
Chemistry Steps


Organic Chemistry
Organic structure determination, nmr practice problems .
In the following examples, we will learn how to solve NMR practice problems step-by-step in over 100 min video solutions which is essential for organic structure determination.
The emphasis is on the 1 H proton NMR and most problems are based on understanding its key principles such as the number of NMR signals , integration , signal splitting (multiplicity) , and, of course, the strategies of putting all of these together to come up with the correct structure.
Aside from the 1 H NMR , we will also go over determining the hydrogen deficiency index (HDI), solving problems where the 13 C NMR, DEPT , and IR are given along with the 1 H NMR spectrum.
The problems are chosen to demonstrate the most common patterns in 1 H NMR spectroscopy , as well as, the situations where you need to consider the possibility of signal overlapping , incorrect absolute values of integrations , as the instrument measures only the relative area for each peak, examples where fairly large molecules give rise to spectra with few signals because of the symmetry elements . We will also discuss the purpose of shaking the sample with deuterated solvents.
How to determine the structure of an unknown compound using NMR spectroscopy
Two things you will need to do very often:
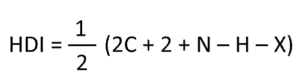
- IR – It will be very beneficial for you to remember key signals in IR spectroscopy so that you can identify the functional groups. Solving IR problems – here .
Before you start working on the first problem or getting stuck on one, let’s go ahead and summarize the most common patterns that you need to recognize.
Common Splitting Patterns in NMR spectroscopy
Start by analyzing the aliphatic region. Many molecules have at least one methyl group and depending on its environment, common splitting patterns are observed.
A triplet with an integration of three represents a methyl with an adjacent CH 2 group. So, look for a triplet and a quartet with integrations 3 and 2 which indicates an ethyl group ( Figure 1 a ).

If there is a singlet with an integration of 3, this represents an isolated methyl group ( b ) . On the other hand, if the singlet integrates to 9 protons, this indicates a tert-Butyl group ( c )
Another common fragment is the combination of a doublet (6H) and a septet ( d ) . This indicates an isopropyl group where the methyl protons are split into a doublet by one adjacent proton and the six equivalent protons of the methyl groups split the signal of that one proton into a septet.
If you see a bunch of triplets each integrating to two protons, think about the -CH 2 -CH 2 – ethylene group ( e ) .
Other Regions and Splitting Patterns
Alkene protons appear at ~5-6 ppm and you can recognize them by the small integration (1 or 2) and complex splitting. This is because all the protons on a double bond are capable of splitting each other and the coupling constants vary anywhere from 5-18 Hz.

Aromatic protons appear at the ~7-8 ppm region. If you have a signal there, the first thing is to check how many groups (or how many protons) are on the aromatic ring. And this is done simply by looking at the integration:

One common splitting pattern in the aromatic region is the presence of two doublets each integrating to two protons. This indicates a symmetrically substituted ring with two groups (1, 4- or para – substituted).

Most other substitution patterns give complex splitting and the easiest for you will be looking at the total integration of all the aromatic signals.
Next functional group recognizable in 1 H NMR spectroscopy is the ~10 ppm signal of aldehydes. Usually, it shows up as a singlet, though splitting with adjacent protons is not uncommon either:

Another way of identifying aldehydes and ketones as well as checking for a ~200 ppm signal on the 13 C NMR. Esters and carboxylic acids appear less downfield – 160-180 ppm.
The OH peak
Like for the IR spectroscopy, the OH peak is a good indicator here as well. Look for a broad peak anywhere from 1-6 ppm. Most often though it will be in the 4-6 range.

Remember also that the OH signal is not split by adjacent protons unless the sample is very dry.
Other groups that give broad, and sometimes, deuterium-exchangeable signals are the amines, amides, and thiols.
Carboxylic acids
If you see a broad signal at 12 ppm, 90% of the time it tells you about a carboxylic acid.

In the following NMR practice problems, we will go over the best strategies you can use for identifying the structure of unknown compounds. As a Chemistry Steps Prime member, you will also get access to the Spectroscopy Summary Sheets in addition to these over 100 min videos of solving NMR problems.
NMR and IR Spectroscopy Summary Sheets
1. How to Solve IR Problems in 3 steps!
2. NMR Spectroscopy – 5 pages that include
- Chemical Shift and Integration
- Number of NMR signals
- Spin-Spin Splitting
- 13 C NMR Spectroscopy
- Determining the Structure of an Unknown
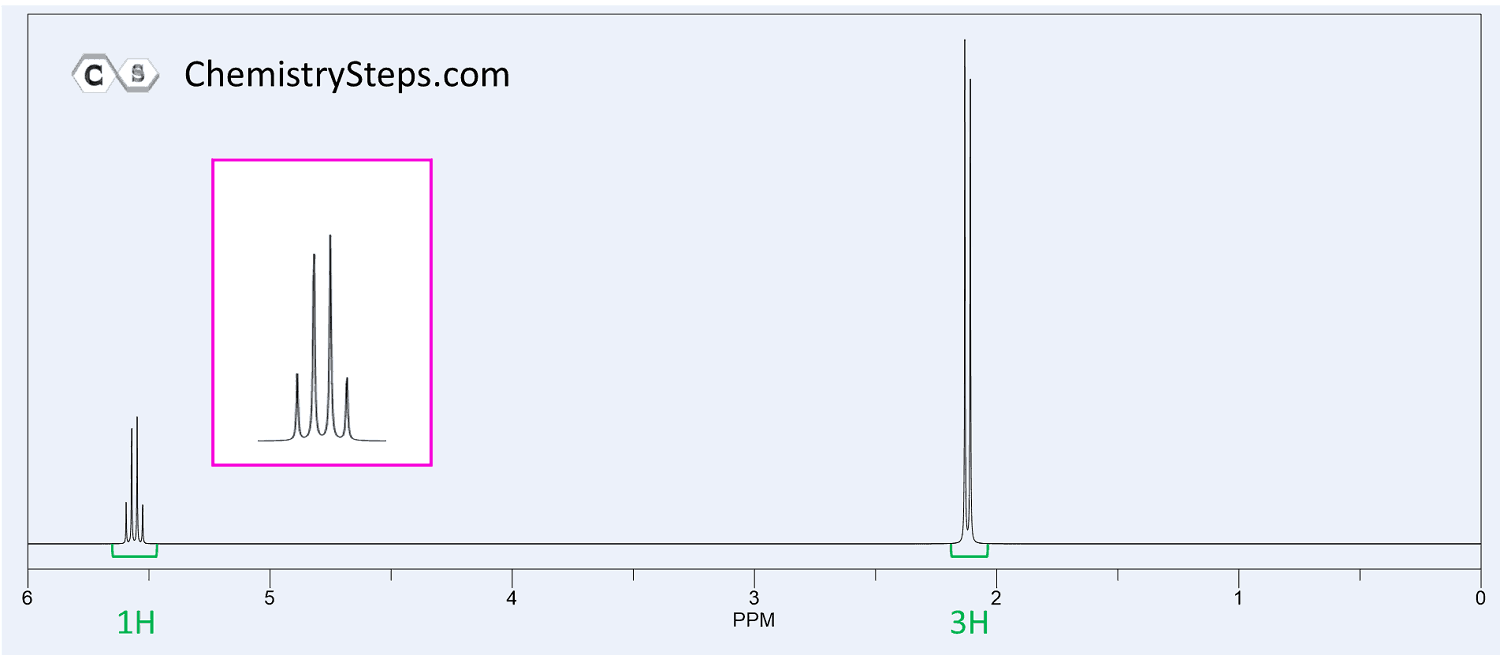
The 1 H NMR spectrum of compound X ( C 4 H 8 O 2 ) is shown below. It also shows a strong IR absorption band near 1730 cm −1 . Propose a structure for X .

This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and t he powerful set of Organic Chemistry 1 and 2 Summary Study Guides .
The 1 H NMR spectrum of compound X ( C 2 H 3 Cl 3 ) is shown below. Propose a structure for X.

The 1 H NMR spectrum of compound X ( C 2 H 4 Cl 2 ) is shown below. Propose a structure for X.
The 1 H NMR and 13 C spectra of compound X ( C 5 H 10 Cl 2 ) are shown below. Propose a structure for X.
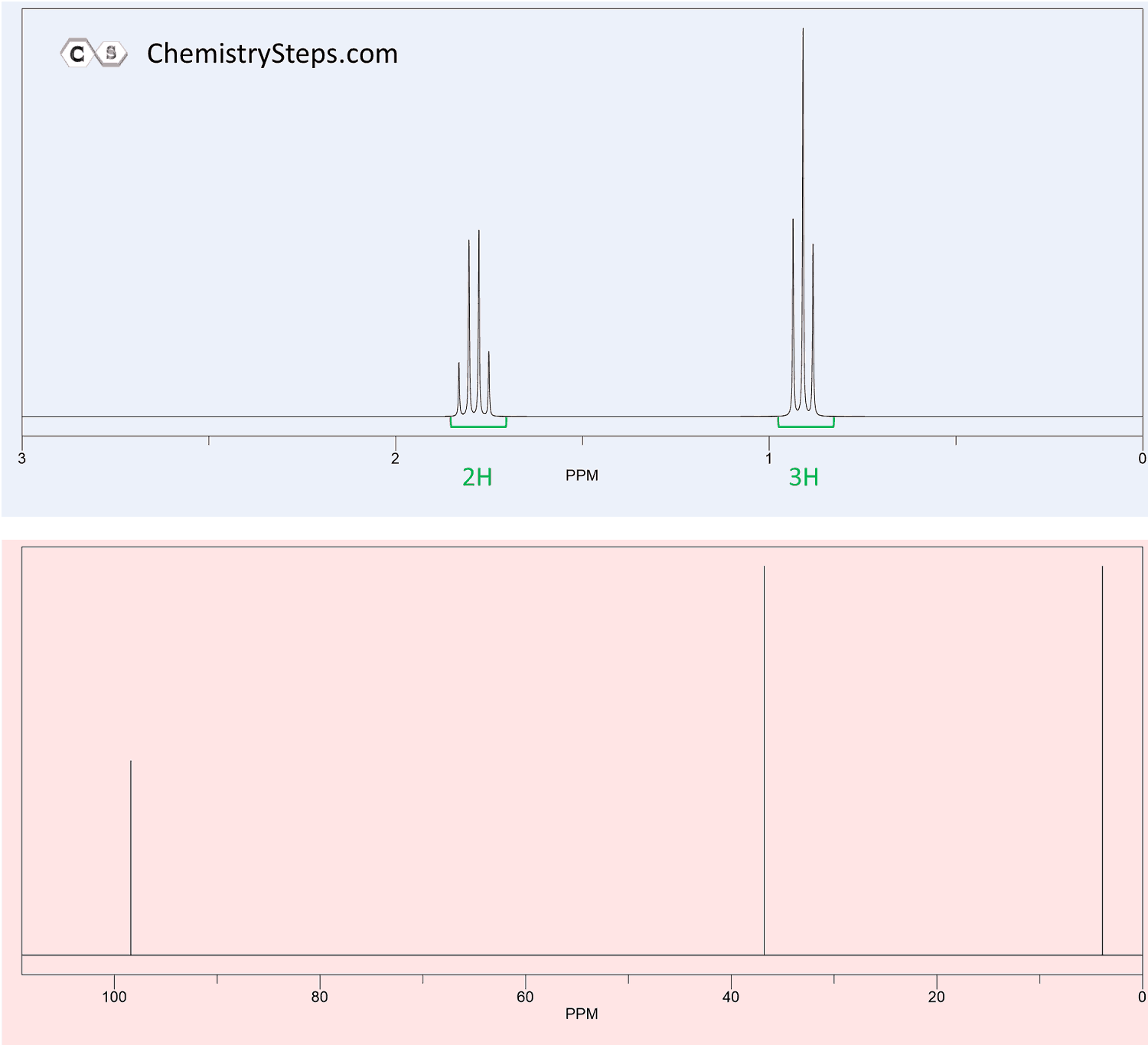
The 1 H NMR and 13 C spectra of compound X (C 6 H 12 O 2 ) are shown below. Propose a structure for X .

