients and Caregivers. Cancer.gov. https://www.cancer.gov/about-cancer/treatment/clinical-trials. Reviewed February 6, 2020. Accessed July 29, 2020.
National Institutes of Health. NIH Clinical Research Trials and You. https://www.nih.gov/health-information/nih-clinical-research-trials-you. Reviewed October 20, 2017. Accessed July 29, 2020.
Taking Part in Cancer Treatment Research Studies . Bethesda, MD: National Cancer Institute, 2016.
Van Norman GA. Drugs, devices, and the FDA: part 1: an overview of approval processes for drugs. JACC Basic Transl Sci . 2016; 1(3):170-179.
Last Revised: August 18, 2020
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy .
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.
Help us end cancer as we know it, for everyone.

If this was helpful, donate to help fund patient support services, research, and cancer content updates.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Science News
- Meetings and Events
- Social Media
- Press Resources
- Email Updates
- Innovation Speaker Series
What are the different types of clinical research?
February 18, 2021
There are many different types of clinical research because researchers study many different things.
Treatment research usually tests an intervention such as medication, psychotherapy, new devices, or new approaches.
Prevention research looks for better ways to prevent disorders from developing or returning. Different kinds of prevention research may study medicines, vitamins, or lifestyle changes.
Diagnostic research refers to the practice of looking for better ways to identify a particular disorder or condition.
Screening research aims to find the best ways to detect certain disorders or health conditions.
Genetic studies aim to improve our ability to predict disorders by identifying and understanding how genes and illnesses may be related. Research in this area may explore ways in which a person’s genes make him or her more or less likely to develop a disorder. This may lead to development of tailor-made treatments based on a patient’s genetic make-up.
Epidemiological studies look at how often and why disorders happen in certain groups of people.
Research studies can be outpatient or inpatient. Outpatient means that participants do not stay overnight at the hospital or research center. Inpatient means that participants will need to stay at least one night in the hospital or research center.
Thank you for your interest in learning more about clinical research!

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
What Are Clinical Trials and Studies?
On this page:
What is clinical research?
Why participate in a clinical trial, what happens in a clinical trial or study, what happens when a clinical trial or study ends, what are the different phases of clinical trials, questions to ask before participating in clinical research, how do researchers decide who will participate, clinical research needs participants with diverse backgrounds.
By participating in clinical research, you can help scientists develop new medications and other strategies to treat and prevent disease. Many effective treatments that are used today, such as chemotherapy, cholesterol-lowering drugs, vaccines, and cognitive-behavioral therapy, would not exist without research participants. Whether you’re healthy or have a medical condition, people of all ages and backgrounds can participate in clinical trials. This article can help you learn more about clinical research, why people choose to participate, and how to get involved in a study.
Mr. Jackson's story
Mr. Jackson is 73 years old and was just diagnosed with Alzheimer’s disease . He is worried about how it will affect his daily life. Will he forget to take his medicine? Will he forget his favorite memories, like the births of his children or hiking the Appalachian Trail? When Mr. Jackson talked to his doctor about his concerns, she told him about a clinical trial that is testing a possible new Alzheimer’s treatment. But Mr. Jackson has concerns about clinical trials. He does not want to feel like a lab rat or take the chance of getting a treatment that may not work or could make him feel worse. The doctor explained that there are both risks and benefits to being part of a clinical trial, and she talked with Mr. Jackson about research studies — what they are, how they work, and why they need volunteers. This information helped Mr. Jackson feel better about clinical trials. He plans to learn more about how to participate.
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials.

Observational studies monitor people in normal settings. Researchers gather information from people and compare changes over time. For example, researchers may ask a group of older adults about their exercise habits and provide monthly memory tests for a year to learn how physical activity is associated with cognitive health . Observational studies do not test a medical intervention, such as a drug or device, but may help identify new treatments or prevention strategies to test in clinical trials.
Clinical trials are research studies that test a medical, surgical, or behavioral intervention in people. These trials are the primary way that researchers determine if a new form of treatment or prevention, such as a new drug, diet, or medical device (for example, a pacemaker), is safe and effective in people. Often, a clinical trial is designed to learn if a new treatment is more effective or has less harmful side effects than existing treatments.
Other aims of clinical research include:
- Testing ways to diagnose a disease early, sometimes before there are symptoms
- Finding approaches to prevent a health problem, including in people who are healthy but at increased risk of developing a disease
- Improving quality of life for people living with a life-threatening disease or chronic health problem
- Studying the role of caregivers or support groups
Learn more about clinical research from MedlinePlus and ClinicalTrials.gov .

People volunteer for clinical trials and studies for a variety of reasons, including:
- They want to contribute to discovering health information that may help others in the future.
- Participating in research helps them feel like they are playing a more active role in their health.
- The treatments they have tried for their health problem did not work or there is no treatment for their health problem.
Whatever the motivation, when you choose to participate in a clinical trial, you become a partner in scientific discovery. Participating in research can help future generations lead healthier lives. Major medical breakthroughs could not happen without the generosity of clinical trial participants — young and old, healthy, or diagnosed with a disease.
Where can I find a clinical trial?
Looking for clinical trials related to aging and age-related health conditions? Talk to your health care provider and use online resources to:
- Search for a clinical trial
- Look for clinical trials on Alzheimer's, other dementias, and caregiving
- Find a registry for a particular diagnosis or condition
- Explore clinical trials and studies supported by NIA
After you find one or more studies that you are interested in, the next step is for you or your doctor to contact the study research staff and ask questions. You can usually find contact information in the description of the study.
Let your health care provider know if you are thinking about joining a clinical trial. Your provider may want to talk to the research team to make sure the study is safe for you and to help coordinate your care.
Joining a clinical trial is a personal decision with potential benefits and some risks. Learn what happens in a clinical trial and how participant safety is protected . Read and listen to testimonials from people who decided to participate in research.
Here’s what typically happens in a clinical trial or study:
- Research staff explain the trial or study in detail, answer your questions, and gather more information about you.
- Once you agree to participate, you sign an informed consent form indicating your understanding about what to expect as a participant and the various outcomes that could occur.
- You are screened to make sure you qualify for the trial or study.
- If accepted into the trial, you schedule a first visit, which is called the “baseline” visit. The researchers conduct cognitive and/or physical tests during this visit.
- For some trials testing an intervention, you are assigned by chance (randomly) to a treatment group or a control group . The treatment group will get the intervention being tested, and the control group will not.
- You follow the trial procedures and report any issues or concerns to researchers.
- You may visit the research site at regularly scheduled times for new cognitive, physical, or other evaluations and discussions with staff. During these visits, the research team collects data and monitors your safety and well-being.
- You continue to see your regular physician(s) for usual health care throughout the study.
How do researchers decide which interventions are safe to test in people?
Before a clinical trial is designed and launched, scientists perform laboratory tests and often conduct studies in animals to test a potential intervention’s safety and effectiveness. If these studies show favorable results, the U.S. Food and Drug Administration (FDA) approves the intervention to be tested in humans. Learn more about how the safety of clinical trial participants is protected.
Once a clinical trial or study ends, the researchers analyze the data to determine what the findings mean and to plan the next steps. As a participant, you should be provided information before the study starts about how long it will last, whether you will continue receiving the treatment after the trial ends (if applicable), and how the results of the research will be shared. If you have specific questions about what will happen when the trial or study ends, ask the research coordinator or staff.
Clinical trials of drugs and medical devices advance through several phases to test safety, determine effectiveness, and identify any side effects. The FDA typically requires Phase 1, 2, and 3 trials to be conducted to determine if the drug or device can be approved for further use. If researchers find the intervention to be safe and effective after the first three phases, the FDA approves it for clinical use and continues to monitor its effects.
Each phase has a different purpose:
- A Phase 1 trial tests an experimental drug or device on a small group of people (around 20 to 80) to judge its safety, including any side effects, and to test the amount (dosage).
- A Phase 2 trial includes more people (around 100 to 300) to help determine whether a drug is effective. This phase aims to obtain preliminary data on whether the drug or device works in people who have a certain disease or condition. These trials also continue to examine safety, including short-term side effects.
- A Phase 3 trial gathers additional information from several hundred to a few thousand people about safety and effectiveness, studying different populations and different dosages, and comparing the intervention with other drugs or treatment approaches. If the FDA agrees that the trial results support the intervention’s use for a particular health condition, it will approve the experimental drug or device.
- A Phase 4 trial takes place after the FDA approves the drug or device. The treatment’s effectiveness and safety are monitored in large, diverse populations. Sometimes, side effects may not become clear until more people have used the drug or device over a longer period of time.
Clinical trials that test a behavior change, rather than a drug or medical device, advance through similar steps, but behavioral interventions are not regulated by the FDA. Learn more about clinical trials , including the types of trials and the four phases.
Choosing to participate in research is an important personal decision. If you are considering joining a trial or study, get answers to your questions and know your options before you decide. Here are questions you might ask the research team when thinking about participating.
- What is this study trying to find out?
- What treatment or tests will I have? Will they hurt? Will you provide me with the test or lab results?
- What are the chances I will be in the experimental group or the control group?
- If the study tests a treatment, what are the possible risks, side effects, and benefits compared with my current treatment?
- How long will the clinical trial last?
- Where will the study take place? Will I need to stay in the hospital?
- Will you provide a way for me to get to the study site if I need it, such as through a ride-share service?
- Will I need a trial or study partner (for example, a family member or friend who knows me well) to come with me to the research site visits? If so, how long will he or she need to participate?
- Can I participate in any part of the trial with my regular doctor or at a clinic closer to my home?
- How will the study affect my everyday life?
- What steps are being taken to ensure my privacy?
- How will you protect my health while I participate?
- What happens if my health problem gets worse during the trial or study?
- Can I take my regular medicines while participating?
- Who will be in charge of my care while I am in the trial or study? Will I be able to see my own doctors?
- How will you keep my doctor informed about my participation?
- If I withdraw from the trial or study, will this affect my normal care?
- Will it cost me anything to be in the trial or study? If so, will I be reimbursed for expenses, such as travel, parking, lodging, or meals?
- Will my insurance pay for costs not covered by the research, or must I pay out of pocket? If I don’t have insurance, am I still eligible to participate?
- Will my trial or study partner be compensated for his or her time?
- Will you follow up on my health after the end of the trial or study?
- Will I continue receiving the treatment after the trial or study ends?
- Will you tell me the results of the research?
- Whom do I contact if I have questions after the trial or study ends?

To be eligible to participate, you may need to have certain characteristics, called inclusion criteria. For example, a clinical trial may need participants to have a certain stage of disease, version of a gene, or family history. Some trials require that participants have a study partner who can accompany them to clinic visits.
Participants with certain characteristics may not be allowed to participate in some trials. These characteristics are called exclusion criteria. They include factors such as specific health conditions or medications that could interfere with the treatment being tested.
Many volunteers must be screened to find enough people who are eligible for a trial or study. Generally, you can participate in only one clinical trial at a time, although this is not necessarily the case for observational studies. Different trials have different criteria, so being excluded from one trial does not necessarily mean you will be excluded from another.

When research only includes people with similar backgrounds, the findings may not apply to or benefit a broader population. The results of clinical trials and studies with diverse participants may apply to more people. That’s why research benefits from having participants of different ages, sexes, races, and ethnicities.
Researchers need older adults to participate in clinical research so that scientists can learn more about how new drugs, tests, and other interventions will work for them. Many older adults have health needs that are different from those of younger people. For example, as people age, their bodies may react differently to certain drugs. Older adults may need different dosages of a drug to have the intended result. Also, some drugs may have different side effects in older people than in younger individuals. Having older adults enrolled in clinical trials and studies helps researchers get the information they need to develop the right treatments for this age group.
Researchers know that it may be challenging for some older adults to join a clinical trial or study. For example, if you have multiple health problems, can you participate in research that is looking at only one condition? If you are frail or have a disability, will you be strong enough to participate? If you no longer drive, how can you get to the research site? Talk to the research coordinator or staff about your concerns. The research team may have already thought about some of the potential obstacles and have a plan to make it easier for you to participate.
Read more about diversity in clinical trials .
You may also be interested in
- Learning more about the benefits, risks, and safety of clinical research
- Finding out about participating in Alzheimer's disease research
- Downloading or sharing an infographic with the benefits of participating in clinical research
Sign up for email updates on healthy aging
For more information about clinical trials.
Alzheimers.gov www.alzheimers.gov Explore the Alzheimers.gov website for information and resources on Alzheimer’s and related dementias from across the federal government.
Clinical Research Trials and You National Institutes of Health www.nih.gov/health-information/nih-clinical-research-trials-you
ClinicalTrials.gov www.clinicaltrials.gov
This content is provided by the NIH National Institute on Aging (NIA). NIA scientists and other experts review this content to ensure it is accurate and up to date.
Content reviewed: March 22, 2023
nia.nih.gov
An official website of the National Institutes of Health
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih clinical research trials and you.
The NIH Clinical Trials and You website is a resource for people who want to learn more about clinical trials. By expanding the below questions, you can read answers to common questions about taking part in a clinical trial.
What are clinical trials and why do people participate?
Clinical research is medical research that involves people like you. When you volunteer to take part in clinical research, you help doctors and researchers learn more about disease and improve health care for people in the future. Clinical research includes all research that involves people. Types of clinical research include:

- Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups.
- Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
- Health services, which looks at how people access health care providers and health care services, how much care costs, and what happens to patients as a result of this care.
- Clinical trials, which evaluate the effects of an intervention on health outcomes.
What are clinical trials and why would I want to take part?
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Clinical trials can study:
- New drugs or new combinations of drugs
- New ways of doing surgery
- New medical devices
- New ways to use existing treatments
- New ways to change behaviors to improve health
- New ways to improve the quality of life for people with acute or chronic illnesses.
The goal of clinical trials is to determine if these treatment, prevention, and behavior approaches are safe and effective. People take part in clinical trials for many reasons. Healthy volunteers say they take part to help others and to contribute to moving science forward. People with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have added (or extra) care and attention from the clinical trial staff. Clinical trials offer hope for many people and a chance to help researchers find better treatments for others in the future
Why is diversity and inclusion important in clinical trials?
People may experience the same disease differently. It’s essential that clinical trials include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances.
See Diversity & Inclusion in Clinical Trials for more information.
How does the research process work?
The idea for a clinical trial often starts in the lab. After researchers test new treatments or procedures in the lab and in animals, the most promising treatments are moved into clinical trials. As new treatments move through a series of steps called phases, more information is gained about the treatment, its risks, and its effectiveness.
What are clinical trial protocols?
Clinical trials follow a plan known as a protocol. The protocol is carefully designed to balance the potential benefits and risks to participants, and answer specific research questions. A protocol describes the following:
- The goal of the study
- Who is eligible to take part in the trial
- Protections against risks to participants
- Details about tests, procedures, and treatments
- How long the trial is expected to last
- What information will be gathered
A clinical trial is led by a principal investigator (PI). Members of the research team regularly monitor the participants’ health to determine the study’s safety and effectiveness.
What is an Institutional Review Board?
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are reduced and are outweighed by potential benefits. IRBs are committees that are responsible for reviewing research in order to protect the rights and safety of people who take part in research, both before the research starts and as it proceeds. You should ask the sponsor or research coordinator whether the research you are thinking about joining was reviewed by an IRB.
What is a clinical trial sponsor?
Clinical trial sponsors may be people, institutions, companies, government agencies, or other organizations that are responsible for initiating, managing or financing the clinical trial, but do not conduct the research.
What is informed consent?
Informed consent is the process of providing you with key information about a research study before you decide whether to accept the offer to take part. The process of informed consent continues throughout the study. To help you decide whether to take part, members of the research team explain the details of the study. If you do not understand English, a translator or interpreter may be provided. The research team provides an informed consent document that includes details about the study, such as its purpose, how long it’s expected to last, tests or procedures that will be done as part of the research, and who to contact for further information. The informed consent document also explains risks and potential benefits. You can then decide whether to sign the document. Taking part in a clinical trial is voluntary and you can leave the study at any time.
What are the types of clinical trials?
There are different types of clinical trials.
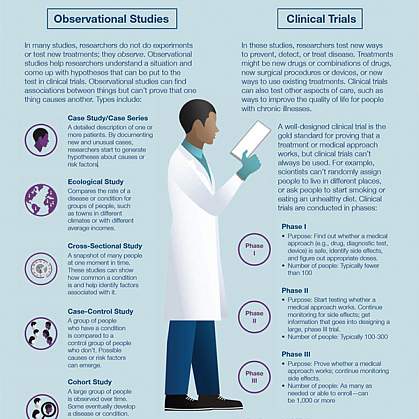
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Approaches may include medicines, vaccines, or lifestyle changes.
- Screening trials test new ways for detecting diseases or health conditions.
- Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with conditions or illnesses.
What are the phases of clinical trials?
Clinical trials are conducted in a series of steps called “phases.” Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials : Researchers test a drug or treatment in a small group of people (20–80) for the first time. The purpose is to study the drug or treatment to learn about safety and identify side effects.
- Phase II trials : The new drug or treatment is given to a larger group of people (100–300) to determine its effectiveness and to further study its safety.
- Phase III trials : The new drug or treatment is given to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or similar treatments, and collect information that will allow the new drug or treatment to be used safely.
- Phase IV trials : After a drug is approved by the FDA and made available to the public, researchers track its safety in the general population, seeking more information about a drug or treatment’s benefits, and optimal use.
What do the terms placebo, randomization, and blinded mean in clinical trials?
In clinical trials that compare a new product or therapy with another that already exists, researchers try to determine if the new one is as good, or better than, the existing one. In some studies, you may be assigned to receive a placebo (an inactive product that resembles the test product, but without its treatment value).
Comparing a new product with a placebo can be the fastest and most reliable way to show the new product’s effectiveness. However, placebos are not used if you would be put at risk — particularly in the study of treatments for serious illnesses — by not having effective therapy. You will be told if placebos are used in the study before entering a trial.
Randomization is the process by which treatments are assigned to participants by chance rather than by choice. This is done to avoid any bias in assigning volunteers to get one treatment or another. The effects of each treatment are compared at specific points during a trial. If one treatment is found superior, the trial is stopped so that the most volunteers receive the more beneficial treatment. This video helps explain randomization for all clinical trials .
" Blinded " (or " masked ") studies are designed to prevent members of the research team and study participants from influencing the results. Blinding allows the collection of scientifically accurate data. In single-blind (" single-masked ") studies, you are not told what is being given, but the research team knows. In a double-blind study, neither you nor the research team are told what you are given; only the pharmacist knows. Members of the research team are not told which participants are receiving which treatment, in order to reduce bias. If medically necessary, however, it is always possible to find out which treatment you are receiving.
Who takes part in clinical trials?
Many different types of people take part in clinical trials. Some are healthy, while others may have illnesses. Research procedures with healthy volunteers are designed to develop new knowledge, not to provide direct benefit to those taking part. Healthy volunteers have always played an important role in research.
Healthy volunteers are needed for several reasons. When developing a new technique, such as a blood test or imaging device, healthy volunteers help define the limits of "normal." These volunteers are the baseline against which patient groups are compared and are often matched to patients on factors such as age, gender, or family relationship. They receive the same tests, procedures, or drugs the patient group receives. Researchers learn about the disease process by comparing the patient group to the healthy volunteers.
Factors like how much of your time is needed, discomfort you may feel, or risk involved depends on the trial. While some require minimal amounts of time and effort, other studies may require a major commitment of your time and effort, and may involve some discomfort. The research procedure(s) may also carry some risk. The informed consent process for healthy volunteers includes a detailed discussion of the study's procedures and tests and their risks.
A patient volunteer has a known health problem and takes part in research to better understand, diagnose, or treat that disease or condition. Research with a patient volunteer helps develop new knowledge. Depending on the stage of knowledge about the disease or condition, these procedures may or may not benefit the study participants.
Patients may volunteer for studies similar to those in which healthy volunteers take part. These studies involve drugs, devices, or treatments designed to prevent,or treat disease. Although these studies may provide direct benefit to patient volunteers, the main aim is to prove, by scientific means, the effects and limitations of the experimental treatment. Therefore, some patient groups may serve as a baseline for comparison by not taking the test drug, or by receiving test doses of the drug large enough only to show that it is present, but not at a level that can treat the condition.
Researchers follow clinical trials guidelines when deciding who can participate, in a study. These guidelines are called Inclusion/Exclusion Criteria . Factors that allow you to take part in a clinical trial are called "inclusion criteria." Those that exclude or prevent participation are "exclusion criteria." These criteria are based on factors such as age, gender, the type and stage of a disease, treatment history, and other medical conditions. Before joining a clinical trial, you must provide information that allows the research team to determine whether or not you can take part in the study safely. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy volunteers. Inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants and keep them safe, and to help ensure that researchers can find new information they need.
What do I need to know if I am thinking about taking part in a clinical trial?

