An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Cancer Prev
- v.25(3); 2020 Sep 30

An Overview of Cancer Prevention: Chemoprevention and Immunoprevention
1 Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
2 College of Natural Sciences, The University of Texas at Austin, Austin, TX, USA
3 Division of Epidemiology, The University of Texas School of Public Health, Houston, TX, USA
Cancer prevention encompasses a broad spectrum of strategies designed to lower the chance of developing cancer and reduce the morbidity of established cancer. There are three levels of cancer prevention. Eliminating or mitigating cancer risk factors by adopting healthy behaviors and lifestyles, such as avoiding tobacco and alcohol use, exercising, eating a healthy diet, and applying sunscreen to protect against UV exposure, belongs to primary prevention and is the easiest and most effective way of preventing cancer for the general public. Secondary prevention includes screening to identify precancerous lesions and taking intervention measures to prevent disease progression to malignancy. Tertiary prevention refers to reducing or controlling the symptoms and morbidity of established cancer or the morbidity caused by cancer therapy. For high-risk populations, chemopreventive agents, such as selective estrogen receptor modulators (including tamoxifan and raloxifene) in breast cancer prevention and non-steroidal anti-inflammatory drugs (aspirin) in colorectal cancer prevention, and immunoprevention using human papillomavirus and hepatitis B virus vaccines in infection-related cancers have shown clear clinical benefits of reducing cancer incidences. In this review, we will summarize the current status of cancer prevention, focusing on the major agents that are clinically used for chemoprevention and immunoprevention.
INTRODUCTION
Cancer is the second leading global cause of death, responsible for 1 in 6 deaths [ 1 ]. About 38.4% of people in the world will be diagnosed with cancer at some stage in their lifetimes, and roughly 1 out of every 3 Americans will develop a malignancy [ 2 ]. Given the prevalence and severity of cancer worldwide, enormous resources and efforts have been devoted to improve cancer screening, diagnosis, and treatment. The progress in the war against cancer has been quite effective, as the cancer death rate in the United States has dropped by 29% from 1991 to 2017 [ 2 ].
Despite considerable progress in cancer therapies such as targeted therapy and immunotherapy, the prognosis of many advanced cancers is still dismal. Effective cancer screening and early detection offer the best hope of further reducing cancer mortality. However, only a few cancer screening tests, for instances, in cervical, breast and colon cancers, are routinely used in clinics and proven to reduce cancer mortality. While cancer treatment is largely ineffective against advanced cancer, and cancer screening is only available for a few cancers, cancer prevention serves as an undervalued approach to reduce cancer incidence and mortality that must be given more attention.
There are three levels of cancer prevention [ 3 , 4 ]. Primary prevention refers to decreasing cancer incidence by eliminating risk factors for cancer, such as exercising, eating a healthy diet, and avoiding tobacco smoke and alcohol drinking [ 5 ]. Secondary prevention includes screening to identify diseases in the earliest stages (e.g., preneoplastic lesions) and blocking disease from progressing to malignancy. Tertiary prevention refers to reducing or controlling the symptoms and morbidity of established cancer or the morbidity caused by cancer therapy. Implementing drugs into the body to prevent tumor onset, malignant progression, or recurrence is known as chemoprevention. While certain chemopreventive agents such as tamoxifan and aspirin have been effective in reducing cancer risks, their intake remains low due to concerns of long-term side effects [ 3 , 4 , 6 ]. Moreover, cancer cells adapt in an evolutionary continuum in response to attempts to control them, while chemopreventive medicine is stagnant in nature. In this regard, immunotherapy has garnered the attention of researchers who seek to utilize the immune system as an adaptive entity that can respond to cancer’s evolution, potentiating immunoprevention as a more efficacious approach in preventing carcinogenesis [ 7 - 10 ]. With the success of vaccines against virally induced cancers, recent progress in the identification of candidate tumor antigens for non-viral cancers, and the application of immune checkpoint inhibitors in premalignant lesions, immunoprevention is rising in popularity and serves as the future of cancer prevention [ 7 - 10 ].
CHEMOPREVENTION
Chemoprevention involves the input of external agents such as medication or supplements to inhibit or delay the onset, progression, or recurrence of cancer. Several agents have been approved by United States Food and Drug Administration (FDA) for clinical use in the setting of cancer prevention [ 3 ]. Table 1 summarizes the chemopreventive agents that are discussed in this article.