The 1 H NMR and 13 C spectra of compound X ( C 10 H 12 O 2 ) are shown below. The 13 C DEPT techniques were also used to identify the carbon types. Propose a structure for X.

The 1 H NMR of compound X ( C 9 H 10 O 2 ) is shown below. Propose a structure for X.

The 1 H NMR and 13 C spectra of compound X ( C 5 H 10 O 2 ) are shown below. Propose a structure for X.

The 1 H NMR and 13 C spectra of compound X ( C 4 H 6 O 2 ) are shown below. Propose a structure for X.

The 1 H NMR of compound X ( C 5 H 8 O 2 ) are shown below. Propose a structure for X.

The 1 H NMR of compound X ( C 5 H 10 O 2 ) are shown below. Propose a structure for X.

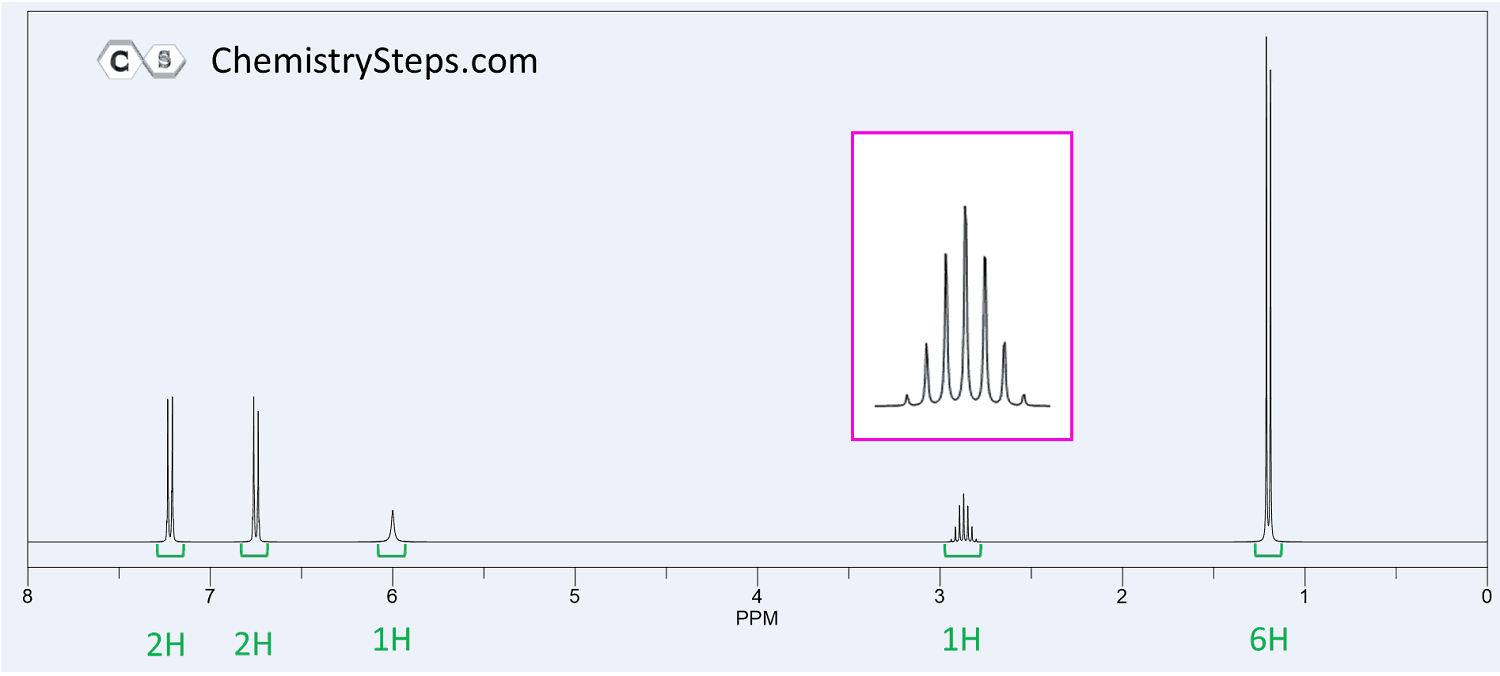
The 1 H NMR of compound X ( C 9 H 12 O ) are shown below. When the sample is mixed and shaken with an excess of deuterium oxide, the signal at 6 ppm disappears. Propose a structure for X.
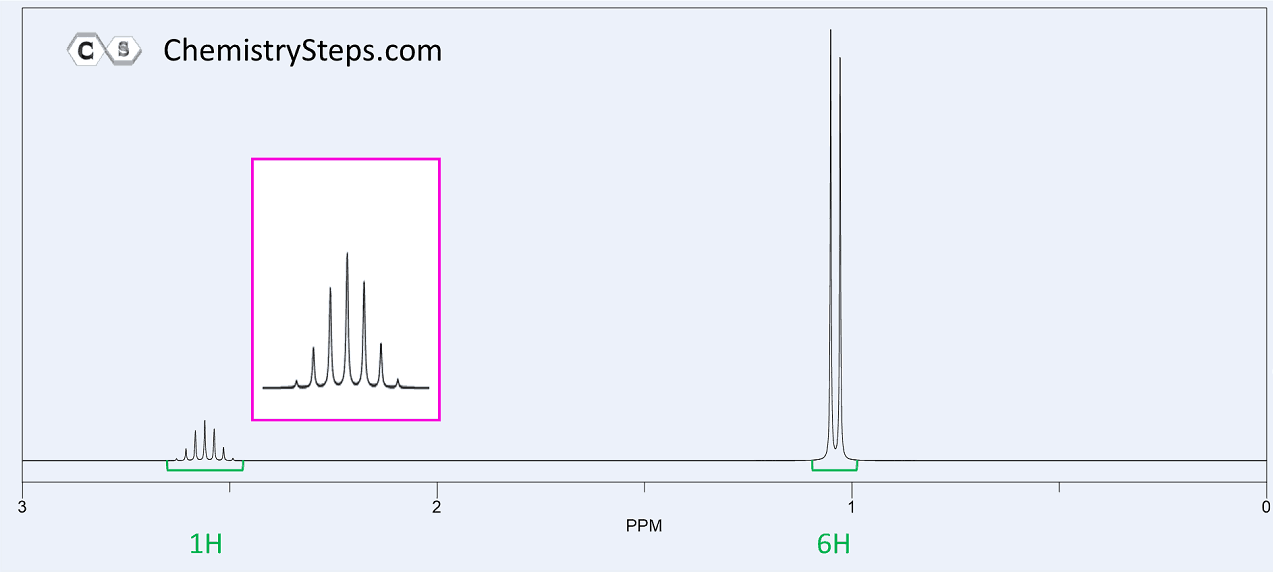
The 1 H NMR of compound X ( C 7 H 14 O ) are shown below. Propose a structure for X.
The 1 H NMR and 13 C spectra of compound X ( C 8 H 10 O ) together with its IR spectrum are shown below. Propose a structure for X.

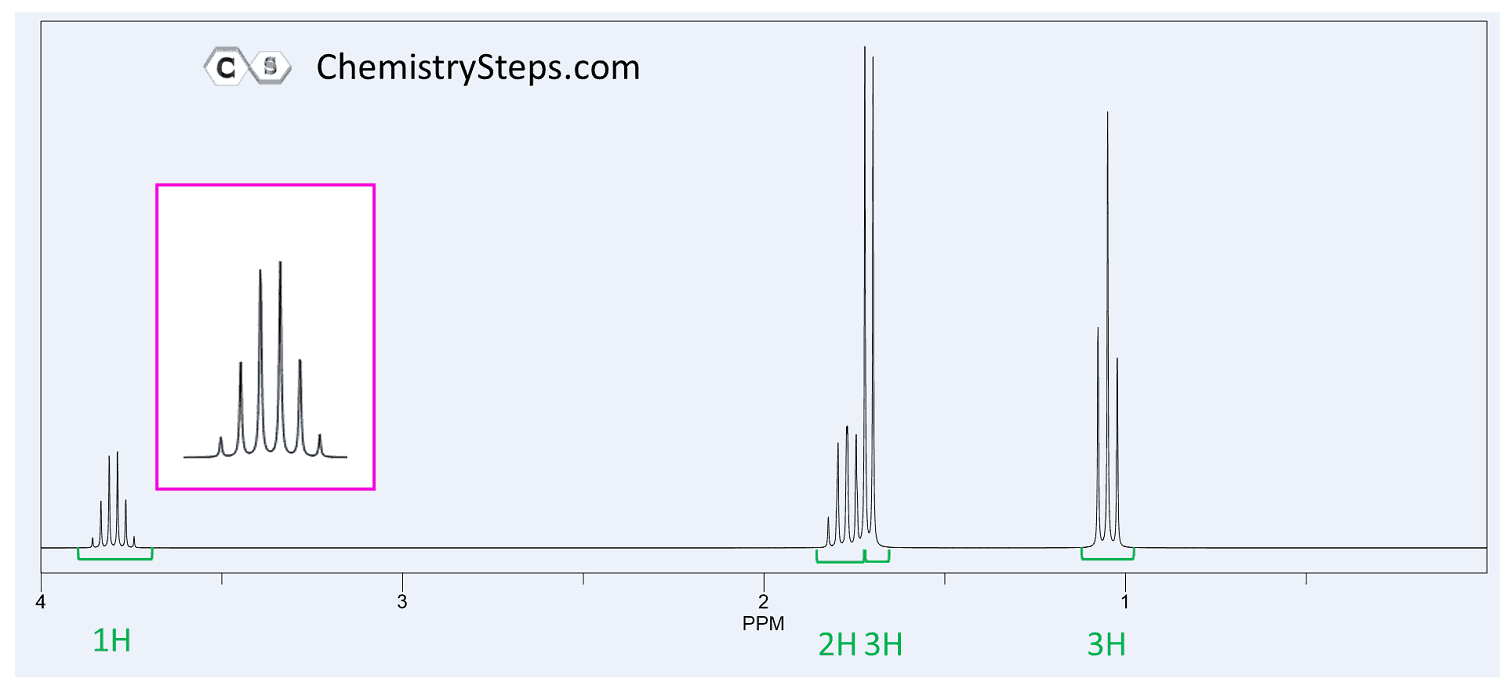
The 1 H NMR of compound X ( C 4 H 9 Br ) are shown below. Propose a structure for X.
The 1 H NMR spectra of three isomers of Butanol are shown below. Draw the structures of all the isomers for butanol and assign each spectrum to the correct isomer.