Risks and potential benefits
Clinical trials may involve risk, as can routine medical care and the activities of daily living. When weighing the risks of research, you can think about these important factors:
- The possible harms that could result from taking part in the study
- The level of harm
- The chance of any harm occurring
Most clinical trials pose the risk of minor discomfort, which lasts only a short time. However, some study participants experience complications that require medical attention. In rare cases, participants have been seriously injured or have died of complications resulting from their participation in trials of experimental treatments. The specific risks associated with a research protocol are described in detail in the informed consent document, which participants are asked to consider and sign before participating in research. Also, a member of the research team will explain the study and answer any questions about the study. Before deciding to participate, carefully consider risks and possible benefits.
Potential benefits
Well-designed and well-executed clinical trials provide the best approach for you to:
- Help others by contributing to knowledge about new treatments or procedures.
- Gain access to new research treatments before they are widely available.
- Receive regular and careful medical attention from a research team that includes doctors and other health professionals.
Risks to taking part in clinical trials include the following:
- There may be unpleasant, serious, or even life-threatening effects of experimental treatment.
- The study may require more time and attention than standard treatment would, including visits to the study site, more blood tests, more procedures, hospital stays, or complex dosage schedules.
What questions should I ask if offered a clinical trial?
If you are thinking about taking part in a clinical trial, you should feel free to ask any questions or bring up any issues concerning the trial at any time. The following suggestions may give you some ideas as you think about your own questions.
- What is the purpose of the study?
- Why do researchers think the approach may be effective?
- Who will fund the study?
- Who has reviewed and approved the study?
- How are study results and safety of participants being monitored?
- How long will the study last?
- What will my responsibilities be if I take part?
- Who will tell me about the results of the study and how will I be informed?
Risks and possible benefits
- What are my possible short-term benefits?
- What are my possible long-term benefits?
- What are my short-term risks, and side effects?
- What are my long-term risks?
- What other options are available?
- How do the risks and possible benefits of this trial compare with those options?
Participation and care
- What kinds of therapies, procedures and/or tests will I have during the trial?
- Will they hurt, and if so, for how long?
- How do the tests in the study compare with those I would have outside of the trial?
- Will I be able to take my regular medications while taking part in the clinical trial?
- Where will I have my medical care?
- Who will be in charge of my care?
Personal issues
- How could being in this study affect my daily life?
- Can I talk to other people in the study?
Cost issues
- Will I have to pay for any part of the trial such as tests or the study drug?
- If so, what will the charges likely be?
- What is my health insurance likely to cover?
- Who can help answer any questions from my insurance company or health plan?
- Will there be any travel or child care costs that I need to consider while I am in the trial?
Tips for asking your doctor about trials
- Consider taking a family member or friend along for support and for help in asking questions or recording answers.
- Plan what to ask — but don't hesitate to ask any new questions.
- Write down questions in advance to remember them all.
- Write down the answers so that they’re available when needed.
- Ask about bringing a tape recorder to make a taped record of what's said (even if you write down answers).
This information courtesy of Cancer.gov.
How is my safety protected?

Ethical guidelines
The goal of clinical research is to develop knowledge that improves human health or increases understanding of human biology. People who take part in clinical research make it possible for this to occur. The path to finding out if a new drug is safe or effective is to test it on patients in clinical trials. The purpose of ethical guidelines is both to protect patients and healthy volunteers, and to preserve the integrity of the science.
Informed consent
Informed consent is the process of learning the key facts about a clinical trial before deciding whether to participate. The process of providing information to participants continues throughout the study. To help you decide whether to take part, members of the research team explain the study. The research team provides an informed consent document, which includes such details about the study as its purpose, duration, required procedures, and who to contact for various purposes. The informed consent document also explains risks and potential benefits.
If you decide to enroll in the trial, you will need to sign the informed consent document. You are free to withdraw from the study at any time.
Most, but not all, clinical trials in the United States are approved and monitored by an Institutional Review Board (IRB) to ensure that the risks are minimal when compared with potential benefits. An IRB is an independent committee that consists of physicians, statisticians, and members of the community who ensure that clinical trials are ethical and that the rights of participants are protected. You should ask the sponsor or research coordinator whether the research you are considering participating in was reviewed by an IRB.
Further reading
For more information about research protections, see:
- Office of Human Research Protection
- Children's Assent to Clinical Trial Participation
For more information on participants’ privacy and confidentiality, see:
- HIPAA Privacy Rule
- The Food and Drug Administration, FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective
For more information about research protections, see: About Research Participation
What happens after a clinical trial is completed?
After a clinical trial is completed, the researchers carefully examine information collected during the study before making decisions about the meaning of the findings and about the need for further testing. After a phase I or II trial, the researchers decide whether to move on to the next phase or to stop testing the treatment or procedure because it was unsafe or not effective. When a phase III trial is completed, the researchers examine the information and decide whether the results have medical importance.
Results from clinical trials are often published in peer-reviewed scientific journals. Peer review is a process by which experts review the report before it is published to ensure that the analysis and conclusions are sound. If the results are particularly important, they may be featured in the news, and discussed at scientific meetings and by patient advocacy groups before or after they are published in a scientific journal. Once a new approach has been proven safe and effective in a clinical trial, it may become a new standard of medical practice.
Ask the research team members if the study results have been or will be published. Published study results are also available by searching for the study's official name or Protocol ID number in the National Library of Medicine's PubMed® database .
How does clinical research make a difference to me and my family?

Only through clinical research can we gain insights and answers about the safety and effectiveness of treatments and procedures. Groundbreaking scientific advances in the present and the past were possible only because of participation of volunteers, both healthy and those with an illness, in clinical research. Clinical research requires complex and rigorous testing in collaboration with communities that are affected by the disease. As research opens new doors to finding ways to diagnose, prevent, treat, or cure disease and disability, clinical trial participation is essential to help us find the answers.
This page last reviewed on October 3, 2022
Connect with Us
- More Social Media from NIH
About Clinical Trials
What is a clinical trial.
Clinical trials look at new ways to prevent, detect, or treat disease. The goal of clinical trials is to determine if a new test or treatment works and is safe.
The idea for a clinical trial —also known as a clinical research study —often originates in the laboratory. After researchers test new therapies or procedures in the laboratory and in animal studies, the most promising experimental treatments are moved into clinical trials, which are conducted in phases. During a trial, more information is gained about an experimental treatment, its risks, and its effectiveness.
Types of Clinical Trials
- Natural history studies provide valuable information about how disease and health progress.
- Prevention trials look for better ways to prevent a disease in people who have never had the disease or to prevent the disease from returning. Better approaches may include medicines, vaccines, or lifestyle changes, among other things.
- Screening trials test the best way to detect certain diseases or health conditions.
- Diagnostic trials determine better tests or procedures for diagnosing a particular disease or condition.
- Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery or radiation therapy.
- Quality of life trials (or supportive care trials) explore and measure ways to improve the comfort and quality of life of people with a chronic illness.
Clinical Trial Phases
Clinical trials are conducted in phases. Each phase has a different purpose and helps researchers answer different questions.
- Phase I trials: Researchers test an experimental drug or treatment in a small group of people (20–80) for the first time. The purpose is to evaluate its safety and identify side effects.
- Phase II trials: The experimental drug or treatment is administered to a larger group of people (100–300) to determine its effectiveness and to further evaluate its safety.
- Phase III trials: The experimental drug or treatment is administered to large groups of people (1,000–3,000) to confirm its effectiveness, monitor side effects, compare it with standard or equivalent treatments, and collect information that will allow the experimental drug or treatment to be used safely.
- Phase IV trials: After a drug is approved by the FDA and made available to the public, researchers track its safety, seeking more information about a drug or treatment’s risks, benefits, and optimal use.
For more information about clinical trials, see the webpage at National Institute of Health.


Different Types of Clinical Research and What They Mean for You
Without clinical research, there would be no new treatments, no improvements in our understanding of how diseases work, and no new options for prevention. In short, clinical research is essential .
Not all research is the same. There are two broad types: observational and investigational. They each have different goals and work in different ways, but they both rely on people like you to participate and spread the word.
Read on for an overview of observational and investigational clinical research and learn how you can get involved.
Observational Research: Observing Health Patterns
Observational research gathers data and looks for patterns. If participants have a medical condition, they will continue their usual treatment plan during an observational study. Participants in this type of research do not receive new treatments. Instead, researchers observe and record various data, depending on the goal of the study, while the participants keep their general routine the same.
Participation may involve filling out a survey, meeting with a researcher for an interview, or providing a blood or tissue sample.
Types of Observational Studies
- Cohort studies : A cohort is a group of people, and this type of research follows a given group over time, collecting information about various health outcomes. For example, a cohort study might examine which participants develop cancer over the length of the study and will look for traits or behaviors they may share.
- Case control studies : In these types of studies, researchers compare two groups: one with a particular disease or outcome, and another without it. They keep other traits as similar as possible to focus on the single factor they are studying. Researchers then ask participants about their past behavior and use this information to identify potential risk factors.
- Cross-sectional studies : These studies compare groups by examining data from a single point in time. Researchers look for patterns and relationships within this data.
Who Can Participate?

Each observational study will have unique requirements based on what the researchers are interested in. In many cases, both healthy volunteers and people with a specific condition are needed.
Since observational research does not require you to take a new medication or change your routine, you can often participate in multiple observational studies at one time.
Why Does Observational Research Matter?
Observational clinical research provides essential information about how diseases work and what risk factors could be. Each new study gives more information, and it takes many studies over a long period of time to clarify possible links between behaviors and diseases.
What does this mean in the real world? For one, it can help people make more informed decisions about their lifestyles to have a better chance of staying healthy. Recommendations like increasing physical activity, maintaining a balanced diet, and avoiding smoking all have their roots in observational research.
Observational studies can also identify factors outside of an individual’s control that increase the chance of developing a certain disease. This gives doctors the information they need to screen for those conditions sooner, potentially improving and saving lives.
Investigating Research: New Treatments
Whereas observational research gathers information about health, investigational research (i.e., clinical trials) puts this information into practice and tests new ways to prevent, diagnose, manage, and cure diseases.
During a clinical trial, researchers monitor participants to ensure safety and track whether the new treatment works correctly. Depending on the trial, participants may go to a specific location (a trial site) to receive treatment, or a health care professional may monitor them while they undergo treatment at home.
Clinical Trial Phases
Clinical trials have four phases , and each serves a specific purpose.
- Phase I tests safety, determines the range of doses, and identifies any side effects
- Phase II gives the treatment to a larger group and tests its effectiveness
- Phase III continues to test effectiveness in an even larger group and compares the new treatment to any others that are currently used
- Phase IV takes place after the new treatment becomes widely available following FDA approval and continues to monitor risks and benefits
Like observational research, different trials look for various traits among participants. Along with looking for people with the health condition the treatment will target, clinical trials may have other requirements depending on the specific goals.
The phase of the trial also changes who can participate. Phase I trials often involve healthy volunteers, as this gives the opportunity to study safety before involving those whose disease may make them more likely to have negative effects.
Why Does Investigational Research Matter?
Any advancement in medicine that you can think of owes its creation to clinical trials, from the most common over-the-counter medications to innovative treatments for serious conditions. Investigational research is essential to determine what treatments are safe and effective. Clinical trials can also provide options for those who don’t have many other treatment choices, like those with rare diseases.
Ready to Get Involved?
Whether you’re interested in taking part in observational or investigational research, you have the power to make a real difference for the future of medicine. You can ask your doctor about studies or trials that may be right for you, or you can use an online tool like Carebox , Antidote , or ClinicalTrials.gov to start your search.
And if participating directly isn’t the right choice for you now, that’s okay too! You still can do your part by sharing information and staying informed . You never know who in your network could benefit from this knowledge.
Similar Posts

Clinical Trial Story Time: A Friendly Intro for Your Child
If you’ve recently enrolled your child in a clinical trial, you may be wondering how to best prepare them for the experience. This blog aims to help you with that effort. The sections below are designed to be read directly to younger children. What Is a Clinical Trial? A clinical trial is when scientists, doctors, and people who are sick get together to find out how well a new medicine helps people feel better. Clinical trials … Why Should You Be Part of a Clinical Trial? In the world of clinical trials, one of the most special things is called “research as care.” When kids like you have health challenges, clinical research can be a great way to get the very best care in the world that can’t be found anywhere else! The trial team helps you feel comfortable and cared for, and you help them find out more about how well the new medicine works. It’s teamwork that’s meant to help you — and lots of other kids around the world — feel better. What Will Happen During the Clinical Trial? At the start of the trial, the doctor’s team will talk to you all about what to expect. You will learn about how often you’ll visit them in person, some things you might get to do from home, and you’ll get to know some of the helpers who will be with you along the way. You’ll also learn about the different ways the study team will find out more about the medicine with your help! Who Can Answer My Questions? You can ask as many questions as you want during the clinical trial. You will have a friendly study buddy who you can always ask any questions you have. And don’t worry! You don’t have to think of all your questions when you meet your helper. Even if you think of a question later, you can ask any time. Final Note for Parents and Caregivers Do you or your child have additional questions or concerns about the clinical trial process? Visit our Resources page, talk to your doctor, and consider working with a patient advocate.

Tips for Searching for Clinical Trials as a Caregiver
If you are a caregiver, you aren’t alone; according to the Caregiver Action Network, over 65 million people in the U.S. (about 29% of the population) provide care for a chronically ill, disabled, or elderly family member or friend. The role caregivers play is different for everyone, whether it involves helping with a few tasks or full-time, live-in care. Across this spectrum, it’s clear that caregiving is essential for our communities and deserves recognition and support. Caregivers help their loved ones in many ways, but one that may not come to mind at first is finding clinical trials. Enrolling the person you are caring for in a clinical trial can help them find new ways to manage their health, while also benefiting others with the same condition. If you’re interested in learning more about how clinical trials work and your role in finding a trial as a caregiver, the information in this article can help you get started. What You Should Know About Clinical Trials Before we explore more details about finding clinical trials as a caregiver, we should first discuss what clinical trials are. A clinical trial is the process of testing a new treatment option, such as a medication or device. Trials happen in different phases, starting with smaller groups and progressing to larger ones. Throughout every step of the process, researchers follow strict standards to make sure they collect accurate data and keep participants safe. If the trial shows the new treatment is effective, it can be approved to become available to more people. What does this mean for your loved one? Clinical trials often provide access to treatments that are not available anywhere else, giving them a new option to manage their condition. In addition to the personal benefits, taking part in a trial is also a valuable gift to others with the same condition. It gives hope for new treatment options that wouldn’t be possible without research. How to Find a Clinical Trial Once you’ve decided that you’re interested in a clinical trial for someone close to you, the next step is exploring trials that could be a good fit. The process can seem overwhelming at first, but the good news is that there are many resources that can guide your journey and support you along the way. Your loved one’s doctor is a great starting point for discussing clinical trials. Although not all doctors are involved in clinical trials, they will be able to answer some of your questions about the process and how it could fit with any current treatment(s). In some cases, they could recommend a trial that they are involved in or familiar with. Even if your loved one’s doctor is not involved in trials, there are still many ways to find options. Sites like Carebox, Antidote, and ClinicalTrials.gov are great resources for searching for trials. You can look based on a condition and see key details about the trials within these tools. From Searching to Finding the Right Fit During this process, there are many elements to consider when deciding whether a trial is a good fit for the person in your care. Each trial has different rules for who can participate. This may include how far along the disease is, whether your loved one has or does not have certain symptoms, other conditions, age, and a variety of other factors. Be sure to have their medical history available as you search. If you have any questions about whether a trial could be a good fit, reach out to the contact for that trial. They can talk through requirements with you and give you more details about the kind of participants the trial is looking for. You should also consider the impact participation may have on you as a caregiver. Is the trial nearby? Will you need to travel to take your care recipient to appointments? Are there options to participate from home? This can help you plan to make sure participating is manageable not only for your loved one, but also for you. Many organizations in the clinical research community are working to improve access. The trial organizers may be able to point you in the direction of resources to help make participation easier, such as patient advocacy networks or support groups for caregivers. Answers to Your Clinical Trial Questions Throughout the process of finding trials for someone in your care, communication is key. If you have questions, be sure to speak up. Researchers understand the key role participants play, and they should support you in finding the answers you need. Our Resource Library is also a valuable tool for finding more information about clinical trials. Whether you are actively searching for trials for your loved one or are just starting to look into clinical research, you can make a life-changing impact by learning more and spreading the word.

Breast Cancer Resources
Source: Touch BBCA

My Journey With Cystic Fibrosis
Source: Ella Balasa

They Gave Me 3 Years to Live. 30 Years Later, Here Are My Tips for Participating in Clinical Trials.
In 1994, I was a 37-year-old Army major stationed in Michigan with my wife, Cecelia, and our five-year-old daughter, Patrice. Little did I know that learning how to participate in clinical trials would save my life. One morning before heading to work in January 1995, I went to the doctor’s office to get results from an MRI I had because of persistent back pain, which had been bothering me for six months. The doctor told me I had chronic myeloid leukemia (CML). He gave me three years to live. He then explained that the only potential cure was a bone marrow transplant. But, as an African American, my chances of finding a match were slim. There were — and still are — few people of color on the marrow donor registry. I took it upon myself to change that. I started organizing marrow drives, visiting churches, malls, colleges, and military bases nationwide. In December 1995, I relocated to Atlanta, Georgia, and continued my efforts. In April 1996, at a marrow drive in Columbus, Georgia, a local businessman with a different type of leukemia approached me. He shared his leukemia story, explaining how an experimental treatment at the MD Anderson Cancer Center in Houston had saved his life. He urged me to visit MD Anderson, so I did. I flew to Houston and met a doctor who enrolled me in a few experimental treatments. But by 1998, after three years, they stopped working. I became extremely fatigued, waking up exhausted and in pain every day. Despite organizing and participating in numerous marrow drives, I had not found a matching donor. Time was running out. As a last resort, I asked my doctor about any other drugs that could help. He mentioned a potential drug that was still in its pre-clinical trial stage. When it was approved for use in humans, I entered the Phase I clinical trial for STI571. I responded exceptionally well. Nine months into the trial, I ran a 26.2-mile marathon in Alaska! Five months later, I cycled 111 miles in Tucson, Arizona, raising funds for leukemia research. Three years passed, and in May 2001, the FDA approved STI571 as Gleevec. Among the hundreds of thousands of lives Gleevec has saved, I am the longest-living survivor. In the decades since, I got to see my daughter grow up, attend Harvard University and Emory Medical School, and become a doctor. I’ve celebrated birthdays and wedding anniversaries and been there for every special moment, all because of a clinical trial. The doctors gave me three years to live. It’s been nearly 30 years — and counting. What Does This Have to Do With You? Gleevec was the first tyrosine kinase inhibitor (TKI) cancer treatment. Now there are 80, seven approved in the last year. I am humbled and honored every day to not only be alive, but to have helped change the cancer treatment game. Being part of something bigger than myself — the clinical trial — felt so good that I decided to dedicate my life to clinical research education and patient advocacy. Because the more people know about clinical trials and how to find a clinical trial, the more lives will be saved today and in the future. Maybe by telling you my story, you’ll be inspired to be part of something bigger than yourself, too. How to Participate in Clinical Trials Clinical research is different from what it once was. Back when I was in the Gleevec trial, I did everything by myself. I had to find a place to live in Houston where I stayed for three months, and then find my way back to Houston every 90 days for about three years. These days, there are patient advocates, support services, and more, all dedicated to making research participation as easy as possible. Despite the differences, there are things that hold true. To participate in clinical trials, you need to know the benefits, do your homework, and start your research journey as soon as possible. In addition, you can enter a trial in a later phase and can withdraw from a trial without penalty. Know the Benefits of Clinical Trials Clinical research is essential! Every prescribed drug in your medicine cabinet has gone through a clinical trial. Clinical trials provide treatment at least equal to the current standard of care. When you’re a clinical trial patient, you not only access the newest care (often for free), but you also get the chance to contribute to the future of medicine. This is especially true for patients of color. Many racial and ethnic minorities aren’t well-represented in clinical research, so it’s even more important for us to step up and participate. In fact, new regulations have been introduced in the industry that put requirements on patient diversity. Otherwise, tomorrow’s treatments won’t be as effective for everyone. Do Your Homework Your doctor may not offer clinical trials unless you ask. You need to do your own research so you can talk to your providers about trial options. Luckily, there are an abundance of clinical trial resources out there, from advocacy groups to government websites. You should research clinical trials even if you’re healthy or happy with your current treatment. First, many trials need healthy volunteers. Second, a clinical trial might not offer you a better outcome or prognosis, but it may help your quality of life or help educate you on your condition. Start Now Trials move slowly, but they go fast once they get going. I first heard about clinical research 18 months before the Gleevec trial. If I hadn’t known to ask the doctor in Houston, I would have missed out. Don’t wait. Begin your research, get on lists, and talk to your doctor now. Turn 3 Years Into 30 My biggest takeaway on how to participate in clinical trials? Leave no stone unturned. If you’re not trying clinical research, you haven’t tried every option. Perhaps a clinical trial won’t mean the difference between life and death for you, but it might make a difference in your quality of life. It might mean survival for someone else today, tomorrow, or in 30 years. I invite you to be part of something bigger than yourself by exploring the resources on Clinical Trials For All to learn more about clinical trials or helping us spread awareness.

I’m About to Start a Clinical Trial. Now What?
So you’ve enrolled in a clinical trial. Congratulations! You’ve just taken an important step toward contributing to better health outcomes for people all over the world. And for many of you, this may also mean that you are receiving research as care for yourself. This is a big, exciting step and we’re here to help you know what to expect. Understand Your Trial Phase There are clinical trials for each phase of pharmaceutical or medical device research. Here is a quick breakdown of what the different phases mean to help you understand more about the trial phase you are participating in. Phase 0: These early exploratory clinical trials test less than 1% of the amount of the drug that will be used in later trial phases. This low-dose trial makes sure the medicine works as expected in the human body before conducting full-dose trials. Phase I: Trials in this phase are all about safety. This type of trial often seeks healthy volunteers. Phase II: Now we’re getting into treatment effectiveness trials. If you’re receiving research as a care option, you may be in a Phase II trial. Phase III: Once a treatment is proved effective, longer-term trials that last several years and involve many more trial volunteers like you take place. This is another trial phase where you may be receiving research as a care option. Post-market: Once new treatments are approved, ongoing studies may continue to observe the long-term effects of the drug. Medical device trials: If you are volunteering for a medical device trial, the safety trial phase is called a pilot study, also known as a feasibility study. The effectiveness trial phase is called a pivotal trial. Then once the device is approved, post-market studies continue just as they do for pharmaceuticals. Informed Consent As a clinical trial volunteer, a research representative will walk you through what is called the “informed consent” process. This is where study staff explain the possible risks and benefits of the trial and everything that is involved in your participation. You will have the opportunity to have any questions answered and confirm that you want to participate after receiving all the information. You can also choose to have a patient advocate support you through this process. Trial Access The sponsor of your trial will discuss the logistics of trial access with you. Some trials require on-site, in-person visits to participate in the study, while others have some on-site visits and some check-ins that can be conducted from your home or from your existing medical practitioner’s office (called “hybrid trials”). In fact, some clinical trials can be conducted completely remotely with data collected from your home or doctor’s office (called “decentralized or virtual trials”). Your study’s sponsor will explain all the details for your particular trial so you know exactly what to expect. If travel to the study site is required, your sponsor can answer questions about support with travel financing and logistics. What Happens When the Trial Is Over? It can take years from the start of a clinical trial to when the study is completed and all data are reviewed and analyzed. Stay in touch with your sponsor contact about study results, realizing that the final results of any study may take quite a long time to be released. In the meantime, work with your sponsor, patient advocate, and/or your trusted medical team to continue your clinical trial journey. Want to learn even more about what it’s like to participate in a clinical trial? Be sure to check out our other blogs to discover firsthand stories from patients like you as well as more information about how and why to participate.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
Clinical Trials: What Patients Need to Know
Learn more about clinical trials and find a trial that might be right for you. Clinical trials are voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments. It is important to remember that the FDA does not conduct Clinical Trials.
Search for a Clinical Trial
Enter a word or phrase, such as the name of a medical condition or intervention. Example: Cancer AND Los Angeles or expanded access AND compassionate use
Learn More About Clinical Trials
Clinical Research Versus Medical Treatment Understand the differences between clinical research and medical treatment and what those differences mean for you. Find answers to your questions about clinical trials, such as why they are done, who should consider participating, and issues to consider before joining a trial.