Summary of major chemopreventive agents
| Agent | Target | Cancer prevention effect | References |
|---|---|---|---|
| Selective estrogen receptor modulators | Estrogen receptor (ER) | Reduce ER-positive breast cancer incidence in high-risk populations | - |
| Aromatase inhibitors | Aromatase | Reduce ER-positive breast cancer incidence in high-risk populations | , , |
| Non-steroidal anti-inflammatory drugs | COX1/COX2 | Reduce the occurrence of several cancers, mainly CRC and less notably stomach and esophageal cancers | - |
| Statins | HMG-CoA reductase | Observational studies showed long term statin use reduces incidences of gastrointestinal cancer | - |
| Metformin | Gluconeogenesis | Observational studies showed metformin use lowers risks of cancer incidence and mortality in diabetic patients | - |
CRC, colorectal cancer; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A.
Selective estrogen receptor modulators (SERMs)
Tamoxifen was the first FDA-approved chemopreventive agent, which can reduce the risk of estrogen receptor (ER)-positive breast cancer [ 6 ]. The second generation SERM, raloxifene, was also approved by FDA for preventing breast cancer in high-risk populations [ 6 ]. Several United States and European phase III cancer prevention trials showed that tamoxifen reduced the risks of ER-positive breast cancer by 30% to 60% in both pre- and post-menopausal women at high-risk of breast cancer [ 11 - 15 ]. The United States Preventive Services Task Force (USPSTF) conducted a systematic review of trials and estimated that compared with placebo, tamoxifen reduced the incidence of invasive breast cancer by 7 events (95% CI, 4-12) per 1,000 women over 5 years [ 16 ]. However, tamoxifen use was associated with increased risks of venous thromboembolic events (e.g., stroke, pulmonary embolus, and deep vein thrombosis), cataract, endometrial cancer, and vasomotor symptoms [ 16 ]. The second generation SERM, raloxifene, produced similar preventive effect on ER-positive breast cancer, and was associated with lower side effects than tamoxifen use [ 17 , 18 ]. FDA approved both drugs for breast cancer prevention in high-risk individuals.
In addition to SERMs, two aromatase inhibitors (AIs), exemestane and anastrozole, have also been shown to significantly reduce ER-positive breast cancer incidence in randomized clinical trials in high-risk post-menopausal women [ 19 , 20 ]. AIs block the enzyme aromatase and hence inhibit the conversion of androgen into estrogen. AIs produced stronger efficacy and had more favorable side effect profiles than SERMs [ 16 ]. The USPSTF recommends that clinicians offer to prescribe risk-reducing drugs, including tamoxifen, raloxifene, or AIs, to women who are at increased risk for breast cancer and at low risk for adverse side effects [ 16 ].
Non-steroidal anti-inflammatory drugs (NSAIDs)
Due to the link between inflammation and cancer development, NSAIDs and COX2 selective inhibitors (COXIBs), have been utilized as chemopreventive agents [ 21 - 23 ]. A large multitude of evidence including observational studies and randomized controlled clinical trials have substantiated that aspirin use can decrease the incidence and mortality of colorectal cancer (CRC) within the general population, while less extensive studies have indicated reductions in incidence for stomach and esophageal cancers, although smaller in magnitude [ 21 - 23 ].
There are several proposed mechanisms through which NSAIDs and COXIBs are hypothesized to lower the risk of CRC. The most compelling mechanism involves the ability of the chemopreventive agents to inhibit COX2, an enzyme involved in the synthesis of inflammatory prostaglandins that stimulate cell proliferation and inflammation [ 24 , 25 ]. COX2 is overexpressed in early stage colon carcinogenesis and colorectal adenomas and increases resistance to apoptosis and promotes angiogenesis. By inhibiting COX2 enzyme, NSAIDs and COXIBs decrease the production of inflammatory prostaglandins, therevy inhibit the downstream tumor-promoting events such as inflammation, cell proliferation, and angiogenesis, and prevent cancer initiation ( Fig. 1 ). Most NSAIDs inhibit both COX1 and COX2, whereas COXIBs selectively inhibit COX2.