- NMR spectroscopy – An Easy Introduction
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic Enantiotopic Diastereotopic and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13 C NMR NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems
6 thoughts on “NMR Practice Problems – Solving Strategies”
Thank you! These strategies are great and I feel more optimistic about NMR questions. Hopefully I won’t get them though… : )
I hope you got the NMR right on the test.
Super helpful for understanding NMR. Thank you!
Great to hear, Pokharel.
This was great! Thank you
You are welcome, Andrew.
Leave a Comment Cancel reply
Notify me of followup comments via e-mail. You can also subscribe without commenting.
Have we been helpful? Please let us know in the Reviews section here.
WassUp 1.9.4.5 timestamp: 2024-09-10 04:33:27PM UTC (11:33AM) If above timestamp is not current time, this page is cached.
When you look at an NMR spectrum, do you see only a bunch of disordered lines or peaks? Then you have come to the right place. This site was established to provide people interested in NMR with a library of NMR spectroscopy problems. Interpretation of spectra is a skill that requires pattern recognition and/or practice to master the chaos. This site provides one dimensional spectra of different nuclei, COSY, HSQC, HMBC and some less common spectra of various compounds for you to interpret, together with worked solutions. Hopefully, these problems will provide a useful resource to help you better understand NMR spectral interpretation.
A series of about 50 problems is available in printable form. The print version will not be developed further. The PDF documents are still available (german only). Get them here:
The step-by-step approach works nearly perfectly on the desktop and most tablets. On cellular phones, PowerPoint documents are sometimes displayed incorrectly. There is a workaround, but it is unfortunately much too complicated to be practical. As an alternative, all documents can be saved locally. Feel free to follow the download links. A free PowerPoint viewer is available in both the iOS and Android AppStores. As a bonus, there would be no need for a permanent internet connection. Of course, this offline method also works with desktop computers.
The author is always grateful for criticism, suggestions, references to errors and information about the design via email:
Pair your accounts.
Export articles to Mendeley
Get article recommendations from ACS based on references in your Mendeley library.
You’ve supercharged your research process with ACS and Mendeley!
Please note: If you switch to a different device, you may be asked to login again with only your ACS ID.
Please login with your ACS ID before connecting to your Mendeley account.
- ACS Publications
- Citation and abstract
- Citation and references
- More citation options
- X (Twitter)
- Expand Collapse
OR SEARCH CITATIONS
You have not visited any articles yet, Please visit some articles to see contents here.
- publications
- Recently Viewed
- Access Options
- Authors & Reviewers
- ACS Members
- Curated Content
- RSS & Mobile
- Products & Services
- Manage My Account
- Website Demos & Tutorials
- Support FAQs
- Live Chat with Agent
- For Advertisers
- For Librarians & Account Managers
- Pair a device
- My Profile Login Logout Pair a device
- ACS & Open Access
CONTENT TYPES
This publication is free to access through this site. Learn More
NMR-Challenge.com: An Interactive Website with Exercises in Solving Structures from NMR Spectra Click to copy article link Article link copied!
- Zuzana Osifová Zuzana Osifová Institute of Organic Chemistry and Biochemistry AS CR, Flemingovo nám. 2, 160 00 Prague, Czech Republic Department of Organic Chemistry, Faculty of Science, Charles University, Hlavova 2030, 128 00 Prague, Czech Republic More by Zuzana Osifová
Journal of Chemical Education
Publication history.
Received
Revised
Published
Copyright © Published 2023 by American Chemical Society and Division of Chemical Education, Inc. This publication is available under these Terms of Use .
Nuclear magnetic resonance (NMR) spectroscopy is a very powerful analytical method that has found many applications in physics, chemistry, biology, and medicine. It is an indispensable tool particularly for synthetic chemists because it can be used for a rapid elucidation of the structures of organic compounds. For this reason, NMR spectral interpretation is a part of the core knowledge of all chemistry students, taught to them from the first years of their undergraduate studies. However, NMR spectral interpretation skills can only be learned by experience. We present a new educational website that includes more than 160 NMR spectral assignments measured for real samples. Each assignment contains NMR spectra of an unknown compound, with the task for the students being to determine its structure. Basic assignments contain only one-dimensional 1 H and 13 C NMR spectra; advanced assignments also offer two-dimensional correlation spectra. The web application is enriched with an interactive chemical structure drawing tool, which gives the users immediate feedback about their proposed structures. The presented assignments are graded according to difficulty to keep both beginners and advanced students motivated. The web page will be continuously updated with new spectral problems.
This publication is licensed for personal use by The American Chemical Society.
Article subjects are automatically applied from the ACS Subject Taxonomy and describe the scientific concepts and themes of the article.
- Chemical structure
- Molecular structure
- Nuclear magnetic resonance spectroscopy
Article keywords are supplied by the authors and highlight key terms and topics of the paper.
- Second-Year Undergraduate
- Organic Chemistry
- Computer-Based Learning
- Distance Learning/Self Instruction
- NMR Spectroscopy
Introduction
Figure 1. (A) Interface of the website. (B) Example of 1D spectra with integral intensities, peak picking, and expansions of the split signals. (C) Interactive drawing web application with an immediate response to the submitted solution.
Figure 2. (A) Structure with the “Correct” response. (B) The interface with the correctly answered assignment marked in green.
Figure 3. Response of the drawing web application to (A) a wrong structure with an incorrect molecular formula and (B) a wrongly assigned structure.
Figure 4. HMBC spectrum with the expansions of the crowded cross-peak regions.
Figure 5. Screenshots of (A) the website interface and (B) the chemical structure-drawing tool on a mobile phone. The assignment can be selected using the dropdown menu in the interface.
Figure 6. 1D and 2D NMR spectra displayed in the (A) day and (B) night modes of the website.
Website Usage
Figure 7. Ten most common structures submitted as solutions to Task 24 (the total number of submissions up to October 31, 2022 is 784). The correct structure is 4-ethoxybenzaldehyde.
Materials and Methods
Supporting information.
Video explanation of an incorrectly assigned structure ( MP4 )
- ed2c01067_si_001.mp4 (13.36 MB)
Terms & Conditions
Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system: http://pubs.acs.org/page/copyright/permissions.html .
Author Information
- Zuzana Osifová - Institute of Organic Chemistry and Biochemistry AS CR, Flemingovo nám. 2, 160 00 Prague, Czech Republic ; Department of Organic Chemistry, Faculty of Science, Charles University, Hlavova 2030, 128 00 Prague, Czech Republic
- Notes The authors declare no competing financial interest.