What are the Different Types of Clinical Research Understand the different types of research and the four clinical trial phases, such as their purpose and how many people participate in each of the phases.
Informed Consent for Clinical Trials Understand what informed consent is and the questions you need to know before signing informed consent.
Diversity in Clinical Trial Participation It is important to test drugs and medical products in the people they are meant to help. Learn about FDASIA 907 and how FDA works to make sure that people of different ages, races, ethnic groups, and genders are included in clinical trials.
What is an Institutional Review Board Understand what Institutional Review Boards are, who is on them and who they protect.
Sexual and Gender Minorities The NIH Sexual and Gender Minority Research Office (SGMRO) coordinates sexual and gender minority (SGM)--related research and activities by working directly with the NIH Institutes, Centers, and Offices. The office was officially established in September 2015 within the NIH Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI).
Resources For You
- NIH Clinical Research Trials and You
- Good Clinical Practice
- HHS Office of Human Research Protections - About Research Participation
- Interactive Patient Education Tutorial On Clinical Trials
- Protecting America's Health Through Human Drugs
- Protection of Human Subjects of Research
- About ClinicalTrials.gov
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
- Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

- Types of clinical trials
Medical research studies involving people are called clinical trials.
There are two main types of trials or studies - interventional and observational.
Interventional trials aim to find out more about a particular intervention, or treatment. A computer puts people taking part into different treatment groups. This is so that the research team can compare the results.
Observational studies aim to find out what happens to people in different situations. The research team observe the people taking part, but they don’t influence what treatments people have. The people taking part aren’t put into treatment groups.
There are different types of trials within these two groups. This page has information about
Pilot studies and feasibility studies
Prevention trials, screening trials, treatment trials, multi-arm multi-stage (mams) trials, cohort studies.
Case control studies
Cross sectional studies
Pilot studies and feasibility studies are small versions of studies which are sometimes done before a large trial takes place.
Feasibility studies are designed to see if it is possible to do the main study. They aim to find out things such as whether patients and doctors are happy to take part, and how long it might take to collect and analyse the information. They don’t answer the main research question about how well a treatment works.
Pilot studies are small versions of the main study. Pilot studies help to test that all the main parts of the study work together. They may also help answer the research question. Sometimes the research team include the information collected during the pilot study in the results of the main study.
Prevention trials look at whether a particular treatment can help prevent cancer. The people taking part don't have cancer.
These trials can be for the general population or for people who have a higher than normal risk of developing a certain cancer. For example, this could include people with a strong family history of cancer.
Screening tests people for the early signs of cancer before they have any symptoms. As with prevention trials, screening trials can be for the general population. Or they can be for a group of people who have a higher than normal risk of developing a certain cancer.
Researchers may plan screening trials to see if new tests are reliable enough to detect particular types of cancer. Or they may try to find out if there is an overall benefit in picking up the cancer early.

For trials that compare two or more treatments, you are put into a treatment group at random. This is a randomised trial. They are the best way to get reliable information about how well a new treatment works. We have more information about randomisation .
A multi arm trial is a trial that has:
- several treatment groups as well as
Multi-arm multi-stage (MAMS) trials have the same control group all the way through. The other treatment groups can change as the trial goes on. As these trials are more complex there are a number of treatments that people might have.
The research team may decide to stop recruiting people to a particular group. This could be because they have enough people to start looking at the results. Or because early results show the treatment isn’t working as well as they’d hoped.
The researchers may add new treatment groups as new drugs become available to look at. This means they don’t have to design and launch a brand new trial each time they want to research a new treatment. So it helps get results quicker.
The Stampede trial for prostate cancer is an example of a MAMS trial.
Observational studies Cohort studies, case control studies and cross sectional studies are all types of observational studies.
A cohort is a group of people, so cohort studies look at groups of people. A cohort study follows the group over a period of time.
A research team may recruit people who do not have cancer and collect information about them for a number of years. The researchers see who in the group develops cancer and who doesn’t. They then look to see whether the people who developed cancer had anything in common.
Cohort studies are very useful ways of finding out more about risk factors. But they are expensive and time consuming. They can be used when it wouldn’t be possible to test a theory any other way.
Case control studies
Case control studies work the opposite way to cohort studies. The research team recruits a group of people who have a disease (cases) and a group of people who don't (controls). They then look back to see how many people in each group were exposed to a certain risk factor.
Researchers want to make the results as reliable as possible. So they try to make sure the people in each group have the same general factors such as age or gender.
Case control studies are useful and they are quicker and cheaper than cohort studies. But the results may be less reliable. The research team often rely on people thinking back and remembering whether they were exposed to a certain risk factor or not. But people may not remember accurately, and this can affect the results.
Another issue is the difference between association and cause. Just because there is an association between a factor and a disease, it doesn’t mean that the factor causes the disease.
For example, a case control study may show that people with a lower income are more likely to develop cancer. But it doesn’t mean that the level of income itself causes cancer. It may mean that they have a poor diet or are more likely to smoke.
Cross sectional studies
Cross sectional studies are carried out at one point in time, or over a short period of time. They find out who has been exposed to a risk factor and who has developed cancer, and see if there is a link.
Cross sectional studies are quicker and cheaper to do. But the results can be less useful. Sometimes researchers do a cross sectional study first to find a possible link. Then they go on to do a case control or cohort study to look at the issue in more detail.
Oxford Handbook of Clinical and Healthcare Research (1st edition) R Sumantra, S Fitzpatrick, R Golubic and others Oxford University Press, 2016
Related information
You may find it helpful to read our information about:
What trials are
- Phases of clinical trials
Finding a clinical trial
Last reviewed
Find a trial, about cancer.
- Spot cancer early
- Talking to your doctor
- What is cancer?
- Randomised trials
- How to find a clinical trial
- How to join a clinical trial
- What you should be told about a clinical trial
- How clinical trials are planned and organised
- Clinical trial results
- What to ask your doctor about clinical trials
- Clinical trial organisations
- Children’s cancers
- How do I check for cancer?
- Welcome to Cancer Chat

Rate this page:


Early Phase Cancer Trials Programme at UCL and UCLH
What Different Types of Clinical Trials Are There?

Different Types of Clinical Trials
In medical research there are two main types of studies:
- Observational Studies – These studies do not involve a new intervention or treatment option. They aim to find out information by observing the people taking part and see what happens in different situations.
- Interventional Trials – These aim to find out information about a particular intervention or treatment. Intervention Trials, called Clinical Trials, are the gold standard for finding out if a new intervention or treatment option(s) is suitable for people.
Clinical Trials:
Researchers will run clinical trials in stages. The early phases aim to find out more about the safety and side effects of a new treatments. Later phases aim to see if a new treatment works better than the current treatment.
DIFFERENT STAGES OF A CLINICAL TRIAL
- Phase I - Trials involves a small number of people (~20-50 people) and looks at what happens to the treatment in the body and if there are any side effects. The trial also looks to find the best dose for the treatment.
- Phase II - Trials involve a medium number of people (~over 100 people) and looks at how well a treatment works.
- Phase III - Trials involve many people (~100s or 1000s of people) at compares the new treatment against the standard treatment option to check if the new treatment is better and/or has fewer side effects.
- Phase IV - These trials involve 100s or 1000s of people depending on the investigation and look at finding out more about the long-term benefits and side effects of the new treatment.
Clinical Trial Designs
There are many different designs to clinical trials. These influence how the study is run and managed, how patients are recruited to the study, the endpoints and results the study is trying to work out and how the study intervention/treatment is given to patients.
Here we described a few different trial designs which you may come across in your visit to the Clinical Research Facility:
First-In-Human (FIH) Studies:
First-in-human (FIH) studies are a type of clinical trial in which a new drug, procedure, or treatment is tested in humans for the first time.
First-in-human studies take place after the new treatment has been tested in laboratory and animal studies and are usually done as Phase I clinical trials.
Dose Escalation Studies:
These trials can sometimes to be referred to as Phase I Dose-Finding Studies.
A study that determines the best dose of a new drug or treatment. In a dose-escalation study, the dose of the test drug is increased a little at a time in different groups of people until the highest dose that does not cause harmful side effects is found - called the Maximum Tolerated Dose (MTD).
The trial will aim to find the MTD which does not exceed a pre-set limit of side-effects - called the Dose Limiting Toxicities (DLT).
Together with these two points of data, the trial can find the Recommended Phase II Dose (RP2D). If successful, this will be the dose which can be used in Phase II.
A dose-escalation study may also measure ways that the drug is used by the body.
This design of trial is usually part of a Phase I clinical trial.
Dose Expansion Studies:
These trials are sometimes done to compliment Dose Escalation Trials by further investigating to find the Recommended Phase II Dose (RP2D).
These trials may have stricter criteria and may look at disease-specific cohorts. They are usually done in the same Phase I trial as Dose Escalation Studies.
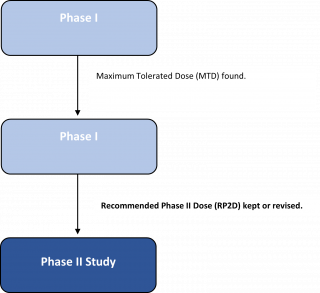
Master Protocol Trials
A Master Protocol Clinical Trial includes multiple subgroups and sub studies, with patients having same or different diseases and that employ one or multiple interventions/drugs to treat it.
This type of Protocol design has been developed for, and is common across, Oncology, in which one investigational drug/treatment and/or more than one cancer type can be investigated within the same trial structure.
There are three types of Master Protocol Designs: Basket Trials, Umbrella Trials and Platform Trials.
Basket Trials:
Also referred to as Multiple Cohort Clinical Trials.
In this trial design patients from different disease groups or subgroups, such as those with different types of cancer, are identified within those groups based on the presence of a specific factor.
Basket designs are intended to study a single investigational regimen in a number of different diseases or disease subtypes.
A basket trial enrols patients with different cancer types and/or different cancer subtypes but who share a common link, usually having the same genetic or molecular abnormality.
For example, the trial would test a new intervention or treatment in a select number of different cancer types (e.g., Colon, Breast, Lung, and others) and/or different cancer subtypes (e.g., Lymphoma Cancers - Hodgkin Lymphoma, Non-Hodgkin Lymphoma, Chronic lymphocytic Leukaemia (CLL), and others).
Patients with that specific ‘common factor’ who take part in Basket Trials are grouped together in one cohort, or “basket,” to receive a new intervention/treatment which is designed to work, or to work best, in that specific category of disease.
Umbrella Trials:
Umbrella trials, on the other hand, evaluate multiple different targeted interventions/treatments for a single cancer type or sub-type that is stratified into subgroups by molecular alternation.
An umbrella trial enrols patients with one cancer type but with different genetic changes within each tumour. It consists of many small sub-trials to test multiple drugs simultaneously in one large trial.
Patients receive different targeting treatments matched to their genetic aberration. The term “umbrella” refers to separation of one alleged cancer into many sub-cancers depending on their molecular features. There is also a “default arm” which assigns patients without a specific marker to receive standard treatment.
Basket trials and umbrella trials both employ a molecular screening protocol that allows either recruitment of different diseases with the common molecular alteration(s) or that differentiates the single disease into different molecular subtypes.
Platform Trials:
Also referred to as Multi-Arm, Multi-Stage (MAMS) design trials.
A multi arm trial is a trial that has:
- several treatment groups as well as
- the standard treatment group (the control group Open a glossary item)
Multi-arm multi-stage (MAMS) trials have the same control group all the way through. The other treatment groups can change as the trial goes on.
The research team may decide to stop recruiting people to a particular group. This could be because they have enough people to start looking at the results. Or because early results show the treatment isn’t working as well as they’d hoped.
The researchers may add new treatment groups as new drugs become available to look at. This means they don’t have to design and launch a brand-new trial each time they want to research a new treatment. So, it helps get results quicker.
Office of Academic Clinical Affairs
Masonic Cancer Center
- Clinical Trials
- Partners and Locations
- For Patients
- For Researchers Overview
- Grant Program
- Brand Guidelines & Logos

Childhood Cancer Awareness Month: Children & Clinical Research
About childhood cancer.
Childhood Cancer Awareness Month is celebrated each September around the world with the goal of increasing awareness of childhood cancer and advocating for pediatric cancer research and funding. It’s estimated that about 400,000 children and adolescents develop cancer globally each year. The most common types of cancer diagnosed in children are leukemia, brain and other central nervous system (CNS) tumors, and lymphoma. More than 100 different subtypes of childhood cancers exist.
Cancer in children isn’t necessarily the same as cancer in adults. For example, cancer in children is almost never caused by lifestyle choices, behaviors, or exposures. For children, cancer is often caused by genetics and DNA mutations, so it is more likely to be random. Sometimes childhood cancer even begins developing before a child is born. Childhood cancer also can be treated differently, and there are several specific drugs created just for pediatric cancers. While these treatments are effective, they can have long-term side effects and lead to secondary cancers later in life as children grow up and live decades beyond their initial childhood cancer.
In the United States, the five-year survival rate for pediatric cancers is 83-88%. This remarkable rate is due to childhood cancer research that developed successful diagnostic tests and treatments. However, not all pediatric populations have equitable access to cancer diagnosis and treatment, especially in lower or middle-income countries. Childhood cancer organizations work to bring awareness and funding to these populations to close treatment and diagnosis gaps.
Children & Clinical Trials
Overall, childhood cancer treatments have advanced quickly due to clinical research. Treating cancer and conducting research with children have unique considerations. It is important that cancer treatments are given to children of a variety of ages in clinical trials. This is because cancer treatments and drugs affect people differently. A child’s body may not react the same way an adult’s body would. As children grow and develop, their responses to medications also change. All age groups are needed in clinical trials to determine safety, effectiveness, side effects, and appropriate dosage for new treatments. Clinical trials for children might also determine the optimal form of a drug or device, like a liquid instead of a pill since it’s easier for children to swallow.
Children are also considered a vulnerable population. All clinical trials have regulations and safety measures in place. But, there must be even more safeguards and ethical considerations in mind when conducting research with children, especially in balancing benefits and risks of a clinical trial. This typically means that children will be the last population a treatment is given to, and the drug will already have been proven safe and effective in animals, cell models, and adults. Because children are under the age of 18, their participation must ultimately be decided by both the child and by a parent or guardian, who will be involved throughout the entire enrollment, informed consent, and clinical trial processes.
Research into Life Beyond Childhood Cancer
Another area of childhood cancer research that does not necessarily involve children is childhood cancer survivorship research. Because the survival rate for childhood cancer is so high, there are many unique concerns and questions about health outcomes for childhood cancer survivors as they reach adulthood and old age. Common concerns and issues for childhood cancer survivors include mental health issues, accelerated aging, infertility, second cancers, and reduced life expectancy. Research is needed to fully understand the consequences and effects of childhood cancer and treatments. Studies in this area typically occur by following a cohort, or group, of childhood cancer survivors over time to track their health outcomes. This type of research is considered an observational study called a cohort study.
One such study, the Childhood Cancer Survivor Study (CCSS), began in 1994 to better understand childhood cancer survivors and issues they experience, increase survival, and minimize harmful health effects of cancer treatments. The study included more than 14,000 childhood cancer survivors who were diagnosed between 1970 and 1986, as well as 4,000 siblings for comparison. A second group of 10,000 survivors that were diagnosed between 1978 and 1999 and 1,000 of their siblings were later added as well. The study participants were surveyed and followed over the years to monitor their long-term health outcomes. Potential late effects of childhood cancer that have been found from the study include premature menopause, stroke, and subsequent cancers. Data from the study has also been used in many other childhood cancer survivorship studies as well.