NSAIDs and COXIBs inhibit COX1/2 enzymes and block the synthesis of inflammatory prostaglandins, therefore inhibit the downstream tumor-promoting events, such as inflammation, cell proliferation, and angiogenesis, and prevent cancer initiation.
Although aspirin, as the most prominent NSAID, has been the poster child in the chemopreventive approach to fighting CRC and other types of cancer, much remains to be clarified regarding the optimal dosage and duration for its use. While daily doses of 75 mg and above have reduced long-term incidence of 7% to 10%, there is no definitive proof of increased reduction with higher dosages [ 22 ]. The harmful side effects of long-term use of aspirin including gastrointestinal ulcerations and cardiovascular toxicities have impacted the uptake and adherence of aspirin use. The USPSTF recommends initiating low-dose aspirin use for the primary prevention of cardiovascular disease (CVD) and CRC in adults aged 50 to 59 years who have a 10% or greater 10-year CVD risk, a life expectancy of at least 10 years, and are not associated with higher risk of bleeding [ 26 ].
Aspirin inhibits both COX1 and COX2. COXIBs selectively inhibit COX2. Celecoxib (celebrex) is the most well-studied COXIB and has demonstrated efficacy in randomized clinical trials for CRC prevention in high risk population [ 3 ]. Celecoxib was approved by the United States FDA as an oral adjunct to usual care for patients with familial adenomatous polyposis (FAP), a rare hereditary disease that has a high likelihood of developing CRC. Celecoxib was the first pharmacological agent to be used for reducing the risk of adenomatous colorectal polyps in patients with FAP. However, because of the observed significantly increased CVD risk in clinical trials, celecoxib is not currently recommended for the primary prevention of CRC in general public [ 3 ].
Statins are a class of drugs that prevent coronary heart disease by reducing blood cholesterol and have gained traction as potential chemopreventive agent. Mechanistically, statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase activity, which inhibits the conversion of HMG-CoA to mevalonate, reducing the amount of products downstream [ 27 - 29 ]. Products of the mevalonate pathway are important for different cellular functions, from cholesterol synthesis to the control of cell growth and differentiation. Observational studies have demonstrated that long term statin use has led to decreased incidence of gastrointestinal cancer, especially CRC and gastric cancer [ 30 ]. However, more randomized clinical trials must be conducted in order to corroborate statins’ chemopreventive efficacy.
Energy metabolism plays an essential role in cell proliferation, linking diabetes with elevated risks for several cancers [ 31 ]. From this association, metformin, a common drug for type II diabetes, was proposed as a chemopreventive drug for its anti-diabetic effects, which have potential value in prevention and therapy. Several meta-analyses have supported metformin’s chemopreventive properties with lowered risks of cancer and mortality in diabetic patients who took metformin [ 32 ]. A 31% decrease in overall cancer incidence and 34% reduction in cancer mortality after adjusting for body mass index were observed for patients using metformin relative to those taking other anti-diabetic drugs [ 33 ]. Many clinical trials are ongoing to investigate the role of metformin in cancer treatment and prevention [ 34 ].
IMMUNOPREVENTION
Genomic instability is a hallmark of cancer and is associated with immune response [ 35 ]. The unstable genomes of tumor cells acquire many mutational changes that produce tumor-specific antigens unique to the tumor genome, which are used as targets for the immune system [ 36 ]. Immunoprevention stimulates the immune system’s recognition and response to tumor-specific antigens in order to prevent tumor onset or malignant transformation. Currently, the most successful implementations of immunoprevention are the human papillomavirus (HPV) and hepatitis B virus (HBV) vaccines for virally induced tumors [ 7 ]. Tumor-specific antigens are still being identified to engineer a vaccine for non-viral tumors. Other forms of immunotherapy like immune checkpoint inhibitors have shown clinical potential in secondary prevention [ 7 , 37 ]. Table 2 summarizes the main immunopreventive agents that are discussed in this article.