Acknowledgments
This work was supported by the Czech Science Foundation (Grant 22-15374S).
This article references 25 other publications.
- 1 Kibrik, E. J. ; Steinhof, O. ; Scherr, G. ; Thiel, W. R. ; Hasse, H. On-Line NMR Spectroscopic Reaction Kinetic Study of Urea-Formaldehyde Resin Synthesis . Ind. Eng. Chem. Res. 2014 , 53 , 12602 – 12613 , DOI: 10.1021/ie5001746 Google Scholar 1 On-Line NMR Spectroscopic Reaction Kinetic Study of Urea-Formaldehyde Resin Synthesis Kibrik, Eleonore J.; Steinhof, Oliver; Scherr, Guenter; Thiel, Werner R.; Hasse, Hans Industrial & Engineering Chemistry Research ( 2014 ), 53 ( 32 ), 12602-12613 CODEN: IECRED ; ISSN: 0888-5885 . ( American Chemical Society ) Quant. online NMR spectroscopy is used to study the kinetics of the reaction of aq. formaldehyde and urea. The investigation focuses on the formation of low mol. mass compds. during the methylolation step. The expts. were carried out at overall formaldehyde to urea molar ratios between 1:1 and 4:1, pH values between 6 and 8, and temps. between 313 and 353 K. The exptl. data were used to develop a kinetic model based on the true species concns. The model describes the exptl. data well and can be used to predict the compn. of the reacting mixt. of aq. formaldehyde and urea during the methylolation step as a function of time. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2cXht1ans77I&md5=970188ca9b518c2b04ca451b0a955df9
- 2 Jaroszewicz, M. J. ; Liu, M. X. ; Kim, J. ; Zhang, G. N. ; Kim, Y. ; Hilty, C. ; Frydman, L. Time- and site-resolved kinetic NMR for real-time monitoring of off-equilibrium reactions by 2D spectrotemporal correlations . Nat. Commun. 2022 , 13 , 833 , DOI: 10.1038/s41467-022-28304-w Google Scholar 2 Time- and site-resolved kinetic NMR for real-time monitoring of off-equilibrium reactions by 2D spectrotemporal correlations Jaroszewicz, Michael J.; Liu, Mengxiao; Kim, Jihyun; Zhang, Guannan; Kim, Yaewon; Hilty, Christian; Frydman, Lucio Nature Communications ( 2022 ), 13 ( 1 ), 833 CODEN: NCAOBW ; ISSN: 2041-1723 . ( Nature Portfolio ) Abstr.: NMR (NMR) spectroscopy provides detailed information about dynamic processes through line-shape changes, which are traditionally limited to equil. conditions. However, a wealth of information is available by studying chem. reactions under off-equil. conditions-e.g., in states that arise upon mixing reactants that subsequently undergo chem. changes-and in monitoring the reactants and products in real time. Herein, we propose and demonstrate a time-resolved kinetic NMR expt. that combines rapid mixing techniques, continuous flow, and single-scan spectroscopic imaging methods, leading in unison to a 2D spectrotemporal NMR correlation that provides high-quality kinetic information of off-equil. chem. reactions. These kinetic 2D NMR spectra possess a high-resoln. spectral dimension revealing the individual chem. sites, correlated with a time-independent, steady-state spatial axis that delivers information concerning temporal changes along the reaction coordinate. A comprehensive description of the kinetic, spectroscopic, and exptl. features assocd. with these spectrotemporal NMR analyses is presented. Exptl. demonstrations are carried out using an enzymically catalyzed reaction leading to site- and time-resolved kinetic NMR data, that are in excellent agreement with control expts. and literature values. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BB38XjsVGrsbg%253D&md5=cc9366e8bde9420adba10a18b8c5a444
- 3 Susanne, F. ; Smith, D. S. ; Codina, A. Kinetic Understanding Using NMR Reaction Profiling . Org. Process Res. Dev. 2012 , 16 , 61 – 64 , DOI: 10.1021/op200202k Google Scholar 3 Kinetic Understanding Using NMR Reaction Profiling Susanne, Flavien; Smith, David S.; Codina, Anna Organic Process Research & Development ( 2012 ), 16 ( 1 ), 61-64 CODEN: OPRDFK ; ISSN: 1083-6160 . ( American Chemical Society ) The combination of kinetic understanding and reaction modeling was successfully applied to the development of processes from lab. to manufg. plant. Although extensively used in bulk chem., polymers, and the oil industry, it was not exploited to its full potential in the pharmaceutical industry. The authors present a fast and efficient methodol. for kinetic modeling of chem. reactions using 1H NMR reaction monitoring that can be used for the process understanding and development of active pharmaceutical ingredients. The parameters that are important for the development of a good, reliable model for the prediction and optimization of reaction conditions are discussed. The hydrolysis of acetic anhydride was chosen to illustrate the methodol. because it is mechanistically and kinetically well established. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3MXhtlOgu77J&md5=4eeafa5413260b38d24f4fdcd1650f87
- 4 Dračínský, M. The Chemical Bond: The Perspective of NMR Spectroscopy . Annu. Rep. NMR Spectrosc. 2017 , 90 , 1 – 40 , DOI: 10.1016/bs.arnmr.2016.07.001 Google Scholar 4 The chemical bond: the perspective of NMR spectroscopy Dracinsky, M. Annual Reports on NMR Spectroscopy ( 2017 ), 90 ( ), 1-40 CODEN: NMRPAJ ; ISSN: 0066-4103 . ( Elsevier Ltd. ) This review article deals with chem. bonding from the perspective of NMR (NMR) spectroscopy. Different types of chem. bonds are briefly discussed and the relation between chem. bonding and NMR observables is overviewed. The various NMR interactions are classified in terms of through-bond and through-space effects on their observable values. The effects of vibrational motion and nuclear delocalization on NMR parameters and the consequences for the exptl. detn. of bond distances are also discussed. Finally, NMR expts. in studies of hydrogen bonding are summarized in Section 5. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2sXhtFSjsb3E&md5=0251de3f684e18d9fa5ffb122b45fb9c
- 5 Lehmann, J. ; Kleinpeter, E. ; Krechl, J. 1 H NMR Spectroscopy as a Probe of Intermolecular Interactions in β-Cyclodextrin Inclusion-Compounds . J. Inclusion Phenom. Mol. Recognit. Chem. 1991 , 10 , 233 – 239 , DOI: 10.1007/BF01066207 Google Scholar 5 Proton NMR spectroscopy as a probe of intermolecular interactions in β-cyclodextrin inclusion compounds Lehmann, J.; Kleinpeter, E.; Krechl, J. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry ( 1991 ), 10 ( 2 ), 233-9 CODEN: JIMCEN ; ISSN: 0923-0750 . 1H NMR spectroscopy was used to probe the formation of inclusion compds. of permethylated and peracetylated β-cyclodextrins as host mols. and a variety of electronically very different guest mols. Complexation, obtained only in water, was estd. quant. by means of chem. shift/concn. curves of relevant protons, and the intermol. forces involved are critically discussed. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADyaK3MXisVKksLk%253D&md5=0ab5b9e7e7f34586a2264c8b16aa6a87
- 6 Xu, Y. J. ; Kumar, V. ; Bradshaw, M. J. Z. ; Bryce, D. L. Chalcogen-Bonded Cocrystals of Substituted Pyridine N-Oxides and Chalcogenodiazoles: An X-ray Diffraction and Solid-State NMR Investigation . Cryst. Growth Des. 2020 , 20 , 7910 – 7920 , DOI: 10.1021/acs.cgd.0c01173 Google Scholar 6 Chalcogen-Bonded Cocrystals of Substituted Pyridine N-Oxides and Chalcogenodiazoles: An X-ray Diffraction and Solid-State NMR Investigation Xu, Yijue; Kumar, Vijith; Bradshaw, Maressa J. Z.; Bryce, David L. Crystal Growth & Design ( 2020 ), 20 ( 12 ), 7910-7920 CODEN: CGDEFU ; ISSN: 1528-7483 . ( American Chemical Society ) Me, MeO, and Ph substituents are introduced at the para-, meta-, and ortho- positions of pyridine N-oxide to study the effect of chem. substitution on the resulting 9 chalcogen-bonded structures formed upon cocrystn. with 3,4-dicyano-1,2,5-selenodiazole and 3,4-dicyano-1,2,5-telluradiazole. Single-crystal x-ray diffraction studies reveal double chalcogen bonding interactions in the cocrystals and demonstrate the impact of the substitution on the geometric features of the chalcogen bonds. 77Se and 125Te solid-state NMR spectroscopy is employed to measure Se and Te chem. shift tensors of the products, and various trends are described. The smallest component of the 77Se chem. shift tensor (δ33) provides the strongest correlation with the chalcogen bond distance. Soln. NMR provides qual. evidence for the persistence of the chalcogen bonds in soln. 1J(77Se,14N) coupling consts. in 3,4-dicyano-1,2,5-selenodiazole and its chalcogen-bonded cocrystals are measured after accounting for residual dipolar coupling between 77Se and 14N; however, changes in 1J(77Se,14N) attributable to chalcogen bonding upon cocrystn. are comparable to the exptl. uncertainties. This systematic study of chalcogen-bonded cocrystals demonstrates the potential utility of the substitution effect for applications of chalcogen bonds in crystal engineering and demonstrates the value of solid-state NMR in characterizing such systems. Chalcogen-bonded cocrystals based on 3,4-dicyano-1,2,5-selenodiazole and 3,4-dicyano-1,2,5-telluradiazole are reported. Various substituted pyridine N-oxides act as electron donors. X-ray diffraction and 77Se/125Te solid-state NMR are employed to characterize these novel materials. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BB3cXitFCqtLjM&md5=86728f3792353c8d645dd45adac74c82
- 7 Chae, Y. ; Min, S. ; Park, E. ; Lim, C. ; Cheon, C. H. ; Jeong, K. ; Kwak, K. ; Cho, M. Real-Time Reaction Monitoring with In Operando Flow NMR and FTIR Spectroscopy: Reaction Mechanism of Benzoxazole Synthesis . Anal. Chem. 2021 , 93 , 2106 – 2113 , DOI: 10.1021/acs.analchem.0c03852 Google Scholar 7 Real-Time Reaction Monitoring with In Operando Flow NMR and FTIR Spectroscopy: Reaction Mechanism of Benzoxazole Synthesis Chae, Yeongseok; Min, Sein; Park, Eunjoon; Lim, Chaiho; Cheon, Cheol-Hong; Jeong, Keunhong; Kwak, Kyungwon; Cho, Minhaeng Analytical Chemistry (Washington, DC, United States) ( 2021 ), 93 ( 4 ), 2106-2113 CODEN: ANCHAM ; ISSN: 0003-2700 . ( American Chemical Society ) In operando observation of reaction intermediates is crucial for unraveling reaction mechanisms. To address the sensitivity limitations of com. ReactIR, a flow cell was integrated with a Fourier transform IR (FTIR) spectrometer yielding a "flow FTIR" device coupled with an NMR spectrometer for the elucidation of reaction mechanisms. The former device detects the low-intensity IR peaks of reaction intermediates by adjusting the path length of the FTIR sample cell, whereas the flow NMR allows the quant. anal. of reaction species, thus offsetting the limitations of IR spectroscopy resulting from different absorption coeffs. of the normal modes. Using the flow NMR and FTIR device, the controversial mechanism of benzoxazole synthesis was conclusively detd. by spectroscopic evaluation of the reaction intermediates. This system enabled the accurate acquisition of previously elusive kinetic data, such as the reaction time and rate-detg. step. The implementation of reaction flow cells into NMR and FTIR systems could be widely applied to study various reaction mechanisms, including dangerous and harsh reactions, thus avoiding contact with potentially harmful reaction intermediates. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BB3MXhtleh&md5=fb2b6f51682e6c1a2082a03d106a1f1c
- 8 Janková, Š. ; Dračínský, M. ; Císařová, I. ; Kotora, M. Synthesis and Rearrangement of Dewar Benzenes Into Biaryls: Experimental Evidence for Conrotary Ring Opening . Eur. J. Org. Chem. 2008 , 2008 , 47 – 51 , DOI: 10.1002/ejoc.200700916 Google Scholar There is no corresponding record for this reference.