Learn More
To learn more about childhood cancer and identify ways to raise awareness and advocate for childhood cancer research, visit the American Childhood Cancer Organization and the Children’s Cancer Research Fund .
- Open access
- Published: 30 August 2024
Re-analysis of single cell and spatial transcriptomics data reveals B cell landscape in gastric cancer microenvironment and its potential crosstalk with tumor cells for clinical prognosis
- Xing Cai 1 , 2 na1 ,
- Jinru Yang 1 , 2 na1 nAff6 ,
- Yusheng Guo 3 , 4 na1 ,
- Yanchao Yu 5 ,
- Chuansheng Zheng 3 , 4 &
- Xiaofang Dai 1 , 2
Journal of Translational Medicine volume 22 , Article number: 807 ( 2024 ) Cite this article
Metrics details
At present, immunotherapy has become a powerful treatment for advanced gastric cancer (AGC), but not all patients can benefit from it. According to the latest research, the impact of B cell subpopulations on the immune microenvironment of gastric cancer (GC) is unknown. Exploring whether the interaction between B cells and tumor cells in GC affects the effectiveness of immunotherapy has attracted our interest.
This study involved the re-analysis of single-cell RNA (scRNA) and spatial transcriptomics (ST) data from publicly available datasets. The focus was on investigating the subpopulations and differentiation trajectories of B cells in the gastric cancer (GC) tumor immune microenvironment (TIME). Spatial transcriptomics (ST) and multiple immunofluorescence (mIF) revealed a clear co-localization pattern between B cells and tumor cells. Multiple immunotherapy datasets were collected to identify unique immunotherapy biomarkers. The unique immunotherapeutic potential of targeting CCL28 was validated through a mouse gastric cancer model. In addition, flow cytometry revealed changes in the tumor immune microenvironment targeting CCL28.
The re-analysis of ST data from multiple cancer types revealed a co-localization pattern between B cells and tumor cells. A significant number of IgA plasma cells were identified in the GC TIME. Five different tumor-infiltrating B cell subpopulations and two unique B cell differentiation trajectories were characterized, along with seven GC-related states. By analyzing the communication between GC cells and B cells, it was further discovered that tumor cells can influence and recruit plasma cells through CCL28-CCR10 signaling. Additionally, there was a crosstalk between GC cells and B cells. Finally, we identified the LAMA/CD44 signaling axis as a potential prognostic marker for immunotherapy through a large amount of immunotherapy data. We also validated through various animal tumor models that targeting CCL28 can significantly promote CD8 + T cell infiltration and function in the TME by regulating B cell and plasma cell functions, and has the ability to synergize immunotherapy.
The co-localization and crosstalk between GC cells and B cells significantly affect the efficacy of immunotherapy, and inhibiting the CCL28-CCR10 signal axis is a potential immunotherapy target for GC. Meanwhile, LAMA/CD44 pair may be a potential adverse indicator for immunotherapy and tumor prognosis.
Introduction
At present, the incidence and mortality rate of gastric cancer (GC) are relatively high worldwide, which seriously threatens the life expenditure and quality of human life. According to Global Cancer Statistics 2020 [ 1 ], the incidence rate ranks fifth and the mortality ranks fourth, which is at a high level in all tumors. The vast majority of GC patients are already in the advanced stage when discovered. Currently, common treatment strategies such as surgery, radiotherapy, and chemotherapy have little efficacy for advanced gastric cancer (AGC) patients. With the improvement of our understanding of the tumor microenvironment (TME), immunotherapy is gradually emerging, greatly improving the survival rate and time of patients with AGC. In 2021, the Food and Drug Administration (FDA) officially approved the combination of nivolumab and chemotherapy for first-line treatment of advanced or metastatic GC, gastroesophageal junction, and esophageal adenocarcinoma patients. This is the first FDA approved first-line immunotherapy for gastric cancer and an important milestone in advanced GC immunotherapy. The immune escape of tumor cells is one of the important mechanisms of GC pathogenesis. Programmed death factor ligand 1 (PD-L1) expressed on tumor cells binds to PD-1 on activated T lymphocytes to provide inhibitory signals, induce T cell apoptosis, and thereby suppress immune response. Immune checkpoint inhibitors (ICIs) such as PD-1/PD-L1 inhibitors specifically block the interaction between the two, enhance the immune activity of T cells, inhibit tumor immune escape, and kill tumor cells. The high expression of PD-L1 in GC tissue provides a possibility for ICIs to be used for the treatment of advanced GC. However, not all patients can benefit from immunotherapy, which attracted our attention. Why do some patients benefit while others do not? Is it due to the different tumor immune microenvironment (TIME)? If the principles and mechanisms can be found, it will greatly help to improve the treatment efficacy of AGC patients.
TME refers to the internal environment in which tumors occur and live, including not only the tumor cells themselves, but also various cells closely related to tumor cells, such as fibroblasts, immune and inflammatory cells, glial cells, etc [ 2 ]. In the past decade or so, the mechanism of immune cells in controlling the occurrence and development of cancer has gradually become clear. The role of T cells in tumor immune monitoring has been widely studied, but there is relatively little research on B cells. In recent years, some studies have found that B cells play a huge role in anti-tumor immunity, and their presence and function can be an important factor in cancer prognosis [ 3 ]. B cells are a type of cell with the ability to secrete antibodies, derived from multifunctional stem cells in the bone marrow. On the one hand, B cells stimulated by antigens will proliferate and differentiate into a large number of plasma cells, secrete antibodies and circulate in the blood, indirectly exercising their functions through antibody mediated cytotoxicity (ADCC). On the other hand, as an antigen presenting cell (APC), B cells can directly activate T cells and macrophages, which is particularly prominent in the TME. In tumor areas infiltrated by plasma cells, even in small quantities, a large number of cytokines and antibodies can be produced, driving ADCC and phagocytosis, complement activation, and enhancing the antigen presentation of dendritic cells (DC), promoting tumor immunity [ 4 ].
In our study, single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics (ST) sequencing techniques were used to investigate the immune landscape of tumor tissue in GC patients and found its correlation with B cells. However, the functional of B cells in tumors is still unclear, especially in the GC microenvironment. The communication between cancer cells and immune elements is a determining factor for tumor progression or regression. In addition, the influence of tumor infiltrating lymphocytes (TIL) on tumor growth and treatment response is becoming increasingly evident. Many studies have focused on the role of T cell response in anti-tumor immunity, and little is known about the role of B cells in solid tumors. This study provides a detailed analysis of the infiltrating B cell status and tumor cell characteristics of GC. The spatial co-localization of B cells and tumor cells further reveals the potential therapeutic value of the signal axis targeting B cells and tumor cells. We also found that targeting CCL28 may promote the therapeutic effect of PD1 monoclonal antibody, and the LAMA-CD44 score may have unique clinical immunotherapy predictive value.
Materials and methods
Gene expression and clinical data acquisition.
In total, gene expression profiles of GC tissues and normal adjacent tissues were obtained from the Cancer Genome Atlas, TCGA-STAD (n = 373) database, and 9 public Gene Expression Omnibus (GEO) datasets GSE84437 (n = 357), GSE115821 (n = 37), GSE126044 (n = 16), GSE135222 (n = 27), GSE179351 (n = 54), GSE19860 (n = 40), GSE35640 (n = 65), GSE78220 (n = 28), and GSE79691 (n = 10), respectively. The metaGSE cohort was combined from all GEO datasets. The single cell and the spatial transcriptome profiles were obtained from OMIX001073 [ 5 ] and GSE203612, respectively. The gene expression profiles with immunotherapy information were obtained from 12 published datasets, including Nathanso cohort (n = 24) [ 6 ], CheckMate (n = 250) [ 7 ], and IMvigor210 (n = 298) [ 8 ]. All data analyzed during this study are freely available in previous publications or the public domain all bulk-seq datasets were converted to transcripts per million (TPM) format and corrected for batch effects using the “combat” function of the “sva” package. Prior to analysis, all data were log-transformed.
Single-cell RNA sequencing (scRNA-seq) data processing
Single-cell RNA sequence (scRNA-seq) data from OMIX001073 [ 5 ] cohorts were obtained in the study. We created Seurat objects for total and individual cell types belonging to the scRNA-seq gene expression matrix using the R package “Seurat”, then ScaleData and RunPCA functions were performed to obtain the number of principal components (PC) based on the Seurat objects. We used uniform manifold approximation and projection (UMAP) dimensionality reduction to further summarize the top principal components. Finally, with the annotated information for each cell in GC supported by the previous article [ 9 , 10 ], the Idents and Dimplot functions were used to annotate and visualize the cells of the major TME cell types or subtypes. The FindMarkers function was used to list the markers of each cluster of each cell type in GC, with selection parameters including logFc.threshold = 1, min.pct = 0.25, only.pos = T. Based on these marker genes among different TME cell types, the “clusterprofiler” R package was used to detect Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database, and gene sets with adjusted p-value < 0.05 were considered significantly enriched. The Cellchat R package was used to calculate the interaction strength of cell–cell communication, and further calculate the L/R signal scores through Lmean + Rmean, representing the average expression value of ligand genes plus receptor gene expression value. In addition, the PAGA, and Slingshot methods in SCP R package were applied to reconstruct the differentiation trajectories and infer cell differentiation states. Furthermore, the pySCENIC package, a Python-based implementation of the SCENIC pipeline was used to investigate the gene regulatory network of transcription factors (TFs) in GC. Furthermore, the ‘SCP’ R package was used during the data visualization process.
Spatial transcriptomics data analysis
To explore the spatial specific immune microenvironment in tumor tissue, we employed 10 × Genomics spatial transcriptome (ST) technology in GSE203612 [ 11 ] cohort, which the tissues were collected from 19 fresh primary untreated patient tumors immediately after surgery, with 9 cancer types, including carcinoma of the ovarian cancer (OVCA), uterine corpus endometrial carcinoma (UCEC), breast invasive carcinoma (BRCA), prostate adenocarcinoma (PRAD), kidney clear cell carcinoma (KIRC), liver hepatocellular carcinoma (LIHC), colon adenocarcinoma (COAD) and pancreatic ductal adenocarcinoma (PDAC), as well as gastrointestinal stromal tumor (GIST).
Multiplex immunofluorescence staining and imaging
We collected section from paraffin-embedded tissues of human gastric cancer and peritumor from Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Multiplex immunofluorescence (mIF) analysis was performed for detection of EPCAM, CCL28, CCR10, and CD138. All the images were captured using a Leica DM 2500 microscope.
Establishment of animal models and flow cytometry
To verify our hypothesis in vivo, we constructed subcutaneous tumor models using two cell lines, the mouse gastric cancer MFC cell line and the mouse colon cancer MC38 cell line, on 615 mice (n = 24) and C57 mice (n = 24), respectively. The shCCL28 cell line was transfected with lentivirus and inoculated (CCL28-1 F:CCGGGCTGTCATCCTTCATGTTAAACTCGAGTTTAACATGAAGGATGACAGCTTTTTG; R:AATTCAAAAAGCTGTCATCCTTCATGTTAAACTCGAGTTTAACATGAAGGATGACAGC; CCL28-2 F:CCGGCCCGCACAATCGTACTTTGAACTCGAGTTCAAAGTACGATTGTGCGGGTTTTTG;R:AATTCAAAAACCCGCACAATCGTACTTTGAACTCGAGTTCAAAGTACGATTGTGCGGG; CCL28-3 F:CCGGCTGAGGTGTCTCATCATGTTTCTCGAGAAACATGATGAGACACCTCAGTTTTTG;R:AATTCAAAAACTGAGGTGTCTCATCATGTTTCTCGAGAAACATGATGAGACACCTCAG). The experiment was divided into four groups: IgG group, shCCL28 group, anti PD-L1 group, and shCCL28 + anti PD-L1 (Comb) group, with 6 mice in each group. Each mouse was inoculated with 3 × 10^5 tumor cells, then the size and weight of the tumor were measured. Further, flow cytometry was used to observe the TME, we detected the extracellular (CD45, CD3, CD4, CD8, CD19, CD20, CD11b, CD11c, F4/80, CD86, and NK1.1) and intracellular indicators (CD206, GZMB, and IFNγ), respectively.
Related in vitro experiments
Besides, the effects of CCL28 gene on MFC and MC38 tumor cells were verified through relevant in vitro experiments, including cell proliferation experiment, colony formation assay, cell cycle checkpoint detection, apoptosis flow cytometry, and cell invasion and migration experiment, the experiment was conducted according to previously published protocols [ 12 ].
Detection of CCL28
Collect CTRL and shCCL28 cell culture supernatant for CCL28 detection. According to the instructions for using the FineTest CCL28 ELISA kit (EM1932), take the kit at room temperature. Add 100 μL diluted standard and test sample, 100 μL Detection Reagent A, 100 μL Detection Reagent B, 90 μL Substrate Solution, and 50 μL Stop Solution to each hole in sequence, and finally measure the absorbance using an enzyme-linked immunosorbent assay (ELISA) reader.
Statistical analysis
R software (version 4.1.3) was used for statistical analysis and draw charts. Correlations between two continuous variables were assessed via spearman’s correlation coefficients. The Chi-square test and student's-t test were used for categorical variables and continuous variables, respectively. The “survminer” package was used to determine the optimal cut-off value. Cox regression and Kaplan–Meier analyses were performed via the survival package. All tests performed were two sided, and P values < 0.05 indicated statistically significant (* meant p value < 0.05, ** meant p value < 0.01, *** meant p value < 0.001, **** meant p value < 0.0001, ns meant p value > 0.05).
Spatial transcriptomic revealed B cell aggregation around tumor cells in various tumor microenvironments
Re-analysis of various tumor spatial transcriptome (ST) data, including breast invasive carcinoma (BRCA), gastrointestinal stromal tumor (GIST), liver hepatocellular carcinoma (LIHC), ovarian cancer (OVCA), pancreatic ductal adenocarcinoma (PDCA), and uterine corpus endometrial carcinoma (UCEC), we found that B cell aggregation typically existed in the tumor microenvironment (TME), and the proximity of the B cell population to tumor cells indicated crosstalk between B cells and tumor cells. As shown in Fig. 1 A and sFig.1A,B, the B cells identified in BRCA sections were very close to the tumor cells in spatial distribution, and the IgG and IgA antibody genes in cancer samples had similar abundance, which was similar to the literature reports [ 13 ]. While in GIST, the expression of IgA gene was dominant (Fig. 1 B), in OVCA, the secretion of IgG antibodies was dominant (Fig. 1 C), in PDCA, the abundance of B cells identified was low (Fig. 1 D), in UCEC, the secretion of IgG antibodies was dominant (Fig. 1 E), and in LIHC, rich expression of IgG antibody genes was observed (sFig.1D) [ 14 , 15 ].

Spatial distribution of B cells in various tumor immune microenvironments and their relationship with regional tumor epithelium. A - E the spatial distribution of tumor epithelial cells, B cells, IgA, and IgG antibody genes in BRCA ( A ), GIST ( B ), OVCA ( C ), PDCA ( D ), UCEC ( E )
ScRNA-seq landscape of infiltrating B cell subsets in GC
To generate a thorough transcriptional atlas of B cells in GC, we first reanalyzed the public scRNA profiles of 166,533 cells from 10 GC patients in a previous study [ 5 ] and identified 12 distinct clusters, including T cells & NK cells (98809), myloid cells (6572), B cells (16247), mast cells (2896), erythrocytes (69), B cells (plasma cells) (6201), epithelial cells (20535), endothelial cells (2911), endocrine cells (1843), fibroblasts (3111), and smooth muscle cells (1424) (Fig. 2 A). After further subcluster analysis by extracting all B cells and plasma cells, we identified naïve_B cells in CD20 + cells, germinal center B cells (GC_B cells), memory_B cells, and at the same time, a group of plasma_cells expressed CD138 were also identified (Fig. 2 B). Being consistent with a previous report [ 16 ], naïve_B cells were characterized by the specific expression of PRPSAP2, MYO1E, ELL3, LRMP, and RGS13, GC_B cells were characterized by YBX3, IL4R, TCL1A, FCER2, and IGHD, memory_B cells were characterized by LDLRAD4, NR4A2, TNFRSF13B, AIM2, CD27, whereas plasma_cells specifically expressed the markers of JCHAIN, FKBP11, DERL3, MZB1, SDC1, SUGCT (Fig. 2 C). As shown in the bar chart (Fig. 2 D), compared to peripheral blood, the TME infiltrates more plasma cells. However, it is interesting that GC_B cells are almost exclusively present in the TME, indicating the presence of a very small number of tertiary lymphoid structures (TLS) in the GC microenvironment, which is consistent with previous reports [ 17 , 18 ].

B cell profiles of immune microenvironment in human gastric cancer. A Unified manifold approximation and projection (UMAP) of 166,533 single cells from 10 patients, colored by main cell types. B Umap plot of B cells. C Bubble plot showing the expression of marker genes in major B cell subpopulations. D Histography of the different B cells components in each tissue. E Scatter plot showing the independent cell distribution based on the expression of IgG and IgA. F Bubble plot showing the expression of marker genes in major plasma cell subpopulations. G Histography of the different plasma cells components in each tissue. H Umap plot showing the gene expression of CCL28 (Up) and CCR10 (Down). I The mIF showed the expression levels of EPCAM, CCL28, CCR10, and CD138 in NR (PFS = 2.07 m, up) and R (PFS = 11.1 m, down)
Antibody class switching to IgA in the GC tumor microenvironment
The distribution of plasma cells and antibody isotypes in the GC TME was not yet clear, therefore, we further analyzed the plasma cell infiltration status of GC patients. Principal component analysis (PCA) was used for dimensionality reduction based on antibody genes. We observed that cells with high expression of IGHG or IGHA constituted almost all principal components (PC), and there was overlap in cells with multiple IgA subtypes [ 19 ] (sFig.2D). The scatter plot also showed significant differences between the two cell types (Fig. 2 E), which indicated that IgA PC was the main PC type for GC. Similarly, from the results of bulk RNA-seq, we found that the expression levels of IgA related genes (IGHA1, IGHA2) in tumor samples were higher than those of IgG related genes (IGHG1, IGHG3, and IGHG4) (sFig.2A). To further explore its functional characteristics, cells with high IGHG and low IGHA expression were defined as IgG PC, while cells with low IGHG and high IGHA expression were defined as IgA PC (Fig. 2 F–G, sFig.2B, C). Among them, IgA PCs were mainly involved in the regulation of phagocytic pathways, such as phagocytosis, and engulfment, while IgG PCs were associated with B cell immune response and MHC class I antigen presentation, such as regulation of B cell activation, indicated that IgG PCs might be more likely to participate in anti-tumor immune regulation, and IgA PCs might be more involved in promoting tumor immunity [ 20 ]. To investigate the effects of IgA and IgG PC on cancer progression and patient survival, we analyzed the correlation between plasma cell infiltration and patient prognosis in the TCGA-STAD cohort. The results showed that the higher the abundance of IgA infiltration, the shorter the patient's survival time. However, IgG plasma cells did not show any correlation with prognosis (sFig.3B). Surprisingly, the bar graph shows that there are no more IgA plasma cells in the GC TME than in the surrounding and peripheral blood, indicating that IgA PC in the GC microenvironment mainly comes from the periphery. Therefore, tumor cells may recruit IgA PC through certain signaling pathways (Fig. 2 G). Therefore, we analyzed the cellular relationship between B cells and tumor cells in the GC microenvironment, and the results showed that CCL28 is mainly expressed by tumor cells, while CCR10 is mainly expressed in plasma cells as shown in Fig. 2 H, which might be one of the mechanisms underlying the abundance of IgA PCs in the GC microenvironment. The multiple immunofluorescence (mIF) also validated the CCL28-CCR10 axis difference between immune therapy responder (R) and non-responder (NR) (Fig. 2 I, sFig.2E). Therefore, targeting the CCL28-CCR10 axis may be one of the potential targets for enhancing immunotherapeutic efficacy in GC. Through patient samples underwent immunotherapy, it was found that CCL28 mainly originated from tumor epithelial cells, while CCR10 and CD138 were co-located. At the same time, patients with high expression of CCL28 had more CD138 + cells and a shorter survival time, while patients with low expression of CCL28 had a longer survival time.
Gastric cancer infiltrating CD20 + B cells exhibited two unique differentiation trajectories
In order to further analyze the subtype status of B cells infiltrating in gastric cancer, we identified five B cell clusters by extracting all CD20 + B cells for further subcluster analysis, including B_cells_C1_RGS13, B_cells_C2_TCL1A, B_cells_C3_CD99, B_cells_C4_GPR183, and B_cells_C5_NR4A1 (Fig. 3 A). For example, B_cells_C1_RGS13 were mainly associated with aerobic respiration, B_cells_C2_TCL1A were mainly associated with B cell activation, B_cells_C3_CD99 were mainly associated with cytoplasmic translation, B_cells_C4_GPR183 were mainly associated with regulation of lymphocyte proliferation, and B_cells_C5_NR4A1 were mainly associated with intrinsic apoptotic signaling pathway (sFig.4A-E). Figure 3 B showed the corresponding markers of each cluster. The bar chart showed that compared to peripheral blood, gastric cancer microenvironment infiltrates more C1, C4, and C5 (Fig. 3 C). Furthermore, we determined the possible involvement of regulons in all five cluster B cells by single-cell regulatory network inference and clustering (SCENIC) analysis (Fig. 3 D), to determine the changes in transcription factors (TFs) during B cell differentiation.(C1: CUX1 [ 21 ], H2AFY [ 22 ], MYBL1 [ 23 ], ZNF236 [ 24 ], SMARCA4 [ 25 ]; C4: FOXO3 [ 26 ], ZFHX2 [ 27 ], RXRA [ 28 ], RXRB [ 29 ], GRHL2 [ 30 ], TGIF1 [ 31 ]). At the same time, the unique state of C1-C5 B cells was also displayed in the functional enrichment analysis (Fig. 3 I, sFig.4F). In pseudotime analysis, we found that C1 cells were located at the beginning of the cell trajectory, while C4 and C5 were located at the end, respectively (Fig. 3 E-G). Figure 3 H showed high abundance of TGFb expression in GC infiltrating B cells, while C4 cells in the terminal differentiation state expressed IL-10, and C5 cells secreted IL-35, further suggesting that GC infiltrating B cells gradually evolved into B cells in different immunosuppressive states. Previous studies have reported the detection of Breg cells in the peripheral blood of GC, which were immunosuppressive cells characterized by IL-10, IL-35, TGFb, and PD1, thereby promoting tumor progression [ 32 , 33 ]. Interestingly, cells exhibited high expression of NR4A1, NR4A2, ZFP35, and EGF1 in lineage 2, which was not present in lineage 1, indicated differences in the function of CD20 + B cells with two different lineages. For example, lineage 2 is associated with response to amphetamine, fat cell differentiation, response to amine, interleukin production, T cell differentiation, etc. (Fig. 3 I). These data indicated that GC infiltrating CD20 + B cells had unique immunosuppressive functions and important subtypes that affect the effectiveness of immunotherapy.

Human gastric cancer CD20 + B cells exhibit two unique differentiation trajectories. A UMAP visualization of 14,536 CD20 + B cells across 10 cancer patients. B Bubble plot showing the expression of tag genes between B cells clusters. C Histography of the different B cells components in each tissue. D Regulons enriched in each B cell cluster detected via pySCENIC analysis. E–H Developmental trajectory of B cells in five different states inferred by PAGA (E) and Slingshot (F) , colored by Lineage1 and Lineage2 (G) . The UMAP plots showed the expression levels of IL10, IL12A, TGFB1, and EBI3 genes in CD20 + B cells (H) . The scale represents the predictive differentiation trajectory. The higher value represents the degree of differentiation. I Heatmap display of the expression of highly variable genes (left) and GO, KEGG pathway functional enrichment (right) along the pseudotime of the Lineage1 trajectory and the Lineage2 trajectory
Identification of seven tumor cell states
Due to the high heterogeneity of tumor cells, we conducted non-negative matrix factorization (NMF) analysis, and identified a total of 40 metagenes that were preferentially co-expressed by subpopulations of malignant cells across tumors. Then, hierarchical clustering was used to characterize these 40 metagenes into gene expression signatures, and high concordance was shown among their signatures (Fig. 4 A, B ). Seven tumor cell states, including Cell Cycle (140), WH (1258), Metal (1560), OP (727), Interferon (159), Epi_dif2 (2711), and Epi_dif1 (2650) in GC were identified and used to assess the intrinsic functional characteristics of tumor cells (Fig. 4 C–K). The tumor cells in different states were found to be involved in different regulatory functions (Fig. 4 M). For example, Cell-Cycle cells were mainly associated with chromosome segregation, WH cells were mainly associated with regulation of leukocyte migration, Metal cells were mainly associated with cellular transition metal ion homeostasis, OP cells were mainly associated with oxidative phosphorylation, Epi_dif1 cells were mainly associated with MHC protein complex assembly, Epi_dif2 cells were mainly associated with response to virus. Additionally, it is worth noting that interferon-like tumor cells were found to be involved in adaptive immune response. Then, we evaluated the impact of different tumor cell states on patient survival (sFig.5A-H). In the non-immunotherapy TCGA-STAD cohort (n = 373), high expression of Epi-dif1 ( P = 0.026), and cell cycle ( P = 0.054) were associated with better OS, while low expression of Wh ( P = 0.0049), interferon ( P = 0.056), OP ( P = 0.19), Epi-dif2 ( P = 0.015), and OS ( P = 0.013) were associated with better OS.