Summary of immunopreventive agents
| Agent | Target | Cancer preventive effect | References |
|---|---|---|---|
| HPV vaccine | Cervarix, a bivalent vaccine, targets HPV 16/18 Gardasil, a quadrivalent vaccine, targets HPV 6/11/16/18 Gardasil 9, a 9-valent vaccine, targets 9 types of HPV | Prevents HPV-related cancers, including cervical, anal, vulvar, vaginal, penile, and oropharyngeal cancer | , - |
| HBV vaccine | HBV | Prevents HBV-related liver cancer | - |
| MUC1 vaccine | MUC1 antigen | MUC1 vaccine produced strong immune response in patients with intestinal polyps and in individuals at high risk for colon cancer in Human clinical trials | - |
| HER2 vaccine | HER2 antigen | Human clinical trials showed DCIS remission, long-term production of Her2 antibodies, and lost expression of Her2 | , |
| Immune checkpoint inhibitors (CLTA-4, PD-1, PD-L1 antibody) | Immune checkpoint proteins (CLTA-4, PD-1, PD-L1) | Preclinical mouse models showed anti-PD-1 treatment significantly prevented malignant progression of oral premalignant lesions | - |
| A case report showed anti-PD-1 inhibited the development of new neoplasm in a Lynch syndrome patient |
HPV, human papillomavirus; HBV, hepatitis B virus; HER2, human epidermal growth factor receptor 2; DCIS, ductal carcinoma in situ; CLTA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1.
Vaccines against virally induced cancers
Cancer preventive vaccines are utilized to stimulate the body’s immune system to produce antibodies against foreign particles like viruses, making them effective agents in primary prevention of virus-induced cancers. HPV infection is the predominant risk factor for a number of cancers, including cervical, oropharyngeal, anal, penile, vulvar, and vaginal cancers [ 38 ], whereas HBV and hepatitis C virus are the major risk factors for hepatocellular carcinoma (HCC). Consequently, vaccines against HPV and HBV can prevent the development of virus-related cancers.
The carcinogenic mechanism of HPV infection mainly involves two oncoproteins, E6 and E7. E6 is responsible for degrading the p53 tumor suppressor gene, causing genetic instability and the buildup of DNA mutations that stimulate unrestrained cell proliferation [ 39 ]. E7 degrades retinoblastoma protein, a tumor suppressor that regulates G1 to S phase transition, and pushes the cell cycle into the S phase by overriding normal cell cycle checkpoints, leading to uncontrolled cell replication [ 40 ].
Currently, the HPV vaccines in clinical use include Cervarix, a bivalent vaccine which targets HPV16 and HPV18, the two most virulent types, Gardasil, a quadrivalent vaccine which targets HPV6/11/16/18, and Gardasil 9 vaccine, a 9-valent vaccine targeting HPV6/11/16/18/31/33/45/52/58. HPV vaccination has proven to be essential to the deterrence of HPV-related illnesses and prevention of HPV-related cancers. Australia was among the first countries to install a government-funded, universal HPV-vaccination program for its population in 2007, starting with 3 doses of Gardasil for 12- to 13-year-old females and later expanding to 12- to 13-year-old males in 2013 [ 41 ]. Since then, significant decreases in HPV-related infections have emanated from school cohorts, showing a decline of 86% in 18 to 24 years old who received 3 doses and decline of 76% in those who received 1 to 2 doses. On a broader scale, vaccine trials conducted across North America, Europe, and Latin America demonstrated that almost 100% of women between 15 to 26 years of age developed antibodies corresponding to HPV 16 and 18 in one month after the third dosage of HPV vaccine, resulting in antibody levels ranging 10 to 104 times higher than that of normal infections [ 42 , 43 ]. The toxicity and side effects of the vaccine were minimal [ 41 ]. Given that HPV vaccination is both safe and effective, universal vaccination could lead to the eradication of cervical cancer. However, vaccination coverage remains low, and the distribution and funding of the vaccine for certain targeted demographic groups, of which adolescent girls are currently the priority, remains limited due to the economic, political, and cultural implications of vaccine implementation. Concerted, coordinated efforts globally are needed to increase HPV vaccine uptake.
Chronic HBV infection is a strong risk factor for HCC, hepatoma, and liver cirrhosis. In areas of high HBV prevalence, chronic HBV is responsible for 60% to 90% of all HCC in adults and almost 100% of HCCs in children [ 44 ]. The HBV virus persists as a “stealth virus” within the host cells by evading the immune system because of its subtle replication methods. The viral DNA template remains in the nucleus while viral mRNA structures resemble that of host cell transcripts, and its own genome remains hidden within viral capsids that stay in the cytoplasm. These replication methods allow HBV to spread throughout host cells of the liver while avoiding immune detection. Exploiting the “memory” of the adaptive immune system, HBV vaccines provide immunity against further re-exposure to HBV by introducing a weakened, harmless virus resembling HBV into the body to induce and elevate Hepatitis B surface antigen (HBsAg)-specific antibody production [ 45 ].