- 9 Merlic, C. A. ; Fam, B. C. ; Miller, M. M. WebSpectra: Online NMR and IR spectra for students . J. Chem. Educ. 2001 , 78 , 118 – 120 , DOI: 10.1021/ed078p118 Google Scholar 9 WebSpectra: online NMR and IR spectra for students Merlic, Craig A.; Fam, Barry C.; Miller, Michael M. Journal of Chemical Education ( 2001 ), 78 ( 1 ), 118-120 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( Division of Chemical Education of the American Chemical Society ) Computers and the World Wide Web (WWW) in particular have become important and powerful tools for teaching chem. WebSpectra is a WWW site implemented at the University of California at Los Angeles (http://www.chem.ucla.edu/webspectra). Through this site, students have convenient and free access to a library of problems in NMR (NMR) and IR (IR) spectroscopy, ranging in difficulty from introductory to advanced. An important feature is that expansions of the high-resoln. spectra can be controlled by the user. The site also includes instructional materials and tools to assist students in learning about NMR and IR spectroscopy. The design and capabilities of this Web-based interface are hereby described. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD3MXptVKj&md5=c5c272e986df00b1e0887761bca78eab
- 10 Kolonko, E. M. ; Kolonko, K. J. Introducing NMR Spectroscopy Using Guided Inquiry and Partial Structure Templating . J. Chem. Educ. 2019 , 96 , 912 – 919 , DOI: 10.1021/acs.jchemed.8b00660 Google Scholar 10 Introducing NMR Spectroscopy Using Guided Inquiry and Partial Structure Templating Kolonko, Erin M.; Kolonko, Kristopher J. Journal of Chemical Education ( 2019 ), 96 ( 5 ), 912-919 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) A review. Strategies for teaching NMR spectral interpretation in the undergraduate org. chem. curriculum are often faculty-centered and can lead to student reliance on rote memorization and "guess and check" methods rather than crit.-thinking skills for structure detn. This article describes a student-focused methodol. for the introduction of NMR spectral interpretation. Guided-inquiry tutorials using NMR prediction tools were developed to enable students to investigate the trends and concepts in 13C and 1H NMR spectral interpretation, with an emphasis on making connections between data and foundational chem. knowledge. A systematic approach to solving unknown structure problems is presented, providing a framework for students to organize spectral data and to build mols. from partial structures. The success of this NMR spectroscopy teaching strategy, which can be adapted for either lab. or lecture environments, was demonstrated both in pos. student survey responses as well as in quant. data showing a significant improvement in exam question scores. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC1MXnsVCnsLY%253D&md5=d3b0b4a4f19b4274fec39ac7ecfe8472
- 11 Henderson, D. E. A Chemical Instrumentation Game for Teaching Critical Thinking and Information Literacy in Instrumental Analysis Courses . J. Chem. Educ. 2010 , 87 , 412 – 415 , DOI: 10.1021/ed800110f Google Scholar 11 A Chemical Instrumentation Game for Teaching Critical Thinking and Information Literacy in Instrumental Analysis Courses Henderson, David E. Journal of Chemical Education ( 2010 ), 87 ( 4 ), 412-415 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) A simulation game is used to teach students in instrumental anal. courses to find the latest developments in the field, use the journal literature, and apply crit. thinking to det. the relative importance of the work they find. They also learn about the business of chem. instruments and to make oral presentations. The competitive aspects of the game provide addnl. motivation and increase student engagement with the literature. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3cXislWktrk%253D&md5=ea38a57811815c289582efef253ffb8f
- 12 Carey, F. A. Organic Chemistry , 11 th ed.; McGraw Hill , 2019 . Google Scholar There is no corresponding record for this reference.
- 13 McMurry, J. Organic Chemistry , 9 th ed.; Cengage Learning , 2015 . Google Scholar There is no corresponding record for this reference.
- 14 Vollhardt, K. P. C. ; Schore, N. E. Organic Chemistry: Structure and Function , 7 th ed.; W. H. Freeman , 2014 . Google Scholar There is no corresponding record for this reference.
- 15 Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry , 3 rd ed.; Wiley-VCH , 2013 . Google Scholar There is no corresponding record for this reference.
- 16 Silverstein, R. M. ; Webster, F. X. ; Kiemle, D. J. ; Bryce, D. L. Spectrometric Identification of Organic Compounds , 8 th ed.; Wiley : Hoboken, NJ , 2014 . Google Scholar There is no corresponding record for this reference.
- 17 Field, L. D. ; Li, H. L. ; Magill, A. M. Organic Structures from Spectra , 6 th ed.; Wiley : Hoboken, NJ , 2020 . Google Scholar There is no corresponding record for this reference.
- 18 Crews, P. ; Rodriguez, J. ; Jaspars, M. Organic Structure Analysis , 2 nd ed.; Oxford University Press , 2009 . Google Scholar There is no corresponding record for this reference.
- 19 Merlic, C. A. ; Fam, B. C. WebSpectra: Problems in NMR and IR Spectroscopy . https://webspectra.chem.ucla.edu/ (accessed October 2022). Google Scholar There is no corresponding record for this reference.
- 20 Yamazaki, T. ; Nabeshima, M. ; Saito, T. ; Yamaji, T. ; Hayamizu, K. ; Yanagisawa, M. ; Yamamoto, O. Spectral Database for Organic Compounds, SDBS . https://sdbs.db.aist.go.jp (accessed October 2022). Google Scholar There is no corresponding record for this reference.
- 21 Flynn, A. B. NMR Interpretation: Getting from Spectrum to Structure . J. Chem. Educ. 2012 , 89 , 1210 – 1212 , DOI: 10.1021/ed3000974 Google Scholar 21 NMR Interpretation: Getting from Spectrum to Structure Flynn, Alison B. Journal of Chemical Education ( 2012 ), 89 ( 9 ), 1210-1212 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) A tactile problem-solving strategy is described that helped students analyze spectral data, primarily from 1H NMR spectra, to det. the structures of unknown compds. Two important elements in that process were (i) students organized their anal. in a table and (ii) they drew each mol. fragment, deduced from their anal., on a sep. sticky note. Possible combinations of the fragments were analyzed by arranging the sticky notes in different ways, comparing back to the given data to det. the most plausible structure of the unknown compd. As part of the structure detn. lesson in a large class, a document camera served to project the sticky notes to the screen and student involvement was encouraged. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC38XhtVOksbjM&md5=721874f5a009af439484dd8104ca86eb
- 22 Haeßner, R. NMR exercises: NMR spectral interpretation based on examples . https://nmr.tips/indexe.html (accessed October 2022). Google Scholar There is no corresponding record for this reference.
- 23 Smith, B. D. ; Boggess, B. ; Zajicek, J. Organic Structure Elucidation: A Workbook of Unknowns . https://structureworkbook.nd.edu/ (accessed October 2022). Google Scholar There is no corresponding record for this reference.
- 24 Alexander, C. W. ; Asleson, G. L. ; Doig, M. T. ; Heldrich, F. J. Spectroscopic instruction in introductory organic chemistry: Results of a national survey . J. Chem. Educ. 1999 , 76 , 1294 – 1296 , DOI: 10.1021/ed076p1294 Google Scholar 24 Spectroscopic instruction in introductory organic chemistry: results of a national survey Alexander, Christopher W.; Asleson, Gary L.; Doig, Marion T.; Heldrich, Frederick J. Journal of Chemical Education ( 1999 ), 76 ( 9 ), 1294-1296 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( Division of Chemical Education of the American Chemical Society ) A national survey was conducted to det. how instructors of the traditional introductory org. chem. courses are handling spectroscopic instruction. A questionnaire was sent to all chem. departments offering ACS-certified degrees and 52% responded. Results showed that spectroscopy has become a core part of the introductory org. course. Significantly, it is more likely to be incorporated into the lab. curriculum when students are able to have hands-on exposure to instruments. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADyaK1MXlslWhtLw%253D&md5=22b7d6fea794584c079c81ec853ab5c1
- 25 Vosegaard, T. iSpec: A Web-Based Activity for Spectroscopy Teaching . J. Chem. Educ. 2018 , 95 , 97 – 103 , DOI: 10.1021/acs.jchemed.7b00482 Google Scholar 25 iSpec: A Web-Based Activity for Spectroscopy Teaching Vosegaard, Thomas Journal of Chemical Education ( 2018 ), 95 ( 1 ), 97-103 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) Students' skills in structure elucidation of org. mols. are developed by training them to understand advanced spectroscopic measurements and elucidate structures of small org. mols. from mass spectrometry (MS) and IR (IR), UV, and 1H and 13C NMR (NMR) spectroscopic data. The present work puts a specific focus on developing student skills to ensure that the students (i) are aware of the resources involved in obtaining the spectral data and (ii) obtain the skills necessary to handle spectra which have not been processed by experts, to better reflect the use of spectroscopic techniques in their later work life. This is achieved through the web-based spectroscopy activity iSpec, in which the basic idea is that every action costs resources. The cost of a resource is given in the form of resource points (RPs), such that different exptl. data can be "purchased" for a no. of RPs. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2sXhsl2ktr7K&md5=a1cb43533f1934db3328914214a5411e
This article is cited by 7 publications.
- Senbin Chen, Zeke Li, Yanggui Wu, Yuping Zhang, Shitao Fu, Xiaomeng Luo, Chengmei Liu, Yonggui Liao, Xingping Zhou, Jintao Zhu . Orthogonal Linking of Gemcitabine by Acyclovir and Bortezomib: A Rational Chemistry Laboratory Introduction of Both Noncovalent and Covalent Dynamics. Journal of Chemical Education 2024 , 101 (9) , 3928-3935. https://doi.org/10.1021/acs.jchemed.4c00468
- Dominik Diermann, Dennis Huber, Steffen J. Glaser, Jenna Koenen . A Digital and Interactive Tool to Learn 1H NMR Spectroscopy: The SpinDrops Learning Environment. Journal of Chemical Education 2024 , 101 (8) , 3202-3215. https://doi.org/10.1021/acs.jchemed.4c00151
- Craig D. Campbell, Thomas C. Birkett, Malcolm I. Stewart . Applying a Guided Inquiry Approach to a Classic Practical on Chemoselective Reduction. Journal of Chemical Education 2024 , 101 (8) , 3434-3444. https://doi.org/10.1021/acs.jchemed.4c00331
- Zuzana Osifová, Ondřej Socha, Martin Dračínský . NMR-Challenge.com: Exploring the Most Common Mistakes in NMR Assignments. Journal of Chemical Education 2024 , 101 (6) , 2561-2569. https://doi.org/10.1021/acs.jchemed.4c00092
- Kyle T. Smith, Christian S. Hamann . Students Constructing for Themselves the Concept of Chemical Shift Correlation for Organic Substructures. Journal of Chemical Education 2024 , 101 (1) , 223-226. https://doi.org/10.1021/acs.jchemed.3c00961
- Maram Kiran, V. V. Krishnan . The Quantum Mechanical Particle-in-a-Box Experiment–A Revisit Using NMR Spectroscopy. Journal of Chemical Education 2023 , 100 (11) , 4543-4548. https://doi.org/10.1021/acs.jchemed.3c00588
- Scott E. Van Bramer, Loyd D. Bastin . Spectroscopy Data for Undergraduate Teaching. Journal of Chemical Education 2023 , 100 (10) , 3897-3902. https://doi.org/10.1021/acs.jchemed.3c00046