Seven unique subtypes were identified in human gastric cancer epithelial cells. A Heatmap depicts pairwise correlations of 40 intra-tumoral programs derived from ten tumors. Clustering identifies seven coherent expression programs across tumors. B UMAP visualization of the 7 tumor cell states. C - J 7 epithelial (tumor) cell state scores calculated by the AUCell R package. K The CNV scores (left) and tumor score (right) were different among cells of the six tumor cell states. M Heatmap display of differential genes (left) and GO, KEGG pathway functional enrichment (right) in 7 tumor transcriptional states
Crosstalk landscape between tumor cells and B cells
Next, we studied the communication between all cells. Generally, fibroblasts and smooth muscle cells (SMC) in stromal cells, and myeloid cells and T cells&NK cells in immune cells were usually the strongest signal senders and receivers, respectively (sFig.6A). B cells and plasma cells were more likely to receive signals from stromal cells and send them to T cells and myeloid cells [ 2 , 3 ] (sFig.6B), which indicated B cells and plasma cells might be an important immune mediator regulating intercellular communication in the TME of GC.
When analyzing the pathway between tumor cells and B cells-based L/R pair, ADGRE5, APP, and macrophage migration inhibitory factor (MIF) signaling pathways were the most significant enrichment pathways (Fig. 5 A, sFig.6C,D). We further found that the ADGRE5 signaling pathway was mainly enriched in tumor cells, rather than in B cells. In the MIF signaling pathway, tumor cells were the main output cells, especially interferon like tumor cells, which were also the main signal cells in all pathways, while B cells were the main receiving cells (sFig.6C). In summary, these data indicated that the communication mode between tumor cells and B cells dynamically changed with the progression of GC.
Crosstalk in cellular communication between gastric cancer cells and B cells. A - B Crosstalk pattern diagram showing signaling from tumor cells to B and plasma cells and from B and plasma cells to tumor cells. The internal heatmap shows the communication probability of the selected L/R pair crosstalk between tumor cells of 7 different states and 4 types of B cells and 4 types of plasma cells. The differentially expressed L/R pairs between tumor and normal samples from the TCGA-STAD cohort are marked in red, those between samples from R versus NR patients from IMvigor210 are marked in green, and those in both cohorts are marked in dark green. C - E Bubble plots showing the change in the expression of L/R pairs between tumor and normal samples from the TCGA-STAD cohort ( C ), GSE84437 ( D ) and IMvigor210 cohort ( E ). Pie chart (right) showing the ratio of upregulated to downregulated molecules in the two groups. F The expression of selected genes in tissue sections. G , H The selected genes expression of 11 main cell types and 7 tumor cell states
The L/R pairs of MIF/(CD74, CD44, or CXCR4) and APP/CD74 were the most prominent interactions involved in signal transduction from tumor cells to B cells (Fig. 5 A, B ). Some studies have reported the tumor promoting effects of MIF and APP, and their potential association with B cells may explain other mechanisms of tumor progression [ 34 , 35 , 36 ]. Some L/R pairs mediated signal transduction from tumor cells to B cells, while others mediated signal transduction from B cells to tumor cells, such as MDK/SDC1 [ 37 , 38 ]. In addition, further research was conducted on the expression levels of 81 L/R pairs involved in significant communication between tumor cells and PC. In the TCGA-STAD cohort, 41 molecules were differentially expressed between tumor and normal samples (FDR < 0.05), of which 35.1% were upregulated in tumor samples (Fig. 5 C). In the GSE84437 cohort [ 39 ], 25 molecules were differentially expressed between peritoneal metastatic and non-metastatic samples (FDR < 0.05), of which 24% were upregulated in peritoneal metastasis samples (Fig. 5 D). In the IMvigor210 cohort treated with anti PD-L1 [ 8 ], 15 molecules were differentially expressed in both treatment responsive and non-responsive patients, but only 1% of the molecules were upregulated in responsive patients (Fig. 5 E). These data suggested that B-cell signal perturbations may significantly affect the response of cancer patients to immunotherapy. Therefore, we speculated that global crosstalk between tumor cells and PC might promote cancer development and be associated with adverse reactions to immune checkpoint blockade (ICB) treatment. In order to further select reliable L/R pairs between tumor cells and B cells, we evaluated their expression levels in tissue slices using ST data analysis. Based on the expression and biological localization analysis of L/R pairs, we identified four pairs of L/R pairs (LAMA3/CD44, MDK/SDC1 (Fig. 5 F), CCL28/CCR10, and GDF/TGFBR2 (sFig.7A,B)) with high reliability between tumor cells and B cells and these L/Rs were also observed in tumor epithelial cells and B cells (Fig. 5 G, H).
LAMA-CD44 crosstalk between GC cells and B cells is a potential predictive marker for immunotherapy and tumor prognosis
To analyze the clinical value of B cell and tumor cell crosstalk, we calculated a score based on 37 L/R pairs and then conducted a univariate COX regression analysis based on average value of receptor gene expression (GeneRmean) and ligand gene expression (GeneLmean). As shown in Fig. 6 E (left), in Nathanso cohorts (n = 24), the crosstalk of LAMA (LAMA3/5), LAMB (LAMB2/3), LAMC (LAMC1/2), and CD44 was associated with poor prognosis of GC, with most of them were significant ( P < 0.05). Both L/R pairs were associated with poor prognosis, indicated that the relationship between tumor epithelial cells and B cells in GC microenvironment might be related to promoting tumor progression. Also, in two large immunotherapy cohorts (CheckMate (n = 250), and IMvigor210 (n = 298)), LAMA/CD44 pairs were associated with poor prognosis. Therefore, we further calculated the AUC values of these L/R pairs for predicting immunotherapy effects in nine immunotherapy cohorts, including GSE115821, GSE126044, GSE135222, GSE179351, GSE19860, GSE35640, GSE78220, GSE79691, and Nathanso cohort (Fig. 6 A (right)). LAMA/CD44 pairs had a higher AUC value (average 0.7), indicated that the LAMA/CD44 axis might be a potential immunotherapy prediction marker for GC immunotherapy. LAMA/CD44 in the TCGA-STAD cohort was also associated with poor disease-free survival (DFS) ( P = 0.026), and in multiple immunotherapy cohorts, LAMA/CD44 pair was also associated with poor OS.

LAMA-CD44 crosstalk between GC cells and B cells is a potential predictive marker for immunotherapy and tumor prognosis. A Forest plot (left) showing the hazard ratio (HR) of 37 L/R pairs based on stepwise Cox regression analysis. Heat map (right) displays a score based on 37 L/R pairs to predict the corresponding AUC values of immunotherapy across 8 immunotherapy cohorts. B Kaplan–Meier curves of DFS according to the LAMA-CD44 pair score in TCGA-STAD cohort. C - F Kaplan–Meier curves of OS according to the LAMA-CD44 pair score in mateGSE cohorts ( C ), checkmate cohorts ( D ), Nathanso cohorts ( E ), and IMvigor210 cohorts ( F ). G Heatmap showing the correlation between LAMA-CD44 pair score and immune checkpoint molecules. (H-M) The distribution of LAMA-CD44 pair score between R and NR of immunotherapy in GSE7961 ( H ), GSE19860 ( I ), CheckMate cohorts ( J ), Nathanso cohorts ( K ) and IMvigor210 cohorts ( M )
To explore the immune status reflected by the LAMA/CD44 score, we analyzed the relationship between the LAMA/CD44 score and immune modulators. As results shown in Fig. 6 G, high LAMA/CD44 score suggested higher levels of immune infiltrating cells and immune modulators, indicated that high LAMA/CD44 was a relatively immunosuppressive microenvironment. The combination of LAMA/CD44 blockers and immunotherapy might be a new treatment method for GC. Subsequently, in GSE7961 ( P = 0.067), GSE19860 ( P = 0.013), CheckMate cohorts ( P = 0.072), Nathanso cohorts ( P = 0.038), and IMvigor210 cohorts ( P = 0.016), compared to immunotherapy non-responders (NR), immunotherapy responders (R) had lower LAMA/CD44 scores (Fig. 6 H-M). These data indicated that LAMA/CD44 had the potential to become a unique prognostic factor for clinical immunotherapy.
Targeting CCL28 significantly inhibited tumor growth, activated TIME, and enhanced anti-PDL1 effect
The specific expression of CCL28 in tumor epithelial cells may be a unique potential target, in order to verify that targeting CCL28 can synergistically enhance the efficacy of immunotherapy, we conducted in vivo and in vitro experiments. In the experiment, we used two cell lines, the mouse gastric cancer MFC cell line and the mouse colon cancer MC38 cell line. The shCCL28 cell line was transfected with lentivirus on both MFC and MC38 cells. In vitro experiments, we conducted cell proliferation experiment, colony formation assay, cell cycle checkpoint detection, apoptosis flow cytometry, and cell invasion and migration experiment (sFig.7). However, there were no statistical differences in all results, which confirmed that inhibiting CCL28 alone did not work in vitro, and it was speculated that it may work within TME. Therefore, we conducted relevant in vivo experiments. We constructed subcutaneous tumor models using MFC and MC38 tumor cells on 615 mice (n = 24) and C57BL/6 J mice (n = 24), respectively. The experiment was divided into four groups: IgG group, shCCL28 group, anti PD-L1 group, and shCCL28 + anti PD-L1 (Comb) group, with 6 mice in each group. Each mouse was inoculated with 1 × 10 6 tumor cells and the size of the tumor was measured. Finally, we took the photographs of the tumor, a statistical map of the tumor weight, and a growth curve (Fig. 7 A–G, J –M). We performed flow cytometry on tumor tissue to detect the infiltration and function of B, CD3 + T, CD8 + T, CD4 + T, IFN + CD8 + T, GZMB + CD8 + T, MDSC and DC (Fig. 7 H, I, sFig.8, sFig.9C, D). The above results showed that knocking down CCL28 can significantly promote immune cell infiltration and enhance the therapeutic effect of anti PD-L1. To sum up, through cell experiments, we have demonstrated that CCL28 alone does not work in vitro, suggesting that it may play a role in TME. Then, tumor cells were implanted into mice, by analyzing the tumor volume, weight and overall survival rate, we found that knock down CCL28 can significantly inhibit tumor growth, this effect is amplified when combined with anti PD-L1. Further, FASC indicated that targeting CCL28 can significantly promote the infiltration and function of CD8 + T cell by regulating B cell function, and has the ability to synergize immunotherapy.