HBV vaccines have proven to be effective through the analysis of vaccine implementation programs over the years. In 1984, Taiwan was the first region to implement a universal HBV vaccination program, which has persisted successfully for more than 2 decades [ 46 ]. The coverage rate of vaccination reached a high of 97%. Infants were given 3 to 4 doses of HBV vaccine during their first week, and those born of high-risk mothers were treated to hepatitis immunoglobulin within 24 hours from their birth. Following 20 years after the program’s initiation, chronic HBV infection rates have decreased significantly in the under-20 population from 10% to 17% to 0.7% to 1.7%. Furthermore, the incidence rate of HCC decreased from 0.52 to 0.54 to 0.13 to 0.20 per 100,000 among the Taiwanese youth of 6 to 14 years of age. Currently, HBV vaccination has been administered in national immunization programs for children in 181 countries [ 47 ]. Hundreds of millions of HBV vaccinations have been given globally and have greatly reduced the disease burden, morbidity, and mortality of HBV-related illnesses wherever administered [ 48 ]. In developed countries, most infants and children are vaccinated with 3 doses of HBV vaccine with the first dose being within 24 hours of birth, and blood donors are screened for HBsAg. In addition, unvaccinated adults and adolescents of high risk groups are encouraged to receive the vaccine as well. However, the people of developing countries experience higher risks of HBV infection because their governments cannot afford the costs of acquiring and administering the vaccine [ 49 ]. Even though cancer vaccines have successfully prevented HBV and its carcinogenic predisposition to HCC, much remains to be accomplished to universalize the vaccine so that humankind, regardless of wealth or privilege, can become immune to the virus.
Prevention for non-viral cancers
Although HPV and HBV vaccines have successfully proven their immunopreventive effects against virally induced cancers, the vast majority of cancers cannot be traced to infectious agents. The progression of non-viral cancers from healthy tissue to malignant tumors involves years of mutational accumulations. For high-risk individuals, many opportunities for immunoprevention arise during the gradual oncogenic formation of non-viral cancers. Much research has been invested in engineering vaccines that can target non-viral cancers. A challenging component of developing such vaccines is the identification of appropriate tumor-specific antigens to target. The optimal antigen should be expressed only in neoplastic cells or lesions, genetically altered during early stages of tumor formation, and essential to tumor survival. However, it is difficult to identify antigens that are both unique to tumors and altered in early stages. Few antigens are specific to only neoplastic or pre-neoplastic cells. The majority of target antigens are expressed to some degree among normal cells as well, making the physiological and biological role of the antigen important.
Numerous antigens have been suggested as candidates for their potential as immunopreventive vaccines based on their tumor specificity and ability to elicit an immune response [ 50 ]. In breast cancers associated with BRCA1 or BRCA2 mutations, recent studies have found a series of antigens including MAGE-A1, NYESO-1, CT10, and GAGE that were expressed in malignant breast tissue, but not in any normal breast tissue [ 7 ]. The overexpression and aberrant glycosylation of MUC1 antigen occur in pancreatic, breast, colon, and various other carcinomas. MUC1 mutations are also responsible for adenomatous polyps, dysplasia, and inflammatory bowl disease [ 51 ]. Mutated human epidermal growth factor receptor 2 (HER2) is another clear oncogene because the product of the HER2 gene is overexpressed in roughly 20% of invasive breast cancers and is associated with greater severity and worse survival [ 52 ].
In several animal models, the prophylactic nature of MUC1 vaccines was corroborated as the vaccines alleviated inflammatory bowel disease and prevented the development of inflammation-induced colon cancer [ 51 ]. Another mouse model showed that prophylactic administration of an anti-EGF receptor (anti-EGFR) vaccine reduced EGFR-driven lung cancer in mice by 76.4% [ 53 ]. In fact, many animal models have shown that the administration of non-viral antibodies could prevent tumor onset in healthy mice susceptible to cancer [ 54 ].