Article Views
25,057 total views
Article Views are the COUNTER-compliant sum of full text article downloads since November 2008 (both PDF and HTML) across all institutions and individuals. These metrics are regularly updated to reflect usage leading up to the last few days.
Citations are the number of other articles citing this article, calculated by Crossref and updated daily. Find more information about Crossref citation counts.
The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a page at altmetric.com with additional details about the score and the social media presence for the given article. Find more information on the Altmetric Attention Score and how the score is calculated.
Recommended Articles
- Supporting Info
- 1 Kibrik, E. J. ; Steinhof, O. ; Scherr, G. ; Thiel, W. R. ; Hasse, H. On-Line NMR Spectroscopic Reaction Kinetic Study of Urea-Formaldehyde Resin Synthesis . Ind. Eng. Chem. Res. 2014 , 53 , 12602 – 12613 , DOI: 10.1021/ie5001746 1 On-Line NMR Spectroscopic Reaction Kinetic Study of Urea-Formaldehyde Resin Synthesis Kibrik, Eleonore J.; Steinhof, Oliver; Scherr, Guenter; Thiel, Werner R.; Hasse, Hans Industrial & Engineering Chemistry Research ( 2014 ), 53 ( 32 ), 12602-12613 CODEN: IECRED ; ISSN: 0888-5885 . ( American Chemical Society ) Quant. online NMR spectroscopy is used to study the kinetics of the reaction of aq. formaldehyde and urea. The investigation focuses on the formation of low mol. mass compds. during the methylolation step. The expts. were carried out at overall formaldehyde to urea molar ratios between 1:1 and 4:1, pH values between 6 and 8, and temps. between 313 and 353 K. The exptl. data were used to develop a kinetic model based on the true species concns. The model describes the exptl. data well and can be used to predict the compn. of the reacting mixt. of aq. formaldehyde and urea during the methylolation step as a function of time. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2cXht1ans77I&md5=970188ca9b518c2b04ca451b0a955df9
- 2 Jaroszewicz, M. J. ; Liu, M. X. ; Kim, J. ; Zhang, G. N. ; Kim, Y. ; Hilty, C. ; Frydman, L. Time- and site-resolved kinetic NMR for real-time monitoring of off-equilibrium reactions by 2D spectrotemporal correlations . Nat. Commun. 2022 , 13 , 833 , DOI: 10.1038/s41467-022-28304-w 2 Time- and site-resolved kinetic NMR for real-time monitoring of off-equilibrium reactions by 2D spectrotemporal correlations Jaroszewicz, Michael J.; Liu, Mengxiao; Kim, Jihyun; Zhang, Guannan; Kim, Yaewon; Hilty, Christian; Frydman, Lucio Nature Communications ( 2022 ), 13 ( 1 ), 833 CODEN: NCAOBW ; ISSN: 2041-1723 . ( Nature Portfolio ) Abstr.: NMR (NMR) spectroscopy provides detailed information about dynamic processes through line-shape changes, which are traditionally limited to equil. conditions. However, a wealth of information is available by studying chem. reactions under off-equil. conditions-e.g., in states that arise upon mixing reactants that subsequently undergo chem. changes-and in monitoring the reactants and products in real time. Herein, we propose and demonstrate a time-resolved kinetic NMR expt. that combines rapid mixing techniques, continuous flow, and single-scan spectroscopic imaging methods, leading in unison to a 2D spectrotemporal NMR correlation that provides high-quality kinetic information of off-equil. chem. reactions. These kinetic 2D NMR spectra possess a high-resoln. spectral dimension revealing the individual chem. sites, correlated with a time-independent, steady-state spatial axis that delivers information concerning temporal changes along the reaction coordinate. A comprehensive description of the kinetic, spectroscopic, and exptl. features assocd. with these spectrotemporal NMR analyses is presented. Exptl. demonstrations are carried out using an enzymically catalyzed reaction leading to site- and time-resolved kinetic NMR data, that are in excellent agreement with control expts. and literature values. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BB38XjsVGrsbg%253D&md5=cc9366e8bde9420adba10a18b8c5a444
- 3 Susanne, F. ; Smith, D. S. ; Codina, A. Kinetic Understanding Using NMR Reaction Profiling . Org. Process Res. Dev. 2012 , 16 , 61 – 64 , DOI: 10.1021/op200202k 3 Kinetic Understanding Using NMR Reaction Profiling Susanne, Flavien; Smith, David S.; Codina, Anna Organic Process Research & Development ( 2012 ), 16 ( 1 ), 61-64 CODEN: OPRDFK ; ISSN: 1083-6160 . ( American Chemical Society ) The combination of kinetic understanding and reaction modeling was successfully applied to the development of processes from lab. to manufg. plant. Although extensively used in bulk chem., polymers, and the oil industry, it was not exploited to its full potential in the pharmaceutical industry. The authors present a fast and efficient methodol. for kinetic modeling of chem. reactions using 1H NMR reaction monitoring that can be used for the process understanding and development of active pharmaceutical ingredients. The parameters that are important for the development of a good, reliable model for the prediction and optimization of reaction conditions are discussed. The hydrolysis of acetic anhydride was chosen to illustrate the methodol. because it is mechanistically and kinetically well established. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3MXhtlOgu77J&md5=4eeafa5413260b38d24f4fdcd1650f87
- 4 Dračínský, M. The Chemical Bond: The Perspective of NMR Spectroscopy . Annu. Rep. NMR Spectrosc. 2017 , 90 , 1 – 40 , DOI: 10.1016/bs.arnmr.2016.07.001 4 The chemical bond: the perspective of NMR spectroscopy Dracinsky, M. Annual Reports on NMR Spectroscopy ( 2017 ), 90 ( ), 1-40 CODEN: NMRPAJ ; ISSN: 0066-4103 . ( Elsevier Ltd. ) This review article deals with chem. bonding from the perspective of NMR (NMR) spectroscopy. Different types of chem. bonds are briefly discussed and the relation between chem. bonding and NMR observables is overviewed. The various NMR interactions are classified in terms of through-bond and through-space effects on their observable values. The effects of vibrational motion and nuclear delocalization on NMR parameters and the consequences for the exptl. detn. of bond distances are also discussed. Finally, NMR expts. in studies of hydrogen bonding are summarized in Section 5. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2sXhtFSjsb3E&md5=0251de3f684e18d9fa5ffb122b45fb9c
- 5 Lehmann, J. ; Kleinpeter, E. ; Krechl, J. 1 H NMR Spectroscopy as a Probe of Intermolecular Interactions in β-Cyclodextrin Inclusion-Compounds . J. Inclusion Phenom. Mol. Recognit. Chem. 1991 , 10 , 233 – 239 , DOI: 10.1007/BF01066207 5 Proton NMR spectroscopy as a probe of intermolecular interactions in β-cyclodextrin inclusion compounds Lehmann, J.; Kleinpeter, E.; Krechl, J. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry ( 1991 ), 10 ( 2 ), 233-9 CODEN: JIMCEN ; ISSN: 0923-0750 . 1H NMR spectroscopy was used to probe the formation of inclusion compds. of permethylated and peracetylated β-cyclodextrins as host mols. and a variety of electronically very different guest mols. Complexation, obtained only in water, was estd. quant. by means of chem. shift/concn. curves of relevant protons, and the intermol. forces involved are critically discussed. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADyaK3MXisVKksLk%253D&md5=0ab5b9e7e7f34586a2264c8b16aa6a87
- 6 Xu, Y. J. ; Kumar, V. ; Bradshaw, M. J. Z. ; Bryce, D. L. Chalcogen-Bonded Cocrystals of Substituted Pyridine N-Oxides and Chalcogenodiazoles: An X-ray Diffraction and Solid-State NMR Investigation . Cryst. Growth Des. 2020 , 20 , 7910 – 7920 , DOI: 10.1021/acs.cgd.0c01173 6 Chalcogen-Bonded Cocrystals of Substituted Pyridine N-Oxides and Chalcogenodiazoles: An X-ray Diffraction and Solid-State NMR Investigation Xu, Yijue; Kumar, Vijith; Bradshaw, Maressa J. Z.; Bryce, David L. Crystal Growth & Design ( 2020 ), 20 ( 12 ), 7910-7920 CODEN: CGDEFU ; ISSN: 1528-7483 . ( American Chemical Society ) Me, MeO, and Ph substituents are introduced at the para-, meta-, and ortho- positions of pyridine N-oxide to study the effect of chem. substitution on the resulting 9 chalcogen-bonded structures formed upon cocrystn. with 3,4-dicyano-1,2,5-selenodiazole and 3,4-dicyano-1,2,5-telluradiazole. Single-crystal x-ray diffraction studies reveal double chalcogen bonding interactions in the cocrystals and demonstrate the impact of the substitution on the geometric features of the chalcogen bonds. 77Se and 125Te solid-state NMR spectroscopy is employed to measure Se and Te chem. shift tensors of the products, and various trends are described. The smallest component of the 77Se chem. shift tensor (δ33) provides the strongest correlation with the chalcogen bond distance. Soln. NMR provides qual. evidence for the persistence of the chalcogen bonds in soln. 1J(77Se,14N) coupling consts. in 3,4-dicyano-1,2,5-selenodiazole and its chalcogen-bonded cocrystals are measured after accounting for residual dipolar coupling between 77Se and 14N; however, changes in 1J(77Se,14N) attributable to chalcogen bonding upon cocrystn. are comparable to the exptl. uncertainties. This systematic study of chalcogen-bonded cocrystals demonstrates the potential utility of the substitution effect for applications of chalcogen bonds in crystal engineering and demonstrates the value of solid-state NMR in characterizing such systems. Chalcogen-bonded cocrystals based on 3,4-dicyano-1,2,5-selenodiazole and 3,4-dicyano-1,2,5-telluradiazole are reported. Various substituted pyridine N-oxides act as electron donors. X-ray diffraction and 77Se/125Te solid-state NMR are employed to characterize these novel materials. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BB3cXitFCqtLjM&md5=86728f3792353c8d645dd45adac74c82
- 7 Chae, Y. ; Min, S. ; Park, E. ; Lim, C. ; Cheon, C. H. ; Jeong, K. ; Kwak, K. ; Cho, M. Real-Time Reaction Monitoring with In Operando Flow NMR and FTIR Spectroscopy: Reaction Mechanism of Benzoxazole Synthesis . Anal. Chem. 2021 , 93 , 2106 – 2113 , DOI: 10.1021/acs.analchem.0c03852 7 Real-Time Reaction Monitoring with In Operando Flow NMR and FTIR Spectroscopy: Reaction Mechanism of Benzoxazole Synthesis Chae, Yeongseok; Min, Sein; Park, Eunjoon; Lim, Chaiho; Cheon, Cheol-Hong; Jeong, Keunhong; Kwak, Kyungwon; Cho, Minhaeng Analytical Chemistry (Washington, DC, United States) ( 2021 ), 93 ( 4 ), 2106-2113 CODEN: ANCHAM ; ISSN: 0003-2700 . ( American Chemical Society ) In operando observation of reaction intermediates is crucial for unraveling reaction mechanisms. To address the sensitivity limitations of com. ReactIR, a flow cell was integrated with a Fourier transform IR (FTIR) spectrometer yielding a "flow FTIR" device coupled with an NMR spectrometer for the elucidation of reaction mechanisms. The former device detects the low-intensity IR peaks of reaction intermediates by adjusting the path length of the FTIR sample cell, whereas the flow NMR allows the quant. anal. of reaction species, thus offsetting the limitations of IR spectroscopy resulting from different absorption coeffs. of the normal modes. Using the flow NMR and FTIR device, the controversial mechanism of benzoxazole synthesis was conclusively detd. by spectroscopic evaluation of the reaction intermediates. This system enabled the accurate acquisition of previously elusive kinetic data, such as the reaction time and rate-detg. step. The implementation of reaction flow cells into NMR and FTIR systems could be widely applied to study various reaction mechanisms, including dangerous and harsh reactions, thus avoiding contact with potentially harmful reaction intermediates. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BB3MXhtleh&md5=fb2b6f51682e6c1a2082a03d106a1f1c