Inhibiting CCL28 can promote the inhibition of tumor progression and the effectiveness of immunotherapy. A-I 615 mice were implanted with 1 × 10 6 MFC cells and received (1) IgG isotype control (IgG); (2) shCCL28, (3) PD-L1 mAb, or (4) shCCL28 plus PD-L1 mAb (Comb). A schematic view of the treatment plan (A) , tumor image (B) , tumor volume (D , E) , weight (F) , and overall survival (G) were measured every 2 days, n = 6 mice per group. C Representative diagram of flow cytometry. H , I The percentages of tumor-infiltrating B, CD3 + T, CD8 + T, CD4 + T, IFN + CD8 + T, GZMB + CD8 + T, MDSC and DC were analyzed by flow cytometry. n = 5–6/group. J-M The tumor volume (J , K) , weight (L) , and overall survival (M) of MC38 cell line in vivo
In recent years, with the rise of immunotherapy, the survival of tumor patients has been greatly improved. Gastric cancer is a malignant tumor with high incidence rate and mortality rate worldwide. Due to the slow progression of the disease, GC rarely presents early symptoms, with over 70% of patients developing advanced diseases, while the five-year survival rate of patients with AGC is only 6%. At present, in the first-line treatment of advanced gastric cancer, immunotherapy has become a consensus [ 40 , 41 , 42 ]. However, not all AGC patients can benefit from immunotherapy. It is necessary to explore the potential mechanisms and powerful biomarkers to predict the efficacy and prognosis of tumor immunotherapy.
Tumor infiltrating T cells and B cells are necessary and synergistic components in TME. However, current research mainly focuses on T cells, and their role in tumor immune monitoring has been widely studied. The mechanism of controlling the occurrence and development of tumors is gradually becoming clear, but there is relatively little research on B cells, which makes their roles in different types of cancer controversial. In this study, we systematically and comprehensively plotted the transcriptional characteristics of gastric cancer infiltrating B cells by deciphering their single-cell transcriptomics and spatial transcriptomics.
By using ST data, we explored the spatial-specific immune microenvironment in tumor tissues, all data were obtained from public cohorts in the GEO database. By analyzing scRNA and ST profiles, we found that B cells and tumor cells have certain co-localization in various cancer species, such as breast invasive carcinoma (BRCA), gastrointestinal stromal tumor (GIST), ovarian cancer (OVCA), pancreatic ductal adenocarcinoma (PDCA), and uterine corpus endometrial carcinoma (UCEC), which has the significance of further studying the interaction between B cells and tumor cells. Therefore, based on scRNA and ST sequence analysis, this study explores relevant scientific issues and elucidates the research potential of targeting B cells and clinical transformation, laying a solid theoretical foundation for clinical research.
Our findings align with previous studies indicating that B cells play significant roles in the TIME [ 43 ]. However, the infiltration and function of B cells vary in different types of cancer. The presence and behavior of B cells in the tumor microenvironment can affect tumor progression and patient prognosis. In some cancers, such as breast cancer and lung cancer, B cells inhibit tumor growth by secreting antibodies, neutralizing tumor antigens, promoting anti-tumor immune response, and activating T cells, helping to improve the prognosis of patients [ 44 , 45 ]. In other cancers, such as gastric cancer and colorectal cancer, specific subgroups of B cells such as regulatory B cells (Breg) and IgA secreting cells may inhibit T cell activity, promote tumor growth and metastasis, and lead to poor prognosis by secreting immunosuppressive cytokines (such as IL-10) [ 46 , 47 ]. Therefore, understanding the mechanism of action of B cells in specific tumor types is of great significance for developing tumor therapies targeting B cells. By regulating the function or specific subgroups of B cells, new strategies and potential for cancer immunotherapy can be provided. Consistent with these studies, our data indicate abundant IgA plasma cell infiltration in the GC TIME. Additionally, we confirmed the recruitment of CCR10 + IgA plasma cells by tumor-secreted CCL28, a mechanism previously suggested but not extensively validated in GC. In gastric cancer, we found that tumor cells can secrete chemokine CCL28, which can attract IgA positive plasma cells expressing CCR10. The enrichment of these CCR10 + IgA + plasma cells in the tumor microenvironment is closely related to poor prognosis of patients. IgA positive plasma cells may inhibit anti-tumor immune responses by secreting immunosuppressive cytokines, thereby promoting tumor progression and metastasis.
Despite these advancements, several questions remain unresolved. The study's limitations include a relatively small sample size and the need for multi-center data to validate the prediction model. Furthermore, no prospective randomized clinical trials (RCTs) have been conducted to confirm these findings. The mechanisms underlying the interaction between GC cells and B cells require deeper exploration. Future research should focus on large-scale studies to validate these results, conduct RCTs to confirm the therapeutic potential of targeting CCL28, and investigate the detailed molecular mechanisms of B cell and tumor cell interactions.
The role of B cells in the tumor microenvironment is complex and multifaceted. Understanding the mechanism of action of B cells in specific tumor types is of great significance for developing tumor therapies targeting B cells. B cell targeted therapy has shown great potential in cancer treatment. By blocking specific signaling pathways, such as CCL28/CCR10, anti-tumor immune responses can be significantly activated, enhancing the efficacy of PD-L1 monoclonal antibodies. In addition, combining with other targeted therapies such as blocking the LAMA/CD44 signaling pathway may further improve the prognosis of gastric cancer and other cancer patients. Future research should focus on large-scale studies and clinical trials to validate the efficacy and safety of these strategies, in order to provide more effective treatment options for cancer patients.
To sum up, in our study, we identified a significant amount of crosstalk between GC cells and B cells through scRNA-seq and ST-seq analyses, revealing two differentiation trajectories of GC B cells, which tended to be inhibitory B cell states with IL-10 and IL-35 terminal states. And by analyzing the L/R pairs, we creatively found that the expression of LAMA/CD44 signal axis is a predictive marker for immunotherapy and tumor prognosis. Animal experiments also confirmed that targeting CCL28 can significantly promote immune cell infiltration and enhance the therapeutic effect of anti PD-L1. The combination of LAMA/CD44 or CCL28/CCR10 blockers and PD-1/PD-L1 may be a new treatment for GC.
Availability data and materials
Publicly available datasets were analyzed in this study. Public data used in this work can be acquired from the TCGA repository ( http://cancergenome.nih.gov/ ), and Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/ ).
Abbreviations
- Gastric cancer
Advanced gastric cancer
Tumor microenvironment
Tumor immune microenvironment
Next-generation sequencing technology
Single-cell RNA sequencing
- Spatial transcriptome
Immunohistochemistry
Multiple immunofluorescence
Tumor infiltrating lymphocytes
Ligand/receptor
The cancer genome Atlas
Gene expression omnibus
Breast invasive carcinoma
Gastrointestinal stromal tumor
Liver hepatocellular carcinoma
Ovarian cancer
Pancreatic ductal adenocarcinoma
Uterine corpus endometrial carcinoma
Germinal center B cells
Tertiary lymphoid structures
Principal component analysis
Principal component
Non-responder
Single-cell regulatory network inference and clustering
Transcription factor
Non-negative matrix factorization
Smooth muscle cells
Migration inhibitory factor
Immune checkpoint blockades
Disease-free survival
Antibody-dependent cell-mediated cytotoxicity
Antigen presenting cell
Dendritic cells
Randomized clinical trials
Global Cancer Statistics. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries - pubmed. Cancer Journal for Clinicians: Published online; 2020. p. 2020.
Google Scholar
Downs-Canner SM, Meier J, Vincent BG, Serody JS. B cell function in the tumor microenvironment. Annu Rev Immunol. 2022;40:169–93. https://doi.org/10.1146/annurev-immunol-101220-015603 .
Article CAS PubMed Google Scholar
Zhang E, Ding C, Li S, et al. Roles and mechanisms of tumour-infiltrating B cells in human cancer: a new force in immunotherapy. Biomark Res. 2023;11(1):28. https://doi.org/10.1186/s40364-023-00460-1 .
Article PubMed PubMed Central Google Scholar
Wei Y. B cell heterogeneity, plasticity, and functional diversity in cancer microenvironments. Oncogene: Published online; 2021.
Book Google Scholar
Sun K, Xu R, Ma F, et al. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat Commun. 2022;13(1):4943. https://doi.org/10.1038/s41467-022-32627-z .
Article CAS PubMed PubMed Central Google Scholar
Nathanson T, Ahuja A, Rubinsteyn A, et al. Somatic mutations and neoepitope homology in melanomas treated with CTLA-4 blockade. Cancer Immunol Res. 2017;5(1):84–91. https://doi.org/10.1158/2326-6066.CIR-16-0019 .
Braun DA, Hou Y, Bakouny Z, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26(6):909–18. https://doi.org/10.1038/s41591-020-0839-y .
Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–8. https://doi.org/10.1038/nature25501 .
Luo H, Xia X, Huang LB, et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat Commun. 2022;13(1):6619. https://doi.org/10.1038/s41467-022-34395-2 .
Li X, Sun Z, Peng G, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022;12(2):620–38. https://doi.org/10.7150/thno.60540 .
Barkley D, Moncada R, Pour M, et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat Genet. 2022;54(8):1192–201. https://doi.org/10.1038/s41588-022-01141-9 .
Zhang B, Li J, Hua Q, et al. Tumor CEMIP drives immune evasion of colorectal cancer via MHC-I internalization and degradation. J Immunother Cancer. 2023;11(1): e005592. https://doi.org/10.1136/jitc-2022-005592 .
Long F. The potential crosstalk between tumor and plasma cells and its association with clinical outcome and immunotherapy response in bladder cancer. J Trans Med. 2023. https://doi.org/10.1186/s12967-023-04151-1 .
Article Google Scholar
Wei Y, Lao XM, Xiao X, et al. Plasma cell polarization to the immunoglobulin G phenotype in hepatocellular carcinomas involves epigenetic alterations and promotes hepatoma progression in mice. Gastroenterology. 2019;156(6):1890-1904.e16. https://doi.org/10.1053/j.gastro.2019.01.250 .
Wu RQ, Lao XM, Chen DP, et al. Immune checkpoint therapy-elicited sialylation of IgG antibodies impairs antitumorigenic type I interferon responses in hepatocellular carcinoma. Immunity. 2023;56(1):180-192.e11. https://doi.org/10.1016/j.immuni.2022.11.014 .
Hamish WK, Nara O, John CR, Andrew JC, Gary W, Sarah AT, Louisa KJ. Single-cell analysis of human B cell maturation predicts how antibody class switching shapes selection dynamics. Sci Immunol. 2021. https://doi.org/10.1126/sciimmunol.abe6291 .
Li Z, Jiang Y, Li B, et al. Development and validation of a machine learning model for detection and classification of tertiary lymphoid structures in gastrointestinal cancers. JAMA Netw Open. 2023;6(1): e2252553. https://doi.org/10.1001/jamanetworkopen.2022.52553 .
Yin YX, Ling YH, Wei XL, et al. Impact of mature tertiary lymphoid structures on prognosis and therapeutic response of Epstein-Barr virus-associated gastric cancer patients. Front Immunol. 2022;13: 973085. https://doi.org/10.3389/fimmu.2022.973085 .
Patil NS, Nabet BY, Müller S, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022;40(3):289-300.e4. https://doi.org/10.1016/j.ccell.2022.02.002 .
Meylan M, Petitprez F, Becht E, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55(3):527-541.e5. https://doi.org/10.1016/j.immuni.2022.02.001 .
Liu N, Sun Q, Wan L, et al. CUX1, a controversial player in tumor development. Front Oncol. 2020;10:738. https://doi.org/10.3389/fonc.2020.00738 .
Huang Y, Huang S, Ma L, et al. Exploring the prognostic value, immune implication and biological function of H2AFY gene in hepatocellular carcinoma. Front Immunol. 2021;12: 723293. https://doi.org/10.3389/fimmu.2021.723293 .
Zhu J, Wu Y, Yu Y, Li Y, Shen J, Zhang R. MYBL1 induces transcriptional activation of ANGPT2 to promote tumor angiogenesis and confer sorafenib resistance in human hepatocellular carcinoma. Cell Death Dis. 2022;13(8):727. https://doi.org/10.1038/s41419-022-05180-2 .
Halama N, Yard-Breedijk A, Vardarli I, et al. The Kruppel-like zinc-finger gene ZNF236 is alternatively spliced and excluded as susceptibility gene for diabetic nephropathy. Genomics. 2003;82(3):406–11. https://doi.org/10.1016/s0888-7543(03)00120-4 .
Tian Y, Xu L, Li X, Li H, Zhao M. SMARCA4: current status and future perspectives in non-small-cell lung cancer. Cancer Lett. 2023;554: 216022. https://doi.org/10.1016/j.canlet.2022.216022 .
Lin Z, Niu Y, Wan A, et al. RNA m6 A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020;39(12): e103181. https://doi.org/10.15252/embj.2019103181 .
Habib AM, Matsuyama A, Okorokov AL, et al. A novel human pain insensitivity disorder caused by a point mutation in ZFHX2. Brain. 2018;141(2):365–76. https://doi.org/10.1093/brain/awx326 .
Article PubMed Google Scholar
Di Martino O, Ferris MA, Hadwiger G, et al. RXRA DT448/9PP generates a dominant active variant capable of inducing maturation in acute myeloid leukemia cells. Haematologica. 2022;107(2):417–26. https://doi.org/10.3324/haematol.2021.278603 .
Chano T, Kita H, Avnet S, Lemma S, Baldini N. Prominent role of RAB39A-RXRB axis in cancer development and stemness. Oncotarget. 2018;9(11):9852–66. https://doi.org/10.18632/oncotarget.23955 .
Wang Z, Coban B, Wu H, et al. GRHL2-controlled gene expression networks in luminal breast cancer. Cell Commun Signal. 2023;21(1):15. https://doi.org/10.1186/s12964-022-01029-5 .
Razzaque MS, Atfi A. TGIF1-Twist1 axis in pancreatic ductal adenocarcinoma. Comput Struct Biotechnol J. 2020;18:2568–72. https://doi.org/10.1016/j.csbj.2020.09.023 .
Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–12. https://doi.org/10.1016/j.immuni.2015.04.005 .
Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–74. https://doi.org/10.1038/cmi.2017.35 .
Klemke L, De Oliveira T, Witt D, et al. Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer. Cell Death Dis. 2021;12(2):155. https://doi.org/10.1038/s41419-021-03426-z .
Sumaiya K, Langford D, Natarajaseenivasan K, Shanmughapriya S. Macrophage migration inhibitory factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. 2022;233: 108024. https://doi.org/10.1016/j.pharmthera.2021.108024 .
Lee HN, Jeong MS, Jang SB. Molecular characteristics of amyloid precursor protein (APP) and its effects in cancer. Int J Mol Sci. 2021;22(9):4999. https://doi.org/10.3390/ijms22094999 .
Hu XF, Yao J, Gao SG, Yang YT, Peng XQ, Feng XS. Midkine and syndecan-1 levels correlate with the progression of malignant gastric cardiac adenocarcinoma. Mol Med Rep. 2014;10(3):1409–15. https://doi.org/10.3892/mmr.2014.2369 .
Akgun H, Metintas S, Ak G, et al. Prognostic value of midkine, syndecan-1, hyaluronan synthase-2, sestrin-1, laminin subunit alpha-4 and fibulin-3 for malignant pleural mesothelioma. Arch Med Sci. 2023;19(2):355–64. https://doi.org/10.5114/aoms/112525 .
Yoon SJ, Park J, Shin Y, et al. Deconvolution of diffuse gastric cancer and the suppression of CD34 on the BALB/c nude mice model. BMC Cancer. 2020;20(1):314. https://doi.org/10.1186/s12885-020-06814-4 .
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. https://doi.org/10.1016/S0140-6736(21)00797-2 .
Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–47. https://doi.org/10.1016/S1470-2045(21)00692-6 .
Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the keynote-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80. https://doi.org/10.1001/jamaoncol.2020.3370 .
Ma J, Wu Y, Ma L, et al. A blueprint for tumor-infiltrating B cells across human cancers. Science. 2024. https://doi.org/10.1126/science.adj4857 .
Gu Y, Liu Y, Fu L, et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med. 2019;25(2):312–22. https://doi.org/10.1038/s41591-018-0309-y .
Hollern DP, Xu N, Thennavan A, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell. 2019;179(5):1191-1206.e21. https://doi.org/10.1016/j.cell.2019.10.028 .
Wang Z, Lu Z, Lin S, et al. Leucine-tRNA-synthase-2-expressing B cells contribute to colorectal cancer immunoevasion. Immunity. 2022;55(6):1067-1081.e8. https://doi.org/10.1016/j.immuni.2022.04.017 .
Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. 2021;54(12):2812-2824.e4. https://doi.org/10.1016/j.immuni.2021.11.003 .
Download references
Acknowledgements
We are grateful to the researchers who provided the original data.
This work was supported by the National Natural Science Foundation of China (No. 82072041 and No. 82372069).
Author information
Present address: Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, 430071, People’s Republic of China
Xing Cai, Jinru Yang and Yusheng Guo have authors contributed equally to this article.
Authors and Affiliations
Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
Xing Cai, Jinru Yang & Xiaofang Dai
Institute of Radiation Oncology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
Yusheng Guo & Chuansheng Zheng
Hubei Key Laboratory of Molecular Imaging, Wuhan, 430022, People’s Republic of China
Department of Urology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
(I) Conception and design: CS Zheng and XF Dai; (II) Administrative support: XF Dai; (III) Provision of study materials or patients:X Cai, JR Yang and YS Guo; (IV) Collection and assembly of data: X Cai and YC Yu; (V) Data analysis and interpretation: X Cai and JR Yang; (VI) Manuscript writing: JR Yang; (VII) Final approval of manuscript: All authors.
Corresponding authors
Correspondence to Chuansheng Zheng or Xiaofang Dai .
Ethics declarations
Ethics approval and consent to participate.
The Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and Institutional Animal Care and Use Committee, Huazhong University of Science and Technology granted approval for the study (No. 2014-041, and No. IACUC [2023] 3525).
Competing interests
All authors have completed the ICMJE uniform disclosure form. The authors have no competing of interest to declare.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12967_2024_5606_moesm1_esm.tiff.
Additional file 1: Supplementary Figure 1. Spatial transcriptomics explained the distribution characteristics of marker genes in various types of cancer epithelium and B cells .Epcam, MS4A1, SDC1 gene expression in multiple cancers, including BRCA, GIST, LIHC, OVCA, PDCA, and UCEC.
12967_2024_5606_MOESM2_ESM.tiff
Additional file 2: Supplementary Figure 2. Single cell landscape of human gastric cancer plasma cells. Box plots showing the expression levels of IgA and IgG related genes.Uniform manifold approximation and projectionof 5518 cells from 10 patients, colored by major cell types. Violin plot showing the expression of antibody-relatedgenes in subclusters of plasma cells.UMAP visualization of antibody-related genes expression showing IgG and IgA cells are the dominated PCs based on dimension reduction of PCA with selected gene.the immunofluorescence quantitative bar chart of CD138 cell, CCR10 + /CD138 + cell and CCL28 + /EPCAM + cell.
12967_2024_5606_MOESM3_ESM.tiff
Additional file 3: Supplementary Figure 3. The correlation between plasma cells and clinical practice. The histogram shows the significantly enriched pathways of plasma cell clusters, including IgA Pc, IgG Pc, IgA high_IgG high Pc, and IgA low_IgG low Pc. The survival curves show the relationship between plasma cell infiltration levels and prognosis in four Pc groups.
12967_2024_5606_MOESM4_ESM.tiff
Additional file 4: Supplementary Figure 4. Functional enrichment and trajectory analysis of gastric cancer B cells. The histogram shows the significantly enriched pathways of B cell clusters. Gaussian process regression curves with a 95% confidence interval showing the dynamic expression of TCL1A, NR4A1, CD99 and GPR183 along the pseudotime of the Lineage1 trajectoryand the Lineage2 trajectory.
12967_2024_5606_MOESM5_ESM.tiff
Additional file 5: Supplementary Figure 5. Correlation between epithelial transcription status and clinical prognosis in gastric cancer. KM plots of seven tumor epithelial cell states, including Epi-dif1, Wh, cell cycle, interferon, OP, Epi-dif2, unknown, and Metal.
12967_2024_5606_MOESM6_ESM.tiff
Additional file 6: Supplementary Figure 6. Disturbance of communication between various cells in the gastric cancer microenvironment. Crosstalk networks show B cells tend to send signals to T&NK cells and myeloid cells but receive signals from stromal cells. Dot plot shows the incoming and outgoing signal strength in different cell types. Dot plots show the incoming and outgoing interaction strength of tumor cell states in all signaling pathways, MK/MIF signaling, ADGRE5/CD55 signaling and APP/CD74 signaling.Heat map shows the signaling pathways enriched by each tumor cell states in both incoming and outgoing signaling patterns.
12967_2024_5606_MOESM7_ESM.tiff
Additional file 7: Supplementary Figure 7. Related cell phenotype experiments on MFC and MC38 tumor cells. The line chart showed no statistical difference in OD valuesbetween shCCL28 and shNC on both MFC and MC38 cells. ELISA showed a decrease in protein levels of CCL28 in MFC-shCCL28 and MC38-shCCL28 cell lines, respectively. The colony formation graphand corresponding bar graphshowed that there was no statistical difference in clone formation between shCCL28 and shNC on both MFC and MC38 cells.Cell cycle checkpoint detection showed no difference in the percentage of G2 between MFC and MC38 cells. Flow cytometry showed no statistically significant difference in the apoptotic effects of shCCL28 and shNC on MFC and MC38 cells. Cell invasion and migration experiments showed no statistical differences between shCCL28 and shNC on MFC and MC38 cells.
12967_2024_5606_MOESM8_ESM.tiff
Additional file 8: Supplementary Figure 8. Gating strategies for flow cytometry in specific immunity and innate immunity, respectively.
12967_2024_5606_MOESM9_ESM.tiff
Additional file 9: Supplementary Figure 9. Inhibiting CCL28 can promote the inhibition of tumor progression and the effectiveness of immunotherapy. C57BL/6J mice were implanted with 1×10 6 MC38 cells and received treatment as 615 mice.A schematic view of the treatment plan.Tumor image, tumors were measured every 2 days, n=6 mice per group.Representative diagram of flow cytometry.The percentages of tumor-infiltrating B, CD3 + T, CD8 + T, CD4 + T, IFN + CD8 + T, GZMB + CD8 + T, MDSC and DC were analyzed by flow cytometry. n=6-8/group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cai, X., Yang, J., Guo, Y. et al. Re-analysis of single cell and spatial transcriptomics data reveals B cell landscape in gastric cancer microenvironment and its potential crosstalk with tumor cells for clinical prognosis. J Transl Med 22 , 807 (2024). https://doi.org/10.1186/s12967-024-05606-9
Download citation
Received : 11 February 2024
Accepted : 14 August 2024
Published : 30 August 2024
DOI : https://doi.org/10.1186/s12967-024-05606-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Single cell RNA sequencing
- Immunotherapy
- CCL28-CCR10
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Acupuncture: Effectiveness and Safety
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What is acupuncture?
Acupuncture is a technique in which practitioners insert fine needles into the skin to treat health problems. The needles may be manipulated manually or stimulated with small electrical currents (electroacupuncture). Acupuncture has been in use in some form for at least 2,500 years. It originated from traditional Chinese medicine but has gained popularity worldwide since the 1970s.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} How widely is acupuncture used?
According to the World Health Organization, acupuncture is used in 103 of 129 countries that reported data.
In the United States, data from the National Health Interview Survey show that the use of acupuncture by U.S. adults more than doubled between 2002 and 2022. In 2002, 1.0 percent of U.S. adults used acupuncture; in 2022, 2.2 percent used it.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What is acupuncture used for?
National survey data indicate that in the United States, acupuncture is most commonly used for pain, such as back, joint, or neck pain.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} How does acupuncture work scientifically?
How acupuncture works is not fully understood. However, there’s evidence that acupuncture may have effects on the nervous system, effects on other body tissues, and nonspecific (placebo) effects.
- Studies in animals and people, including studies that used imaging methods to see what’s happening in the brain, have shown that acupuncture may affect nervous system function.
- Acupuncture may have direct effects on the tissues where the needles are inserted. This type of effect has been seen in connective tissue.
- Acupuncture has nonspecific effects (effects due to incidental aspects of a treatment rather than its main mechanism of action). Nonspecific effects may be due to the patient’s belief in the treatment, the relationship between the practitioner and the patient, or other factors not directly caused by the insertion of needles. In many studies, the benefit of acupuncture has been greater when it was compared with no treatment than when it was compared with sham (simulated or fake) acupuncture procedures, such as the use of a device that pokes the skin but does not penetrate it. These findings suggest that nonspecific effects contribute to the beneficial effect of acupuncture on pain or other symptoms.
- In recent research, a nonspecific effect was demonstrated in a unique way: Patients who had experienced pain relief during a previous acupuncture session were shown a video of that session and asked to imagine the treatment happening again. This video-guided imagery technique had a significant pain-relieving effect.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What does research show about the effectiveness of acupuncture for pain?
Research has shown that acupuncture may be helpful for several pain conditions, including back or neck pain, knee pain associated with osteoarthritis, and postoperative pain. It may also help relieve joint pain associated with the use of aromatase inhibitors, which are drugs used in people with breast cancer.
An analysis of data from 20 studies (6,376 participants) of people with painful conditions (back pain, osteoarthritis, neck pain, or headaches) showed that the beneficial effects of acupuncture continued for a year after the end of treatment for all conditions except neck pain.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Back or Neck Pain
- In a 2018 review, data from 12 studies (8,003 participants) showed acupuncture was more effective than no treatment for back or neck pain, and data from 10 studies (1,963 participants) showed acupuncture was more effective than sham acupuncture. The difference between acupuncture and no treatment was greater than the difference between acupuncture and sham acupuncture. The pain-relieving effect of acupuncture was comparable to that of nonsteroidal anti-inflammatory drugs (NSAIDs).
- A 2017 clinical practice guideline from the American College of Physicians included acupuncture among the nondrug options recommended as first-line treatment for chronic low-back pain. Acupuncture is also one of the treatment options recommended for acute low-back pain. The evidence favoring acupuncture for acute low-back pain was judged to be of low quality, and the evidence for chronic low-back pain was judged to be of moderate quality.
For more information, see the NCCIH webpage on low-back pain .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Osteoarthritis
- In a 2018 review, data from 10 studies (2,413 participants) showed acupuncture was more effective than no treatment for osteoarthritis pain, and data from 9 studies (2,376 participants) showed acupuncture was more effective than sham acupuncture. The difference between acupuncture and no treatment was greater than the difference between acupuncture and sham acupuncture. Most of the participants in these studies had knee osteoarthritis, but some had hip osteoarthritis. The pain-relieving effect of acupuncture was comparable to that of NSAIDs.
- A 2018 review evaluated 6 studies (413 participants) of acupuncture for hip osteoarthritis. Two of the studies compared acupuncture with sham acupuncture and found little or no difference between them in terms of effects on pain. The other four studies compared acupuncture with a variety of other treatments and could not easily be compared with one another. However, one of the trials indicated that the addition of acupuncture to routine care by a physician may improve pain and function in patients with hip osteoarthritis.
- A 2019 clinical practice guideline from the American College of Rheumatology and the Arthritis Foundation conditionally recommends acupuncture for osteoarthritis of the knee, hip, or hand. The guideline states that the greatest number of studies showing benefits have been for knee osteoarthritis.
For more information, see the NCCIH webpage on osteoarthritis .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Headache and Migraine
- A 2020 review of nine studies that compared acupuncture with various drugs for preventing migraine found that acupuncture was slightly more effective, and study participants who received acupuncture were much less likely than those receiving drugs to drop out of studies because of side effects.
- There’s moderate-quality evidence that acupuncture may reduce the frequency of migraines (from a 2016 evaluation of 22 studies with almost 5,000 people). The evidence from these studies also suggests that acupuncture may be better than sham acupuncture, but the difference is small. There is moderate- to low-quality evidence that acupuncture may reduce the frequency of tension headaches (from a 2016 evaluation of 12 studies with about 2,350 people).
For more information, see the NCCIH webpage on headache .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Myofascial Pain Syndrome
- Myofascial pain syndrome is a common form of pain derived from muscles and their related connective tissue (fascia). It involves tender nodules called “trigger points.” Pressing on these nodules reproduces the patient’s pattern of pain.
- A combined analysis of a small number of studies of acupuncture for myofascial pain syndrome showed that acupuncture applied to trigger points had a favorable effect on pain intensity (5 studies, 215 participants), but acupuncture applied to traditional acupuncture points did not (4 studies, 80 participants).
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Sciatica
- Sciatica involves pain, weakness, numbness, or tingling in the leg, usually on one side of the body, caused by damage to or pressure on the sciatic nerve—a nerve that starts in the lower back and runs down the back of each leg.
- Two 2015 evaluations of the evidence, one including 12 studies with 1,842 total participants and the other including 11 studies with 962 total participants, concluded that acupuncture may be helpful for sciatica pain, but the quality of the research is not good enough to allow definite conclusions to be reached.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Postoperative Pain
- A 2016 evaluation of 11 studies of pain after surgery (with a total of 682 participants) found that patients treated with acupuncture or related techniques 1 day after surgery had less pain and used less opioid pain medicine after the operation.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Cancer Pain
- A 2016 review of 20 studies (1,639 participants) indicated that acupuncture was not more effective in relieving cancer pain than conventional drug therapy. However, there was some evidence that acupuncture plus drug therapy might be better than drug therapy alone.
- A 2017 review of 5 studies (181 participants) of acupuncture for aromatase inhibitor-induced joint pain in breast cancer patients concluded that 6 to 8 weeks of acupuncture treatment may help reduce the pain. However, the individual studies only included small numbers of women and used a variety of acupuncture techniques and measurement methods, so they were difficult to compare.
- A larger 2018 study included 226 women with early-stage breast cancer who were taking aromatase inhibitors. The study found that the women who received 6 weeks of acupuncture treatment, given twice each week, reported less joint pain than the participants who received sham or no acupuncture.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Chronic Prostatitis/Chronic Pelvic Pain Syndrome
- Chronic prostatitis/chronic pelvic pain syndrome is a condition in men that involves inflammation of or near the prostate gland; its cause is uncertain.
- A review of 3 studies (204 total participants) suggested that acupuncture may reduce prostatitis symptoms, compared with a sham procedure. Because follow-up of the study participants was relatively brief and the numbers of studies and participants were small, a definite conclusion cannot be reached about acupuncture’s effects.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Irritable Bowel Syndrome
- A 2019 review of 41 studies (3,440 participants) showed that acupuncture was no more effective than sham acupuncture for symptoms of irritable bowel syndrome, but there was some evidence that acupuncture could be helpful when used in addition to other forms of treatment.
For more information, see the NCCIH webpage on irritable bowel syndrome .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Fibromyalgia
- A 2019 review of 12 studies (824 participants) of people with fibromyalgia indicated that acupuncture was significantly better than sham acupuncture for relieving pain, but the evidence was of low-to-moderate quality.
For more information, see the NCCIH webpage on fibromyalgia .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What does research show about acupuncture for conditions other than pain?
In addition to pain conditions, acupuncture has also been studied for at least 50 other health problems. There is evidence that acupuncture may help relieve seasonal allergy symptoms, stress incontinence in women, and nausea and vomiting associated with cancer treatment. It may also help relieve symptoms and improve the quality of life in people with asthma, but it has not been shown to improve lung function.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Seasonal Allergies (Allergic Rhinitis or Hay Fever)
- A 2015 evaluation of 13 studies of acupuncture for allergic rhinitis, involving a total of 2,365 participants, found evidence that acupuncture may help relieve nasal symptoms. The study participants who received acupuncture also had lower medication scores (meaning that they used less medication to treat their symptoms) and lower blood levels of immunoglobulin E (IgE), a type of antibody associated with allergies.
- A 2014 clinical practice guideline from the American Academy of Otolaryngology–Head and Neck Surgery included acupuncture among the options health care providers may offer to patients with allergic rhinitis.
For more information, see the NCCIH webpage on seasonal allergies .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Urinary Incontinence
- Stress incontinence is a bladder control problem in which movement—coughing, sneezing, laughing, or physical activity—puts pressure on the bladder and causes urine to leak.
- In a 2017 study of about 500 women with stress incontinence, participants who received electroacupuncture treatment (18 sessions over 6 weeks) had reduced urine leakage, with about two-thirds of the women having a decrease in leakage of 50 percent or more. This was a rigorous study that met current standards for avoiding bias.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Treatment-Related Nausea and Vomiting in Cancer Patients
- Experts generally agree that acupuncture is helpful for treatment-related nausea and vomiting in cancer patients, but this conclusion is based primarily on research conducted before current guidelines for treating these symptoms were adopted. It’s uncertain whether acupuncture is beneficial when used in combination with current standard treatments for nausea and vomiting.
For more information, see the NCCIH webpage on cancer .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Asthma
- In a study conducted in Germany in 2017, 357 participants receiving routine asthma care were randomly assigned to receive or not receive acupuncture, and an additional 1,088 people who received acupuncture for asthma were also studied. Adding acupuncture to routine care was associated with better quality of life compared to routine care alone.
- A review of 9 earlier studies (777 participants) showed that adding acupuncture to conventional asthma treatment improved symptoms but not lung function.
For more information, see the NCCIH webpage on asthma .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Depression
- A 2018 review of 64 studies (7,104 participants) of acupuncture for depression indicated that acupuncture may result in a moderate reduction in the severity of depression when compared with treatment as usual or no treatment. However, these findings should be interpreted with caution because most of the studies were of low or very low quality.
For more information, see the NCCIH webpage on depression .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Quitting Smoking
- In recommendations on smoking cessation treatment issued in 2021, the U.S. Preventive Services Task Force, a panel of experts that makes evidence-based recommendations about disease prevention, did not make a recommendation about the use of acupuncture as a stop-smoking treatment because only limited evidence was available. This decision was based on a 2014 review of 9 studies (1,892 participants) that looked at the effect of acupuncture on smoking cessation results for 6 months or more and found no significant benefit. Some studies included in that review showed evidence of a possible small benefit of acupuncture on quitting smoking for shorter periods of time.
For more information, see the NCCIH webpage on quitting smoking .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Infertility
- A 2021 review evaluated 6 studies (2,507 participants) that compared the effects of acupuncture versus sham acupuncture on the success of in vitro fertilization as a treatment for infertility. No difference was found between the acupuncture and sham acupuncture groups in rates of pregnancy or live birth.
- A 2020 review evaluated 12 studies (1,088 participants) on the use of acupuncture to improve sperm quality in men who had low sperm numbers and low sperm motility. The reviewers concluded that the evidence was inadequate for firm conclusions to be drawn because of the varied design of the studies and the poor quality of some of them.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Carpal Tunnel Syndrome
- A 2018 review of 12 studies with 869 participants concluded that acupuncture and laser acupuncture (a treatment that uses lasers instead of needles) may have little or no effect on carpal tunnel syndrome symptoms in comparison with sham acupuncture. It’s uncertain how the effects of acupuncture compare with those of other treatments for this condition.
- In a 2017 study not included in the review described above, 80 participants with carpal tunnel syndrome were randomly assigned to one of three interventions: (1) electroacupuncture to the more affected hand; (2) electroacupuncture at “distal” body sites, near the ankle opposite to the more affected hand; and (3) local sham electroacupuncture using nonpenetrating placebo needles. All three interventions reduced symptom severity, but local and distal acupuncture were better than sham acupuncture at producing desirable changes in the wrist and the brain.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Hot Flashes Associated With Menopause
- A 2018 review of studies of acupuncture for vasomotor symptoms associated with menopause (hot flashes and related symptoms such as night sweats) analyzed combined evidence from an earlier review of 15 studies (1,127 participants) and 4 newer studies (696 additional participants). The analysis showed that acupuncture was better than no acupuncture at reducing the frequency and severity of symptoms. However, acupuncture was not shown to be better than sham acupuncture.
For more information, see the NCCIH webpage on menopause .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What is auricular acupuncture good for?
- Auricular acupuncture is a type of acupuncture that involves stimulating specific areas of the ear.
- In a 2019 review of 15 studies (930 participants) of auricular acupuncture or auricular acupressure (a form of auricular therapy that does not involve penetration with needles), the treatment significantly reduced pain intensity, and 80 percent of the individual studies showed favorable effects on various measures related to pain.
- A 2020 review of 9 studies (783 participants) of auricular acupuncture for cancer pain showed that auricular acupuncture produced better pain relief than sham auricular acupuncture. Also, pain relief was better with a combination of auricular acupuncture and drug therapy than with drug therapy alone.
- An inexpensive, easily learned form of auricular acupuncture called “battlefield acupuncture” has been used by the U.S. Department of Defense and Department of Veterans Affairs to treat pain. However, a 2021 review of 9 studies (692 participants) of battlefield acupuncture for pain in adults did not find any significant improvement in pain when this technique was compared with no treatment, usual care, delayed treatment, or sham battlefield acupuncture.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Is acupuncture safe?
- Relatively few complications from using acupuncture have been reported. However, complications have resulted from use of nonsterile needles and improper delivery of treatments.
- When not delivered properly, acupuncture can cause serious adverse effects, including infections, punctured organs, and injury to the central nervous system.
- The U.S. Food and Drug Administration (FDA) regulates acupuncture needles as medical devices and requires that they be sterile and labeled for single use only.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Is acupuncture covered by health insurance?
- Some health insurance policies cover acupuncture, but others don’t. Coverage is often limited based on the condition being treated.
- An analysis of data from the Medical Expenditure Panel Survey, a nationally representative U.S. survey, showed that the share of adult acupuncturist visits with any insurance coverage increased from 41.1 percent in 2010–2011 to 50.2 percent in 2018–2019.
- Medicare covers acupuncture only for the treatment of chronic low-back pain. Coverage began in 2020. Up to 12 acupuncture visits are covered, with an additional 8 visits available if the first 12 result in improvement. Medicaid coverage of acupuncture varies from state to state.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Do acupuncturists need to be licensed?
- Most states license acupuncturists, but the requirements for licensing vary from state to state. To find out more about licensing of acupuncturists and other complementary health practitioners, visit the NCCIH webpage Credentialing, Licensing, and Education .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} NCCIH-Funded Research
NCCIH funds research to evaluate acupuncture’s effectiveness for various kinds of pain and other conditions and to further understand how the body responds to acupuncture and how acupuncture might work. Some recent NCCIH-supported studies involve:
- Evaluating the feasibility of using acupuncture in hospital emergency departments.
- Testing whether the effect of acupuncture on chronic low-back pain can be enhanced by combining it with transcranial direct current stimulation.
- Evaluating a portable acupuncture-based nerve stimulation treatment for anxiety disorders.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} More To Consider
- Don’t use acupuncture to postpone seeing a health care provider about a health problem.
- Take charge of your health—talk with your health care providers about any complementary health approaches you use. Together, you can make shared, well-informed decisions.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} For More Information
Nccih clearinghouse.
The NCCIH Clearinghouse provides information on NCCIH and complementary and integrative health approaches, including publications and searches of Federal databases of scientific and medical literature. The Clearinghouse does not provide medical advice, treatment recommendations, or referrals to practitioners.
Toll-free in the U.S.: 1-888-644-6226
Telecommunications relay service (TRS): 7-1-1
Website: https://www.nccih.nih.gov
Email: [email protected] (link sends email)
Know the Science
NCCIH and the National Institutes of Health (NIH) provide tools to help you understand the basics and terminology of scientific research so you can make well-informed decisions about your health. Know the Science features a variety of materials, including interactive modules, quizzes, and videos, as well as links to informative content from Federal resources designed to help consumers make sense of health information.
Explaining How Research Works (NIH)
Know the Science: How To Make Sense of a Scientific Journal Article
Understanding Clinical Studies (NIH)
A service of the National Library of Medicine, PubMed® contains publication information and (in most cases) brief summaries of articles from scientific and medical journals. For guidance from NCCIH on using PubMed, see How To Find Information About Complementary Health Approaches on PubMed .
Website: https://pubmed.ncbi.nlm.nih.gov/
NIH Clinical Research Trials and You
The National Institutes of Health (NIH) has created a website, NIH Clinical Research Trials and You, to help people learn about clinical trials, why they matter, and how to participate. The site includes questions and answers about clinical trials, guidance on how to find clinical trials through ClinicalTrials.gov and other resources, and stories about the personal experiences of clinical trial participants. Clinical trials are necessary to find better ways to prevent, diagnose, and treat diseases.
Website: https://www.nih.gov/health-information/nih-clinical-research-trials-you
Research Portfolio Online Reporting Tools Expenditures & Results (RePORTER)
RePORTER is a database of information on federally funded scientific and medical research projects being conducted at research institutions.
Website: https://reporter.nih.gov
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Key References
- Befus D, Coeytaux RR, Goldstein KM, et al. Management of menopause symptoms with acupuncture: an umbrella systematic review and meta-analysis . Journal of Alternative and Complementary Medicine. 2018;24(4):314-323.
- Bleck R, Marquez E, Gold MA, et al. A scoping review of acupuncture insurance coverage in the United States . Acupuncture in Medicine. 2020;964528420964214.
- Briggs JP, Shurtleff D. Acupuncture and the complex connections between the mind and the body. JAMA. 2017;317(24):2489-2490.
- Brinkhaus B, Roll S, Jena S, et al. Acupuncture in patients with allergic asthma: a randomized pragmatic trial. Journal of Alternative and Complementary Medicine. 2017;23(4):268-277.
- Chan MWC, Wu XY, Wu JCY, et al. Safety of acupuncture: overview of systematic reviews. Scientific Reports. 2017;7(1):3369.
- Coyle ME, Stupans I, Abdel-Nour K, et al. Acupuncture versus placebo acupuncture for in vitro fertilisation: a systematic review and meta-analysis. Acupuncture in Medicine. 2021;39(1):20-29.
- Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA. 2018;320(2):167-176.
- Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database of Systematic Reviews. 2016;(6):CD001218. Accessed at cochranelibrary.com on February 12, 2021.
- Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of tension-type headache. Cochrane Database of Systematic Reviews. 2016;(4):CD007587. Accessed at cochranelibrary.com on February 12, 2021.
- MacPherson H, Vertosick EA, Foster NE, et al. The persistence of the effects of acupuncture after a course of treatment: a meta-analysis of patients with chronic pain . Pain. 2017;158(5):784-793.
- Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine. 2017;166(7):514-530.
- Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngology—Head and Neck Surgery. 2015;152(suppl 1):S1-S43.
- Vickers AJ, Vertosick EA, Lewith G, et al. Acupuncture for chronic pain: update of an individual patient data meta-analysis . The Journal of Pain. 2018;19(5):455-474.
- White AR, Rampes H, Liu JP, et al. Acupuncture and related interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2014;(1):CD000009. Accessed at cochranelibrary.com on February 17, 2021.
- Zia FZ, Olaku O, Bao T, et al. The National Cancer Institute’s conference on acupuncture for symptom management in oncology: state of the science, evidence, and research gaps. Journal of the National Cancer Institute. Monographs. 2017;2017(52):lgx005.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Other References
- Adams D, Cheng F, Jou H, et al. The safety of pediatric acupuncture: a systematic review. Pediatrics. 2011;128(6):e1575-1587.
- Candon M, Nielsen A, Dusek JA. Trends in insurance coverage for acupuncture, 2010-2019. JAMA Network Open. 2022;5(1):e2142509.
- Cao J, Tu Y, Orr SP, et al. Analgesic effects evoked by real and imagined acupuncture: a neuroimaging study. Cerebral Cortex. 2019;29(8):3220-3231.
- Centers for Medicare & Medicaid Services. Decision Memo for Acupuncture for Chronic Low Back Pain (CAG-00452N). Accessed at https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=295 on June 25, 2021.
- Chen L, Lin C-C, Huang T-W, et al. Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials . The Breast. 2017;33:132-138.
- Choi G-H, Wieland LS, Lee H, et al. Acupuncture and related interventions for the treatment of symptoms associated with carpal tunnel syndrome. Cochrane Database of Systematic Reviews. 2018;(12):CD011215. Accessed at cochranelibrary.com on January 28, 2021.
- Cui J, Wang S, Ren J, et al. Use of acupuncture in the USA: changes over a decade (2002–2012). Acupuncture in Medicine. 2017;35(3):200-207.
- Federman DG, Zeliadt SB, Thomas ER, et al. Battlefield acupuncture in the Veterans Health Administration: effectiveness in individual and group settings for pain and pain comorbidities. Medical Acupuncture. 2018;30(5):273-278.
- Feng S, Han M, Fan Y, et al. Acupuncture for the treatment of allergic rhinitis: a systematic review and meta-analysis. American Journal of Rhinology & Allergy. 2015;29(1):57-62.
- Franco JV, Turk T, Jung JH, et al. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database of Systematic Reviews. 2018;(5):CD012551. Accessed at cochranelibrary.com on January 28, 2021.
- Freeman MP, Fava M, Lake J, et al. Complementary and alternative medicine in major depressive disorder: the American Psychiatric Association task force report. The Journal of Clinical Psychiatry . 2010;71(6):669-681.
- Giovanardi CM, Cinquini M, Aguggia M, et al. Acupuncture vs. pharmacological prophylaxis of migraine: a systematic review of randomized controlled trials. Frontiers in Neurology. 2020;11:576272.
- Hu C, Zhang H, Wu W, et al. Acupuncture for pain management in cancer: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2016;2016;1720239.
- Jiang C, Jiang L, Qin Q. Conventional treatments plus acupuncture for asthma in adults and adolescent: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine . 2019;2019:9580670.
- Ji M, Wang X, Chen M, et al. The efficacy of acupuncture for the treatment of sciatica: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2015;2015:192808.
- Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Annals of Internal Medicine . 2002;136(5):374-383.
- Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care & Research. 2020;72(2):149-162.
- Langevin H. Fascia mobility, proprioception, and myofascial pain. Life. 2021;11(7):668.
- Liu Z, Liu Y, Xu H, et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: a randomized clinical trial. JAMA. 2017;317(24):2493-2501.
- MacPherson H, Hammerschlag R, Coeytaux RR, et al. Unanticipated insights into biomedicine from the study of acupuncture. Journal of Alternative and Complementary Medicine. 2016;22(2):101-107.
- Maeda Y, Kim H, Kettner N, et al. Rewiring the primary somatosensory cortex in carpal tunnel syndrome with acupuncture. Brain. 2017;140(4):914-927.
- Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for hip osteoarthritis. Cochrane Database of Systematic Reviews. 2018;(5):CD013010. Accessed at cochranelibrary.com on February 17, 2021.
- Moura CC, Chaves ECL, Cardoso ACLR, et al. Auricular acupuncture for chronic back pain in adults: a systematic review and metanalysis. Revista da Escola de Enfermagem da U S P. 2019;53:e03461.
- Nahin RL, Rhee A, Stussman B. Use of complementary health approaches overall and for pain management by US adults. JAMA. 2024;331(7):613-615.
- Napadow V. Neuroimaging somatosensory and therapeutic alliance mechanisms supporting acupuncture. Medical Acupuncture. 2020;32(6):400-402.
- Patnode CD, Henderson JT, Coppola EL, et al. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(3):280-298.
- Qin Z, Liu X, Wu J, et al. Effectiveness of acupuncture for treating sciatica: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2015;2015;425108.
- Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database of Systematic Reviews. 2018;(3):CD004046. Accessed at cochranelibrary.com on January 20, 2021.
- US Preventive Services Task Force. Interventions for tobacco smoking cessation in adults, including pregnant persons. US Preventive Services Task Force recommendation statement. JAMA. 2021;325(3):265-279.
- Vase L, Baram S, Takakura N, et al. Specifying the nonspecific components of acupuncture analgesia. Pain. 2013;154(9):1659-1667.
- Wang R, Li X, Zhou S, et al. Manual acupuncture for myofascial pain syndrome: a systematic review and meta-analysis. Acupuncture in Medicine. 2017;35(4):241-250.
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Geneva, Switzerland: World Health Organization, 2013. Accessed at https://www.who.int/publications/i/item/9789241506096 on February 2, 2021.
- Wu M-S, Chen K-H, Chen I-F, et al. The efficacy of acupuncture in post-operative pain management: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150367.
- Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evidence-Based Complementary and Alternative Medicine. 2013;2013:581203.
- Yang J, Ganesh R, Wu Q, et al. Battlefield acupuncture for adult pain: a systematic review and meta-analysis of randomized controlled trials. The American Journal of Chinese Medicine. 2021;49(1):25-40.
- Yang Y, Wen J, Hong J. The effects of auricular therapy for cancer pain: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2020;2020:1618767.
- Yeh CH, Morone NE, Chien L-C, et al. Auricular point acupressure to manage chronic low back pain in older adults: a randomized controlled pilot study. Evidence-Based Complementary and Alternative Medicine. 2014;2014;375173.
- You F, Ruan L, Zeng L, et al. Efficacy and safety of acupuncture for the treatment of oligoasthenozoospermia: a systematic review. Andrologia. 2020;52(1):e13415.
- Zhang X-C, Chen H, Xu W-T, et al. Acupuncture therapy for fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. Journal of Pain Research. 2019;12:527-542.
- Zheng H, Chen R, Zhao X, et al. Comparison between the effects of acupuncture relative to other controls on irritable bowel syndrome: a meta-analysis. Pain Research and Management. 2019;2019:2871505.
Acknowledgments
NCCIH thanks Pete Murray, Ph.D., David Shurtleff, Ph.D., and Helene M. Langevin, M.D., NCCIH for their review of the 2022 update of this fact sheet.
This publication is not copyrighted and is in the public domain. Duplication is encouraged.
NCCIH has provided this material for your information. It is not intended to substitute for the medical expertise and advice of your health care provider(s). We encourage you to discuss any decisions about treatment or care with your health care provider. The mention of any product, service, or therapy is not an endorsement by NCCIH.
Related Topics
Pain: Considering Complementary Approaches (eBook)
For Consumers
6 Things To Know When Selecting a Complementary Health Practitioner
For Health Care Providers
Mind and Body Approaches for Chronic Pain
Complementary Psychological and/or Physical Approaches for Cancer Symptoms and Treatment Side Effects
Research Results
New Findings Suggest Acupuncture Stimulation Reduces Systemic Inflammation
How the Body and Brain Achieve Carpal Tunnel Pain Relief via Acupuncture
Related Fact Sheets
Low-Back Pain and Complementary Health Approaches: What You Need To Know
Osteoarthritis: In Depth
Cancer and Complementary Health Approaches: What You Need To Know
Chronic Pain and Complementary Health Approaches
Traditional Chinese Medicine: What You Need To Know
Credentialing, Licensing, and Education
Paying for Complementary and Integrative Health Approaches
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Pediatr Investig
- v.3(4); 2019 Dec

Clinical research study designs: The essentials
Ambika g. chidambaram.
1 Children's Hospital of Philadelphia, Philadelphia Pennsylvania, USA
Maureen Josephson
In clinical research, our aim is to design a study which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and governed by ethical clinical principles. The purpose of this review is to provide the readers an overview of the basic study designs and its applicability in clinical research.
Introduction
In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the “real world” setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of the population being studied. The conclusions derived from a research study can either improve health care or result in inadvertent harm to patients. Hence, this requires a well‐designed clinical research study that rests on a strong foundation of a detailed methodology and is governed by ethical principles. 2
From an epidemiological standpoint, there are two major types of clinical study designs, observational and experimental. 3 Observational studies are hypothesis‐generating studies, and they can be further divided into descriptive and analytic. Descriptive observational studies provide a description of the exposure and/or the outcome, and analytic observational studies provide a measurement of the association between the exposure and the outcome. Experimental studies, on the other hand, are hypothesis testing studies. It involves an intervention that tests the association between the exposure and outcome. Each study design is different, and so it would be important to choose a design that would most appropriately answer the question in mind and provide the most valuable information. We will be reviewing each study design in detail (Figure 1 ).

Overview of clinical research study designs
Observational study designs
Observational studies ask the following questions: what, who, where and when. There are many study designs that fall under the umbrella of descriptive study designs, and they include, case reports, case series, ecologic study, cross‐sectional study, cohort study and case‐control study (Figure 2 ).

Classification of observational study designs
Case reports and case series
Every now and then during clinical practice, we come across a case that is atypical or ‘out of the norm’ type of clinical presentation. This atypical presentation is usually described as case reports which provides a detailed and comprehensive description of the case. 4 It is one of the earliest forms of research and provides an opportunity for the investigator to describe the observations that make a case unique. There are no inferences obtained and therefore cannot be generalized to the population which is a limitation. Most often than not, a series of case reports make a case series which is an atypical presentation found in a group of patients. This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore. However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity.
Ecologic study
Ecological studies are observational studies that provide a description of population group characteristics. That is, it describes characteristics to all individuals within a group. For example, Prentice et al 5 measured incidence of breast cancer and per capita intake of dietary fat, and found a correlation that higher per capita intake of dietary fat was associated with an increased incidence of breast cancer. But the study does not conclude specifically which subjects with breast cancer had a higher dietary intake of fat. Thus, one of the limitations with ecologic study designs is that the characteristics are attributed to the whole group and so the individual characteristics are unknown.
Cross‐sectional study
Cross‐sectional studies are study designs used to evaluate an association between an exposure and outcome at the same time. It can be classified under either descriptive or analytic, and therefore depends on the question being answered by the investigator. Since, cross‐sectional studies are designed to collect information at the same point of time, this provides an opportunity to measure prevalence of the exposure or the outcome. For example, a cross‐sectional study design was adopted to estimate the global need for palliative care for children based on representative sample of countries from all regions of the world and all World Bank income groups. 6 The limitation of cross‐sectional study design is that temporal association cannot be established as the information is collected at the same point of time. If a study involves a questionnaire, then the investigator can ask questions to onset of symptoms or risk factors in relation to onset of disease. This would help in obtaining a temporal sequence between the exposure and outcome. 7
Case‐control study
Case‐control studies are study designs that compare two groups, such as the subjects with disease (cases) to the subjects without disease (controls), and to look for differences in risk factors. 8 This study is used to study risk factors or etiologies for a disease, especially if the disease is rare. Thus, case‐control studies can also be hypothesis testing studies and therefore can suggest a causal relationship but cannot prove. It is less expensive and less time‐consuming than cohort studies (described in section “Cohort study”). An example of a case‐control study was performed in Pakistan evaluating the risk factors for neonatal tetanus. They retrospectively reviewed a defined cohort for cases with and without neonatal tetanus. 9 They found a strong association of the application of ghee (clarified butter) as a risk factor for neonatal tetanus. Although this suggests a causal relationship, cause cannot be proven by this methodology (Figure 3 ).

Case‐control study design
One of the limitations of case‐control studies is that they cannot estimate prevalence of a disease accurately as a proportion of cases and controls are studied at a time. Case‐control studies are also prone to biases such as recall bias, as the subjects are providing information based on their memory. Hence, the subjects with disease are likely to remember the presence of risk factors compared to the subjects without disease.
One of the aspects that is often overlooked is the selection of cases and controls. It is important to select the cases and controls appropriately to obtain a meaningful and scientifically sound conclusion and this can be achieved by implementing matching. Matching is defined by Gordis et al as ‘the process of selecting the controls so that they are similar to the cases in certain characteristics such as age, race, sex, socioeconomic status and occupation’ 7 This would help identify risk factors or probable etiologies that are not due to differences between the cases and controls.
Cohort study
Cohort studies are study designs that compare two groups, such as the subjects with exposure/risk factor to the subjects without exposure/risk factor, for differences in incidence of outcome/disease. Most often, cohort study designs are used to study outcome(s) from a single exposure/risk factor. Thus, cohort studies can also be hypothesis testing studies and can infer and interpret a causal relationship between an exposure and a proposed outcome, but cannot establish it (Figure 4 ).

Cohort study design
Cohort studies can be classified as prospective and retrospective. 7 Prospective cohort studies follow subjects from presence of risk factors/exposure to development of disease/outcome. This could take up to years before development of disease/outcome, and therefore is time consuming and expensive. On the other hand, retrospective cohort studies identify a population with and without the risk factor/exposure based on past records and then assess if they had developed the disease/outcome at the time of study. Thus, the study design for prospective and retrospective cohort studies are similar as we are comparing populations with and without exposure/risk factor to development of outcome/disease.
Cohort studies are typically chosen as a study design when the suspected exposure is known and rare, and the incidence of disease/outcome in the exposure group is suspected to be high. The choice between prospective and retrospective cohort study design would depend on the accuracy and reliability of the past records regarding the exposure/risk factor.
Some of the biases observed with cohort studies include selection bias and information bias. Some individuals who have the exposure may refuse to participate in the study or would be lost to follow‐up, and in those instances, it becomes difficult to interpret the association between an exposure and outcome. Also, if the information is inaccurate when past records are used to evaluate for exposure status, then again, the association between the exposure and outcome becomes difficult to interpret.
Case‐control studies based within a defined cohort
Case‐control studies based within a defined cohort is a form of study design that combines some of the features of a cohort study design and a case‐control study design. When a defined cohort is embedded in a case‐control study design, all the baseline information collected before the onset of disease like interviews, surveys, blood or urine specimens, then the cohort is followed onset of disease. One of the advantages of following the above design is that it eliminates recall bias as the information regarding risk factors is collected before onset of disease. Case‐control studies based within a defined cohort can be further classified into two types: Nested case‐control study and Case‐cohort study.
Nested case‐control study
A nested case‐control study consists of defining a cohort with suspected risk factors and assigning a control within a cohort to the subject who develops the disease. 10 Over a period, cases and controls are identified and followed as per the investigator's protocol. Hence, the case and control are matched on calendar time and length of follow‐up. When this study design is implemented, it is possible for the control that was selected early in the study to develop the disease and become a case in the latter part of the study.
Case‐cohort Study
A case‐cohort study is similar to a nested case‐control study except that there is a defined sub‐cohort which forms the groups of individuals without the disease (control), and the cases are not matched on calendar time or length of follow‐up with the control. 11 With these modifications, it is possible to compare different disease groups with the same sub‐cohort group of controls and eliminates matching between the case and control. However, these differences will need to be accounted during analysis of results.
Experimental study design
The basic concept of experimental study design is to study the effect of an intervention. In this study design, the risk factor/exposure of interest/treatment is controlled by the investigator. Therefore, these are hypothesis testing studies and can provide the most convincing demonstration of evidence for causality. As a result, the design of the study requires meticulous planning and resources to provide an accurate result.
The experimental study design can be classified into 2 groups, that is, controlled (with comparison) and uncontrolled (without comparison). 1 In the group without controls, the outcome is directly attributed to the treatment received in one group. This fails to prove if the outcome was truly due to the intervention implemented or due to chance. This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion.
Experimental study designs can be divided into 3 broad categories: clinical trial, community trial, field trial. The specifics of each study design are explained below (Figure 5 ).