- 8 Janková, Š. ; Dračínský, M. ; Císařová, I. ; Kotora, M. Synthesis and Rearrangement of Dewar Benzenes Into Biaryls: Experimental Evidence for Conrotary Ring Opening . Eur. J. Org. Chem. 2008 , 2008 , 47 – 51 , DOI: 10.1002/ejoc.200700916 There is no corresponding record for this reference.
- 9 Merlic, C. A. ; Fam, B. C. ; Miller, M. M. WebSpectra: Online NMR and IR spectra for students . J. Chem. Educ. 2001 , 78 , 118 – 120 , DOI: 10.1021/ed078p118 9 WebSpectra: online NMR and IR spectra for students Merlic, Craig A.; Fam, Barry C.; Miller, Michael M. Journal of Chemical Education ( 2001 ), 78 ( 1 ), 118-120 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( Division of Chemical Education of the American Chemical Society ) Computers and the World Wide Web (WWW) in particular have become important and powerful tools for teaching chem. WebSpectra is a WWW site implemented at the University of California at Los Angeles (http://www.chem.ucla.edu/webspectra). Through this site, students have convenient and free access to a library of problems in NMR (NMR) and IR (IR) spectroscopy, ranging in difficulty from introductory to advanced. An important feature is that expansions of the high-resoln. spectra can be controlled by the user. The site also includes instructional materials and tools to assist students in learning about NMR and IR spectroscopy. The design and capabilities of this Web-based interface are hereby described. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BD3MXptVKj&md5=c5c272e986df00b1e0887761bca78eab
- 10 Kolonko, E. M. ; Kolonko, K. J. Introducing NMR Spectroscopy Using Guided Inquiry and Partial Structure Templating . J. Chem. Educ. 2019 , 96 , 912 – 919 , DOI: 10.1021/acs.jchemed.8b00660 10 Introducing NMR Spectroscopy Using Guided Inquiry and Partial Structure Templating Kolonko, Erin M.; Kolonko, Kristopher J. Journal of Chemical Education ( 2019 ), 96 ( 5 ), 912-919 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) A review. Strategies for teaching NMR spectral interpretation in the undergraduate org. chem. curriculum are often faculty-centered and can lead to student reliance on rote memorization and "guess and check" methods rather than crit.-thinking skills for structure detn. This article describes a student-focused methodol. for the introduction of NMR spectral interpretation. Guided-inquiry tutorials using NMR prediction tools were developed to enable students to investigate the trends and concepts in 13C and 1H NMR spectral interpretation, with an emphasis on making connections between data and foundational chem. knowledge. A systematic approach to solving unknown structure problems is presented, providing a framework for students to organize spectral data and to build mols. from partial structures. The success of this NMR spectroscopy teaching strategy, which can be adapted for either lab. or lecture environments, was demonstrated both in pos. student survey responses as well as in quant. data showing a significant improvement in exam question scores. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC1MXnsVCnsLY%253D&md5=d3b0b4a4f19b4274fec39ac7ecfe8472
- 11 Henderson, D. E. A Chemical Instrumentation Game for Teaching Critical Thinking and Information Literacy in Instrumental Analysis Courses . J. Chem. Educ. 2010 , 87 , 412 – 415 , DOI: 10.1021/ed800110f 11 A Chemical Instrumentation Game for Teaching Critical Thinking and Information Literacy in Instrumental Analysis Courses Henderson, David E. Journal of Chemical Education ( 2010 ), 87 ( 4 ), 412-415 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) A simulation game is used to teach students in instrumental anal. courses to find the latest developments in the field, use the journal literature, and apply crit. thinking to det. the relative importance of the work they find. They also learn about the business of chem. instruments and to make oral presentations. The competitive aspects of the game provide addnl. motivation and increase student engagement with the literature. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC3cXislWktrk%253D&md5=ea38a57811815c289582efef253ffb8f
- 12 Carey, F. A. Organic Chemistry , 11 th ed.; McGraw Hill , 2019 . There is no corresponding record for this reference.
- 13 McMurry, J. Organic Chemistry , 9 th ed.; Cengage Learning , 2015 . There is no corresponding record for this reference.
- 14 Vollhardt, K. P. C. ; Schore, N. E. Organic Chemistry: Structure and Function , 7 th ed.; W. H. Freeman , 2014 . There is no corresponding record for this reference.
- 15 Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry , 3 rd ed.; Wiley-VCH , 2013 . There is no corresponding record for this reference.
- 16 Silverstein, R. M. ; Webster, F. X. ; Kiemle, D. J. ; Bryce, D. L. Spectrometric Identification of Organic Compounds , 8 th ed.; Wiley : Hoboken, NJ , 2014 . There is no corresponding record for this reference.
- 17 Field, L. D. ; Li, H. L. ; Magill, A. M. Organic Structures from Spectra , 6 th ed.; Wiley : Hoboken, NJ , 2020 . There is no corresponding record for this reference.
- 18 Crews, P. ; Rodriguez, J. ; Jaspars, M. Organic Structure Analysis , 2 nd ed.; Oxford University Press , 2009 . There is no corresponding record for this reference.
- 19 Merlic, C. A. ; Fam, B. C. WebSpectra: Problems in NMR and IR Spectroscopy . https://webspectra.chem.ucla.edu/ (accessed October 2022). There is no corresponding record for this reference.
- 20 Yamazaki, T. ; Nabeshima, M. ; Saito, T. ; Yamaji, T. ; Hayamizu, K. ; Yanagisawa, M. ; Yamamoto, O. Spectral Database for Organic Compounds, SDBS . https://sdbs.db.aist.go.jp (accessed October 2022). There is no corresponding record for this reference.
- 21 Flynn, A. B. NMR Interpretation: Getting from Spectrum to Structure . J. Chem. Educ. 2012 , 89 , 1210 – 1212 , DOI: 10.1021/ed3000974 21 NMR Interpretation: Getting from Spectrum to Structure Flynn, Alison B. Journal of Chemical Education ( 2012 ), 89 ( 9 ), 1210-1212 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) A tactile problem-solving strategy is described that helped students analyze spectral data, primarily from 1H NMR spectra, to det. the structures of unknown compds. Two important elements in that process were (i) students organized their anal. in a table and (ii) they drew each mol. fragment, deduced from their anal., on a sep. sticky note. Possible combinations of the fragments were analyzed by arranging the sticky notes in different ways, comparing back to the given data to det. the most plausible structure of the unknown compd. As part of the structure detn. lesson in a large class, a document camera served to project the sticky notes to the screen and student involvement was encouraged. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC38XhtVOksbjM&md5=721874f5a009af439484dd8104ca86eb
- 22 Haeßner, R. NMR exercises: NMR spectral interpretation based on examples . https://nmr.tips/indexe.html (accessed October 2022). There is no corresponding record for this reference.
- 23 Smith, B. D. ; Boggess, B. ; Zajicek, J. Organic Structure Elucidation: A Workbook of Unknowns . https://structureworkbook.nd.edu/ (accessed October 2022). There is no corresponding record for this reference.
- 24 Alexander, C. W. ; Asleson, G. L. ; Doig, M. T. ; Heldrich, F. J. Spectroscopic instruction in introductory organic chemistry: Results of a national survey . J. Chem. Educ. 1999 , 76 , 1294 – 1296 , DOI: 10.1021/ed076p1294 24 Spectroscopic instruction in introductory organic chemistry: results of a national survey Alexander, Christopher W.; Asleson, Gary L.; Doig, Marion T.; Heldrich, Frederick J. Journal of Chemical Education ( 1999 ), 76 ( 9 ), 1294-1296 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( Division of Chemical Education of the American Chemical Society ) A national survey was conducted to det. how instructors of the traditional introductory org. chem. courses are handling spectroscopic instruction. A questionnaire was sent to all chem. departments offering ACS-certified degrees and 52% responded. Results showed that spectroscopy has become a core part of the introductory org. course. Significantly, it is more likely to be incorporated into the lab. curriculum when students are able to have hands-on exposure to instruments. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADyaK1MXlslWhtLw%253D&md5=22b7d6fea794584c079c81ec853ab5c1
- 25 Vosegaard, T. iSpec: A Web-Based Activity for Spectroscopy Teaching . J. Chem. Educ. 2018 , 95 , 97 – 103 , DOI: 10.1021/acs.jchemed.7b00482 25 iSpec: A Web-Based Activity for Spectroscopy Teaching Vosegaard, Thomas Journal of Chemical Education ( 2018 ), 95 ( 1 ), 97-103 CODEN: JCEDA8 ; ISSN: 0021-9584 . ( American Chemical Society and Division of Chemical Education, Inc. ) Students' skills in structure elucidation of org. mols. are developed by training them to understand advanced spectroscopic measurements and elucidate structures of small org. mols. from mass spectrometry (MS) and IR (IR), UV, and 1H and 13C NMR (NMR) spectroscopic data. The present work puts a specific focus on developing student skills to ensure that the students (i) are aware of the resources involved in obtaining the spectral data and (ii) obtain the skills necessary to handle spectra which have not been processed by experts, to better reflect the use of spectroscopic techniques in their later work life. This is achieved through the web-based spectroscopy activity iSpec, in which the basic idea is that every action costs resources. The cost of a resource is given in the form of resource points (RPs), such that different exptl. data can be "purchased" for a no. of RPs. >> More from SciFinder ® https://chemport.cas.org/services/resolver?origin=ACS&resolution=options&coi=1%3ACAS%3A528%3ADC%252BC2sXhsl2ktr7K&md5=a1cb43533f1934db3328914214a5411e
System Message
Please be aware that pubs.acs.org is undergoing maintenance on Friday, September 6, at 6 p.m. EDT that may have an impact on your experience. During this time, you may not be able to access certain features like login, purchasing single articles, saving searches or running existing saved searches, modifying your e-Alert preferences, or accessing Librarian administrative functions. We appreciate your patience as we continue to improve the ACS Publications platform.
We are undergoing maintenance which may impact PDF downloads for some users. This update will complete shortly and we appreciate your patience while we improve the ACS Publications Platform.
On Tuesday, July 23, we will complete an update to our article pages. You may find that some elements are not aligned on these pages while the final work is completed. Thank you for your patience.
This website uses cookies to improve your user experience. By continuing to use the site, you are accepting our use of cookies. Read the ACS privacy policy .
The OChem Whisperer
Guide to Solving NMR Questions
How to solve any nmr question.
Solving NMR questions is easier than you think. All you need is a step-by-step process to help guide you through each question. And here it is…
Most NMR questions on an exam involve determining a specific structure rather than memorizing and repeating various NMR values. Typically, you will be given an NMR spectra and a molecular formula (sometimes an IR spectra will be provided). I have put together a few ideas that might make this process a bit easier. I am in the process of putting together a more concise document than this as a study aid. This post is meant to walk you through the thought process of how to tackle this type of problem. The description is a bit long (….so hold on!), but once you get it, you can just use the algorithm to solve your NMR problems. Here are some reference values and a couple of proton NMR spectra:
Proton NMR Reference Values

(cem.msu.edu)

(process-nmr.com)

(Our example 1H NMR spectra for this post; unknown source)
Start with an algorithm to get you on track
When staring an NMR question, you can use the following algorithm to help guide you through the thought process:

(above should say C2H5Cl = C2H6)
Calculate Degrees of Freedom
We notice the first thing says calculate degrees of unsaturation… what is that ? This allows us to determine if there are any double bonds or rings (cyclic structures) in the compound. Let’s look at an example; the formula is C 4 H 8 O 2 . Now, we need to compare this formula with the formula of a completely saturated hydrocarbon (all single bonds…no double bonds):
This formula tells us how many hydrogens we need to have a carbon compound with NO double bonds or rings. Let’s look at our example…
If I have a compound with 4 carbons, this 4 refers to n. Now, if we plug this in the formula, we get
C 4 H 2(4)+2 = C 4 H 10
This says that if we have a compound with only 4 carbons, we need 10 hydrogens to have a compound with no double bonds or rings. If we look at our example, we have C 4 H 8 O 2 . For now, all we need to look at is the C 4 H 8 when dealing with degrees of unsaturation (we will discuss what to do with heteroatoms a bit later). What we now want to do is subtract the hydrogens in our example ( C 4 H 8 ) from the saturated formula ( C 4 H 10 ):
C 4 H 10 – C 4 H 8 = 2 hydrogens
This leaves us with a value of 2. Now, the last things we do to get our degrees of unsaturation is divide this number by 2:
How to use the degrees of unsaturation to get the answer
This leaves us with 1; therefore, we have 1° of unsaturation. So, what does this mean? Each degree of unsaturation equates to a double bond or ring. Here are a few examples to further clarify:
1° of unsaturation = 1 double bond or 1 cyclic structure
2° of unsaturation = 2 double bonds; 1 alkyne; 1 double bond and 1 cyclic structure; 2 cyclic structures
3° of unsaturation = 3 double bonds; 2 double bonds and 1 cyclic structure; etc…
When you see 4° of unsaturation, think benzene; 3° of unsaturation for the 3 double bonds and 1° of unsaturation for the ring.
In our example, it means we have one double bond or one cyclic structure in our compound. Let’s look at few ring systems:

The larger the ring, the more stable the ring (with this series). Three and four-membered rings are rare. Usually, you will hear more about 5- and 6-membered rings. Since this is the case, we more than likely have a double bond (but never rule out a ring until you have looked at the NMR spectra).
Next, if we look at the algorithm, we need to consider the other atoms (other than carbon and hydrogen) in our formula…oxygen. Since we have degrees of freedom…think carbonyl. That is all we can do for now with our algorithm. Let’s now look at our spectra and see if we can start to determine the structure from the peaks:
Interpreting the spectra – splitting patterns

We have three peaks: a quartet around 4 ppm; a singlet around 2 ppm; and a triplet around 1 ppm. At this point, let’s review singlet, doublet, triplet, quartet, and multiplet:

What do these peaks refer to and how to we get the specific peak pattern? Well, we use the n+1 rule to figure out the pattern:

Putting the fragments together
Once you have your fragments, it is a matter of figuring out how to put them together. By looking at the spectra and where the peaks show up (ppm), you can figure out how the fragments go together.

Click NMR pictures to see the images as a PDF.
Don’t forget IR Spectroscopy!
Here is a simple guide showing you the most common IR values
Share this:
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
Notify me of new posts by email.
13.9 Uses of 1 H NMR Spectroscopy
13.9 • Uses of 1 H NMR Spectroscopy
NMR is used to help identify the product of nearly every reaction run in the laboratory. For example, we said in Section 8.5 that hydroboration–oxidation of alkenes occurs with non-Markovnikov regiochemistry to yield the less highly substituted alcohol. With the help of NMR, we can now prove this statement.
Does hydroboration–oxidation of methylenecyclohexane yield cyclohexylmethanol or 1-methylcyclohexanol?
The 1 H NMR spectrum of the reaction product is shown in Figure 13.16 a. The spectrum shows a two-proton peak at 3.40 δ , indicating that the product has a –CH 2 – group bonded to an electronegative oxygen atom (–CH 2 OH). Furthermore, the spectrum shows no large three-proton singlet absorption near 1 δ , where we would expect the signal of a quaternary –CH 3 group to appear. ( Figure 13.16 b) gives the spectrum of 1-methylcyclohexanol, the alternative product.) Thus, it’s clear that cyclohexylmethanol is the reaction product.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution-NonCommercial-ShareAlike License and you must attribute OpenStax.
Access for free at https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Authors: John McMurry, Professor Emeritus
- Publisher/website: OpenStax
- Book title: Organic Chemistry
- Publication date: Sep 20, 2023
- Location: Houston, Texas
- Book URL: https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Section URL: https://openstax.org/books/organic-chemistry/pages/13-9-uses-of-1h-nmr-spectroscopy
© Aug 5, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
- 3311-100/200 (Luca)
- 3311-300 (Toner)
- 3331-100 (Richardson)
- 3471/3491 (Wang)
- Loudon Chapter Reviews
- 3311 (OChem I)
- 3331 (OChem II)
- 3451 (OChem I for Majors)
- 3471 & 3491 (OChem II for Majors)
- 3321 (OChem I Lab)
- 3341 (OChem II Lab)
- 3381 (OChem II Majors Lab)
- Chemical Information
- Lab Equipment
- Procedures and Techniques
- Structural Determination
Spectroscopy Problems
In each of these problems you are given the IR, NMR, and molecular formula. Using this information, your task is to determine the structure of the compound. The best approach for spectroscopy problems is the following steps:
- Calculate the degree of unsaturation to limit the number of possible structures. Remember, each degree of unsaturation is a ring or pi bond (likely an alkene or carbonyl). An alkyne has two degrees of unsaturation (2 pi bonds), and an aromatic ring has four (3 pi bonds plus a ring.) Although there's no guarantee, if your structure has more than four degrees of unsaturation it's quite likely to have an aromatic ring.
- Look at the IR absorption bands at wavenumbers above 1500 cm -1 to determine what functional groups are likely in the compound. Remember that these functional groups must be consistent with the degree of unsaturation.
- Look at the NMR to determine the connectivity of the compound. If you can't figure out the entire structure at once, it helps to come up with fragments of the molecule that you can stick together into larger and larger groups until you have the entire structure.
- Approach this as a puzzle - it can be fun!
You can use the buttons at the bottom of each page reveal part or all of the answer.
Back to Spectroscopy page

IMAGES
VIDEO
COMMENTS
NMR Practice Problems In the following examples, we will learn how to solve NMR practice problems step-by-step in over 100 min video solutions which is essential for organic structure determination.. The emphasis is on the 1 H proton NMR and most problems are based on understanding its key principles such as the number of NMR signals, integration, signal splitting (multiplicity), and, of ...
This video covers H-NMR Problems with detailed solutions. This is the problem solving video that we covered after the theory review of HNMR. If you have an...
The following exercises are designed to help you become familiar with predicting the 1H NMR spectra of simple organic molecules. For each example you should find the number of signals you expect, where they should show on the scale (chemical shift), and what shape they should be (splitting patterns). Use the spectroscopy sheet to become ...
Welcome to WebSpectra - This site was established to provide chemistry students with a library of spectroscopy problems. Interpretation of spectra is a technique that requires practice - this site provides 1 H NMR and 13 C NMR, DEPT, COSY and IR spectra of various compounds for students to interpret. Hopefully, these problems will provide a useful resource to better understand spectroscopy.
This site was established to provide interested people in NMR with a library of NMR spectroscopy problems. Interpretation of spectra is a technique that requires pattern recognition and/or practice to order the chaos. This site provides one dimensional spectra of different nucleus, COSY, HSQC, HMBC and some less common spectra of various compounds to interprete.
This organic chemistry video provides a review of H NMR spectroscopy. It provides plenty of examples and multiple choice practice problems that you might en...
http://Leah4sci.com/NMR presents: Proton NMR Practice on Predicting Molecular Structure Using Formula + GraphNeed help with Orgo? Download my free guide '10 ...
Compound W has an empirical formula of C11H10O2. Given are the following spectra. Show all your work (= label peaks in the spectra!) Determine the degree of unsaturation for the compound. Assign the six pertinent peaks in the infrared spectrum. Suggest a structure for compound W based on the spectra given. Place your final answer in the box ...
For example, in the 1 H NMR spectrum of bromoethane shown in Figure 13.8, the -CH 2 Br protons appear as four peaks ... It's best to begin solving structural problems by calculating a molecule's degree of unsaturation (we'll see this again in Worked Example 13.4).
Organic Chemistry 307 - Solving NMR Problems - H. D. Roth. Now we go to high field (right): there is a triplet (3 H) at 0.9 ppm; 3 H at high field is almost always a methyl group. The signal is a triplet (n + 1 = 3); therefore, the methyl group must have (n =) 2 1H neighbors; that must be a CH2group. The CH2 signal is a quartet (n + 1 = 4 ...
A tactile problem-solving strategy is described that helped students analyze spectral data, primarily from 1H NMR spectra, to det. the structures of unknown compds. Two important elements in that process were (i) students organized their anal. in a table and (ii) they drew each mol. fragment, deduced from their anal., on a sep. sticky note.
The description is a bit long (….so hold on!), but once you get it, you can just use the algorithm to solve your NMR problems. Here are some reference values and a couple of proton NMR spectra: Proton NMR Reference Values (cem.msu.edu) (mhhe.com) (process-nmr.com) (1H NMR of Taxol; unknown source) (Our example 1H NMR spectra for this post ...
In this video, we will go over the strategies for solving NMR problems step by step. This practice problem involves determination of Hydrogen Deficiency Inde...
13.9 • Uses of 1 H NMR Spectroscopy NMR is used to help identify the product of nearly every reaction run in the laboratory. For example, we said in Section 8.5 that hydroboration-oxidation of alkenes occurs with non-Markovnikov regiochemistry to yield the less highly substituted alcohol. With the help of NMR, we can now prove this statement.
The best approach for spectroscopy problems is the following steps: Calculate the degree of unsaturation to limit the number of possible structures. Remember, each degree of unsaturation is a ring or pi bond (likely an alkene or carbonyl). An alkyne has two degrees of unsaturation (2 pi bonds), and an aromatic ring has four (3 pi bonds plus a ...