Experimental study designs
Clinical trial
Clinical trials are also known as therapeutic trials, which involve subjects with disease and are placed in different treatment groups. It is considered a gold standard approach for epidemiological research. One of the earliest clinical trial studies was performed by James Lind et al in 1747 on sailors with scurvy. 12 Lind divided twelve scorbutic sailors into six groups of two. Each group received the same diet, in addition to a quart of cider (group 1), twenty‐five drops of elixir of vitriol which is sulfuric acid (group 2), two spoonfuls of vinegar (group 3), half a pint of seawater (group 4), two oranges and one lemon (group 5), and a spicy paste plus a drink of barley water (group 6). The group who ate two oranges and one lemon had shown the most sudden and visible clinical effects and were taken back at the end of 6 days as being fit for duty. During Lind's time, this was not accepted but was shown to have similar results when repeated 47 years later in an entire fleet of ships. Based on the above results, in 1795 lemon juice was made a required part of the diet of sailors. Thus, clinical trials can be used to evaluate new therapies, such as new drug or new indication, new drug combination, new surgical procedure or device, new dosing schedule or mode of administration, or a new prevention therapy.
While designing a clinical trial, it is important to select the population that is best representative of the general population. Therefore, the results obtained from the study can be generalized to the population from which the sample population was selected. It is also as important to select appropriate endpoints while designing a trial. Endpoints need to be well‐defined, reproducible, clinically relevant and achievable. The types of endpoints include continuous, ordinal, rates and time‐to‐event, and it is typically classified as primary, secondary or tertiary. 2 An ideal endpoint is a purely clinical outcome, for example, cure/survival, and thus, the clinical trials will become very long and expensive trials. Therefore, surrogate endpoints are used that are biologically related to the ideal endpoint. Surrogate endpoints need to be reproducible, easily measured, related to the clinical outcome, affected by treatment and occurring earlier than clinical outcome. 2
Clinical trials are further divided into randomized clinical trial, non‐randomized clinical trial, cross‐over clinical trial and factorial clinical trial.
Randomized clinical trial
A randomized clinical trial is also known as parallel group randomized trials or randomized controlled trials. Randomized clinical trials involve randomizing subjects with similar characteristics to two groups (or multiple groups): the group that receives the intervention/experimental therapy and the other group that received the placebo (or standard of care). 13 This is typically performed by using a computer software, manually or by other methods. Hence, we can measure the outcomes and efficacy of the intervention/experimental therapy being studied without bias as subjects have been randomized to their respective groups with similar baseline characteristics. This type of study design is considered gold standard for epidemiological research. However, this study design is generally not applicable to rare and serious disease process as it would unethical to treat that group with a placebo. Please see section “Randomization” for detailed explanation regarding randomization and placebo.
Non‐randomized clinical trial
A non‐randomized clinical trial involves an approach to selecting controls without randomization. With this type of study design a pattern is usually adopted, such as, selection of subjects and controls on certain days of the week. Depending on the approach adopted, the selection of subjects becomes predictable and therefore, there is bias with regards to selection of subjects and controls that would question the validity of the results obtained.
Historically controlled studies can be considered as a subtype of non‐randomized clinical trial. In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature. 1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Cross‐over clinical trial
In cross‐over clinical trial study design, there are two groups who undergoes the same intervention/experiment at different time periods of the study. That is, each group serves as a control while the other group is undergoing the intervention/experiment. 14 Depending on the intervention/experiment, a ‘washout’ period is recommended. This would help eliminate residuals effects of the intervention/experiment when the experiment group transitions to be the control group. Hence, the outcomes of the intervention/experiment will need to be reversible as this type of study design would not be possible if the subject is undergoing a surgical procedure.
Factorial trial
A factorial trial study design is adopted when the researcher wishes to test two different drugs with independent effects on the same population. Typically, the population is divided into 4 groups, the first with drug A, the second with drug B, the third with drug A and B, and the fourth with neither drug A nor drug B. The outcomes for drug A are compared to those on drug A, drug A and B and to those who were on drug B and neither drug A nor drug B. 15 The advantages of this study design that it saves time and helps to study two different drugs on the same study population at the same time. However, this study design would not be applicable if either of the drugs or interventions overlaps with each other on modes of action or effects, as the results obtained would not attribute to a particular drug or intervention.
Community trial
Community trials are also known as cluster‐randomized trials, involve groups of individuals with and without disease who are assigned to different intervention/experiment groups. Hence, groups of individuals from a certain area, such as a town or city, or a certain group such as school or college, will undergo the same intervention/experiment. 16 Hence, the results will be obtained at a larger scale; however, will not be able to account for inter‐individual and intra‐individual variability.
Field trial
Field trials are also known as preventive or prophylactic trials, and the subjects without the disease are placed in different preventive intervention groups. 16 One of the hypothetical examples for a field trial would be to randomly assign to groups of a healthy population and to provide an intervention to a group such as a vitamin and following through to measure certain outcomes. Hence, the subjects are monitored over a period of time for occurrence of a particular disease process.
Overview of methodologies used within a study design
Randomization.
Randomization is a well‐established methodology adopted in research to prevent bias due to subject selection, which may impact the result of the intervention/experiment being studied. It is one of the fundamental principles of an experimental study designs and ensures scientific validity. It provides a way to avoid predicting which subjects are assigned to a certain group and therefore, prevent bias on the final results due to subject selection. This also ensures comparability between groups as most baseline characteristics are similar prior to randomization and therefore helps to interpret the results regarding the intervention/experiment group without bias.
There are various ways to randomize and it can be as simple as a ‘flip of a coin’ to use computer software and statistical methods. To better describe randomization, there are three types of randomization: simple randomization, block randomization and stratified randomization.
Simple randomization
In simple randomization, the subjects are randomly allocated to experiment/intervention groups based on a constant probability. That is, if there are two groups A and B, the subject has a 0.5 probability of being allocated to either group. This can be performed in multiple ways, and one of which being as simple as a ‘flip of a coin’ to using random tables or numbers. 17 The advantage of using this methodology is that it eliminates selection bias. However, the disadvantage with this methodology is that an imbalance in the number allocated to each group as well as the prognostic factors between groups. Hence, it is more challenging in studies with a small sample size.
Block randomization
In block randomization, the subjects of similar characteristics are classified into blocks. The aim of block randomization is to balance the number of subjects allocated to each experiment/intervention group. For example, let's assume that there are four subjects in each block, and two of the four subjects in each block will be randomly allotted to each group. Therefore, there will be two subjects in one group and two subjects in the other group. 17 The disadvantage with this methodology is that there is still a component of predictability in the selection of subjects and the randomization of prognostic factors is not performed. However, it helps to control the balance between the experiment/intervention groups.
Stratified randomization
In stratified randomization, the subjects are defined based on certain strata, which are covariates. 18 For example, prognostic factors like age can be considered as a covariate, and then the specified population can be randomized within each age group related to an experiment/intervention group. The advantage with this methodology is that it enables comparability between experiment/intervention groups and thus makes result analysis more efficient. But, with this methodology the covariates will need to be measured and determined before the randomization process. The sample size will help determine the number of strata that would need to be chosen for a study.
Blinding is a methodology adopted in a study design to intentionally not provide information related to the allocation of the groups to the subject participants, investigators and/or data analysts. 19 The purpose of blinding is to decrease influence associated with the knowledge of being in a particular group on the study result. There are 3 forms of blinding: single‐blinded, double‐blinded and triple‐blinded. 1 In single‐blinded studies, otherwise called as open‐label studies, the subject participants are not revealed which group that they have been allocated to. However, the investigator and data analyst will be aware of the allocation of the groups. In double‐blinded studies, both the study participants and the investigator will be unaware of the group to which they were allocated to. Double‐blinded studies are typically used in clinical trials to test the safety and efficacy of the drugs. In triple‐blinded studies, the subject participants, investigators and data analysts will not be aware of the group allocation. Thus, triple‐blinded studies are more difficult and expensive to design but the results obtained will exclude confounding effects from knowledge of group allocation.
Blinding is especially important in studies where subjective response are considered as outcomes. This is because certain responses can be modified based on the knowledge of the experiment group that they are in. For example, a group allocated in the non‐intervention group may not feel better as they are not getting the treatment, or an investigator may pay more attention to the group receiving treatment, and thereby potentially affecting the final results. However, certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention such as quitting smoking.
Placebo is defined in the Merriam‐Webster dictionary as ‘an inert or innocuous substance used especially in controlled experiments testing the efficacy of another substance (such as drug)’. 20 A placebo is typically used in a clinical research study to evaluate the safety and efficacy of a drug/intervention. This is especially useful if the outcome measured is subjective. In clinical drug trials, a placebo is typically a drug that resembles the drug to be tested in certain characteristics such as color, size, shape and taste, but without the active substance. This helps to measure effects of just taking the drug, such as pain relief, compared to the drug with the active substance. If the effect is positive, for example, improvement in mood/pain, then it is called placebo effect. If the effect is negative, for example, worsening of mood/pain, then it is called nocebo effect. 21
The ethics of placebo‐controlled studies is complex and remains a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “The benefits, risks, burdens and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances:
Where no proven intervention exists, the use of placebo, or no intervention, is acceptable; or
Where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the patients who receive any intervention less effective than the best proven one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention.
Extreme care must be taken to avoid abuse of this option”. 22
Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Bias has been defined as “any systematic error in the design, conduct or analysis of a study that results in a mistaken estimate of an exposure's effect on the risk of disease”. 23 There are multiple types of biases and so, in this review we will focus on the following types: selection bias, information bias and observer bias. Selection bias is when a systematic error is committed while selecting subjects for the study. Selection bias will affect the external validity of the study if the study subjects are not representative of the population being studied and therefore, the results of the study will not be generalizable. Selection bias will affect the internal validity of the study if the selection of study subjects in each group is influenced by certain factors, such as, based on the treatment of the group assigned. One of the ways to decrease selection bias is to select the study population that would representative of the population being studied, or to randomize (discussed in section “Randomization”).
Information bias is when a systematic error is committed while obtaining data from the study subjects. This can be in the form of recall bias when subject is required to remember certain events from the past. Typically, subjects with the disease tend to remember certain events compared to subjects without the disease. Observer bias is a systematic error when the study investigator is influenced by the certain characteristics of the group, that is, an investigator may pay closer attention to the group receiving the treatment versus the group not receiving the treatment. This may influence the results of the study. One of the ways to decrease observer bias is to use blinding (discussed in section “Blinding”).
Thus, while designing a study it is important to take measure to limit bias as much as possible so that the scientific validity of the study results is preserved to its maximum.
Overview of drug development in the United States of America
Now that we have reviewed the various clinical designs, clinical trials form a major part in development of a drug. In the United States, the Food and Drug Administration (FDA) plays an important role in getting a drug approved for clinical use. It includes a robust process that involves four different phases before a drug can be made available to the public. Phase I is conducted to determine a safe dose. The study subjects consist of normal volunteers and/or subjects with disease of interest, and the sample size is typically small and not more than 30 subjects. The primary endpoint consists of toxicity and adverse events. Phase II is conducted to evaluate of safety of dose selected in Phase I, to collect preliminary information on efficacy and to determine factors to plan a randomized controlled trial. The study subjects consist of subjects with disease of interest and the sample size is also small but more that Phase I (40–100 subjects). The primary endpoint is the measure of response. Phase III is conducted as a definitive trial to prove efficacy and establish safety of a drug. Phase III studies are randomized controlled trials and depending on the drug being studied, it can be placebo‐controlled, equivalence, superiority or non‐inferiority trials. The study subjects consist of subjects with disease of interest, and the sample size is typically large but no larger than 300 to 3000. Phase IV is performed after a drug is approved by the FDA and it is also called the post‐marketing clinical trial. This phase is conducted to evaluate new indications, to determine safety and efficacy in long‐term follow‐up and new dosing regimens. This phase helps to detect rare adverse events that would not be picked up during phase III studies and decrease in the delay in the release of the drug in the market. Hence, this phase depends heavily on voluntary reporting of side effects and/or adverse events by physicians, non‐physicians or drug companies. 2
We have discussed various clinical research study designs in this comprehensive review. Though there are various designs available, one must consider various ethical aspects of the study. Hence, each study will require thorough review of the protocol by the institutional review board before approval and implementation.
CONFLICT OF INTEREST
Chidambaram AG, Josephson M. Clinical research study designs: The essentials . Pediatr Invest . 2019; 3 :245‐252. 10.1002/ped4.12166 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Living in tree-filled neighborhoods may reduce risk of heart disease, study shows
Living in a tree-filled neighborhood may be as beneficial to the heart as regular exercise, new research shows.
Researchers at the University of Louisville designed a clinical trial that followed hundreds of people living in six low- to middle-income neighborhoods in South Louisville, Kentucky. They used blood and other samples to better understand how their heart risks changed before and after the team planted thousands of mature trees near their homes.
Results from the Green Heart Louisville Project ’s HEAL Study , released Tuesday, showed that people living in neighborhoods with twice as many trees and shrubs had lower levels of a blood marker associated with heart disease, diabetes and some types of cancer compared with those who lived in more tree-bare neighborhoods.

“We are trying to see if we can decrease the rates of heart disease in a community,” said Aruni Bhatnagar, a professor of medicine at the University of Louisville, who led the project.
Most previous studies showing the effects of nature on mental and physical health are observational and can’t answer whether people who live in green communities are healthier because they’re wealthier and have access to better health care.
The HEAL study was set up with a control group and an intervention, meaning something measurable that some of the participants were exposed to during the study but not before.

Bhatnagar and his team recruited about 750 people living in a 4-mile area of South Louisville cut by a highway. The residents were 25 to 75 years old.
Nearly 80% were white, and 60% identified as female. Half reported average household incomes of $50,000.
The researchers collected blood, urine, nail and hair samples, as well as health data, from each person before they began their intervention.
Then, from 2019 to 2022, they planted nearly 8,500 evergreen trees, 630 deciduous trees — the type that lose leaves in the fall — and 45 different types of shrubs in parts of the 4-mile study area, leaving others untouched.
Last year and this year, they took new samples from residents living in both areas.
People living in the intervention areas had 13% lower levels of high-sensitivity C-reactive protein , a blood marker associated with heart disease, including stroke, coronary artery disease and heart attack. The drop was similar to starting a regular exercise routine, Bhatnagar said.
“I wouldn’t have expected such a strong biomarker response, and that speaks to maybe something truly is causal here with how trees impact health,” said Peter James, director of the Center for Occupational and Environmental Health at the University of California, Davis School of Medicine, who wasn’t involved in the new research.

How trees can improve physical health
Previous research has shown spending time in green spaces boosts mental health .
The new study showed the connection between living among more trees and physical health.
Trees provide shade and cool the areas where they’re planted, helping quell the urban heat effect that disproportionately affects low-income neighborhoods and neighborhoods of color. Hot weather aggravates heart disease and can cause heatstroke in people without pre-existing conditions.
Trees also buffer noise, which is linked to higher rates of cardiovascular disease, James said.
“They provide areas for people to relax, exercise, and probably more importantly, socialize,” Joan Casey, an environmental epidemiologist and associate professor of environmental and occupational health sciences at the University of Washington, said in an email.
“They also replace other health-harmful land uses, like industrial sites,” she said.
Because one of the city’s major highways cuts through the study area, Bhatnagar and his team believe, trees’ ability to filter air pollution and buffer neighborhoods from constantly breathing in harmful particles could be a primary way the tree-planting intervention appeared to lower inflammation markers in people living in greened areas.
During the study, the project planted trees only in the parts of South Louisville that had the worst air quality. It took air quality samples before the project, and it is still analyzing how the new tree cover has affected pollution. It’s a complex undertaking, because air quality fluctuates based on the weather — a windy day might increase or decrease air pollution in certain areas, depending on the direction of the wind, and air pollution is worse on hotter days.
The project plans to plant trees in the control group neighborhoods in another three or four years if the intervention neighborhoods continue to show positive results. It also wants to determine whether tree cover improves sleep or children’s immune systems by encouraging outside play.
“There is no sort of ultimate proof,” Bhatnagar said. “But this is the strongest evidence of any study that’s ever been done on trees and their relationship to health.”
Growing evidence shows the importance of ensuring green spaces are equitably distributed around cities, which is currently not the case .
Casey said it’s important that city planners be careful not to create “green gentrification” when they create more equitable access to green spaces in cities — that is, when spaces such as water fronts are restored and housing prices increase as a result, making it unaffordable for current residents to continue living there once a green space is completed.
“The take-home message here is that nature is not an amenity; green spaces are not a perk for the wealthy. They are essential for us as human beings,” James said.
Kaitlin Sullivan is a contributor for NBCNews.com who has worked with NBC News Investigations. She reports on health, science and the environment and is a graduate of the Craig Newmark Graduate School of Journalism at City University of New York.
Anne Thompson is NBC News’ chief environmental affairs correspondent.

IMAGES
VIDEO
COMMENTS
Different kinds of prevention research may study medicines, vitamins, vaccines, minerals, or lifestyle changes. Diagnostic Research refers to the practice of looking for better ways to identify a ...
Observational study. A type of study in which people are observed or certain outcomes are measured. No attempt is made by the researcher to affect the outcome — for example, no treatment is given by the researcher. Clinical trial (interventional study). During clinical trials, researchers learn if a new test or treatment works and is safe.
There are various types of scientific studies such as experiments and comparative analyses, observational studies, surveys, or interviews. The choice of study type will mainly depend on the research question being asked. When making decisions, patients and doctors need reliable answers to a number of questions. Depending on the medical condition and patient's personal situation, the following ...
There are different types of clinical trials that include those which are conducted for treatment, prevention, early detection/screening, and diagnosis. ... Among the various types of clinical research, observational research using a cross-sectional study design is the most frequently performed clinical research. This type of research is ...
There are several types of cancer clinical trials. Each type of trial is designed to answer different research questions and will help researchers learn things that can help people in the future. Treatment Trials. Most cancer clinical trials are treatment studies that involve people who have cancer.
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Phase I trials usually include a small number of people (up to a few dozen). Phase I trials most often include people with different types of cancer. These studies are usually done in major cancer centers. Phase I trials carry the most potential risk. But phase I studies do help some patients.
Understanding Clinical Studies. Part of the challenge of explaining clinical research to the public is describing the important points of a study without going into a detailed account of the study's design. There are many different kinds of clinical studies, each with their own strengths and weaknesses, and no real shorthand way to explain them.
Transcript. ANNOUNCER: There are many different types of clinical research because researchers study many different things. Treatment research usually tests an intervention such as medication, psychotherapy, new devices, or new approaches. Prevention research looks for better ways to prevent disorders from developing or returning.
Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current ...
Scientists may have many reasons for doing a clinical study, such as: • To explore the cause of a disease or a set of symptoms. • To test if a treatment will help with a symptom or condition. • To learn how a certain behavior affects people's health. Diferent types of clinical studies are used in diferent circumstances.
Clinical research is the study of health and illness in people. There are two main types of clinical research: observational studies and clinical trials. Read and share this infographic (PDF, 317K) to learn why researchers do different kinds of clinical studies. Observational studies monitor people in normal settings.
Types of study design. Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. ... data of different trials can be plotted with the point estimate and 95% ...
Clinical research includes all research that involves people. Types of clinical research include: Epidemiology, which improves the understanding of a disease by studying patterns, causes, and effects of health and disease in specific groups. Behavioral, which improves the understanding of human behavior and how it relates to health and disease.
Clinical trials look at new ways to prevent, detect, or treat disease. The goal of clinical trials is to determine if a new test or treatment works and is safe. The idea for a clinical trial —also known as a clinical research study —often originates in the laboratory. After researchers test new therapies or procedures in the laboratory and ...
Clinical trials have four phases, and each serves a specific purpose. Phase I tests safety, determines the range of doses, and identifies any side effects. Phase II gives the treatment to a larger group and tests its effectiveness. Phase III continues to test effectiveness in an even larger group and compares the new treatment to any others ...
Clinical trials, informed consent and ... Understand the different types of research and the four clinical trial phases, such as their purpose and how many people participate in each of the phases.
Email. Clinical research is the study of health and illness in people. Scientists may have many reasons for doing a clinical study, such as: To explore the cause of a disease or a set of symptoms. To test if a treatment will help with a symptom or condition. To learn how a certain behavior affects people's health.
Medical research studies involving people are called clinical trials. There are two main types of trials or studies - interventional and observational. Interventional trials aim to find out more about a particular intervention, or treatment. A computer puts people taking part into different treatment groups.
Different Types of Clinical Trials. In medical research there are two main types of studies: Observational Studies - These studies do not involve a new intervention or treatment option. They aim to find out information by observing the people taking part and see what happens in different situations.
The study types in this area can be found under clinical research and epidemiology. Basic research Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials.
Types of Clinical Research. This page will provide definitions for the following design types: ... Cohort analysis is highly flexible and can provide insight into effects over time and related to a variety of different types of changes [e.g., social, cultural, political, economic, etc.]. ... Historical sources can be used over and over to study ...
About Childhood CancerChildhood Cancer Awareness Month is celebrated each September around the world with the goal of increasing awareness of childhood cancer and advocating for pediatric cancer research and funding. It's estimated that about 400,000 children and adolescents develop cancer globally each year. The most common types of cancer diagnosed in children are leukemia, brain and other ...
However, current research mainly focuses on T cells, and their role in tumor immune monitoring has been widely studied. The mechanism of controlling the occurrence and development of tumors is gradually becoming clear, but there is relatively little research on B cells, which makes their roles in different types of cancer controversial.
The site includes questions and answers about clinical trials, guidance on how to find clinical trials through ClinicalTrials.gov and other resources, and stories about the personal experiences of clinical trial participants. Clinical trials are necessary to find better ways to prevent, diagnose, and treat diseases.
The types of endpoints include continuous, ordinal, rates and time‐to‐event, and it is typically classified as primary, secondary or tertiary. 2 An ideal endpoint is a purely clinical outcome, for example, cure/survival, and thus, the clinical trials will become very long and expensive trials. Therefore, surrogate endpoints are used that ...
Then, from 2019 to 2022, they planted nearly 8,500 evergreen trees, 630 deciduous trees — the type that lose leaves in the fall — and 45 different types of shrubs in parts of the 4-mile study ...