The first immunopreventive vaccine targeting non-viral cancers in human clinical trial was the MUC1 peptide vaccine in patients with intestinal polyps [ 55 , 56 ]. The results showed high production levels of tumor-specific, cytotoxic anti-MUC1 antibodies that induced the immune response. The vaccine was also tested in healthy individuals at high risk for colon cancer due to history of colon adenoma, and 43% of patients responded with strong and long-term immunity as indicated by high antibody levels [ 57 ]. For breast cancer immunoprevention, the promising target is the Her2 antigen. In a pilot clinical trial, 27 patients with ductal carcinoma in situ (DCIS) that overexpressed HER2 were administered the dendritic cell HER2 vaccine once a week for 4 weeks before surgery [ 58 ]. Upon surgery, 5 out 27 had no disease present, and 11 out of the 22 that still had DCIS experienced a decrease in lesion size and lost expression of HER2. HER2 antibodies were observed up to 52 months after initial vaccination. In another neoadjuvant clinical trial, vaccines comprised of autologous dendritic cells displaying HER2 peptides were administered to women with Her2 positive DCIS who were going to have surgery in 4-6 weeks [ 59 ]. By the time of surgery, 25% of patients experienced complete tumor regression, with the best rate being 38% among the ER-negative group.
Though clinical trials have shown promise, the use of immunopreventive vaccines in non-viral cancers comes with limitations and challenges [ 10 ]. Vaccines that target tumor-specific antigens are restricted to patients with pre-neoplastic lesions at high risk. These non-viral vaccines cannot be administered to healthy individuals because they may induce inflammatory responses and unnecessary toxicities. The use of single target vaccines may not be sufficient enough because tumors can overcome an immune response directed towards a single antigen. Therefore, the development of multivalent vaccines may be necessary for more effective immunopreventive effects in non-viral cancers.
Immune checkpoint inhibitors
In addition to using viral and non-viral vaccines to amplify the antitumor response, antibodies against immune checkpoints, such as the programmed death ligand-1 (PD-L1), programmed cell death protein-1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), work on the other end of the spectrum by inhibiting immunosuppressive mechanisms through which tumors evade immune detection.
To prevent autoimmunity, normal cells contain surface proteins that bind to “checkpoint” proteins on immune cells to switch them off so that the immune system does not target healthy cells. Cancer cells can adopt this same protective mechanism by producing surface proteins that bond with checkpoint proteins on immune cells to avoid being targeted by T-cells of the immune system.
The major checkpoints of the adaptive immune response include CTLA-4 and PD-1 receptors, which are both located on the surface of T cells [ 60 ]. T cell activity occurs through the binding of the T cell receptor (TCR) to antigens present on the major histocompatibility complex (MHC) located on the antigen-presenting cell (APC) surface [ 61 ]. T cell activation and proliferation requires co-stimulatory binding of its CD28 molecules with B7-1 or B7-2 molecules on the APC. CTLA-4 inhibits normal T cell activity by competing against CD28 for B7-1 and B7-2 in the early stages of the adaptive immune response [ 62 ]. The CTLA-4 inhibitor prevents CTLA-4 checkpoint protein from binding to B7 on APCs. By eliminating competitive inhibition brought about by CTLA-4:B7, CD28:B7 binding predominates. CD28:B7 and TCR bound to tumor-specific antigens on the MHC co-stimulate T cell activation and proliferation, inducing more potent antitumor response. An anti-CTLA-4 monoclonal antibody, Ipilimumab, was the first FDA-approved immune checkpoint inhibitor for cancer therapy [ 63 ].
PD-1 is another inhibitory transmembrane protein expressed on the surface of T cells and other immune cells [ 61 ]. Tumor cells express PD-L1 that binds to PD-1 on effector T cells, weakening T cell immune activity even though the TCR recognizes specific antigens on the MHC complex of the tumor cell. PD-1 and PD-L1 antibodies elicit antitumor response by preventing PD-1 and PD-L1 checkpoints from binding to each other ( Fig. 2 ). Immune checkpoint inhibitors have revolutionized the treatment of many advanced stage cancer. Since the FDA approval of ipilimumab in 2011, six more checkpoint inhibitors have been approved for the treatment of various cancers, including PD-1 inhibitors nivolumab, pembrolizumab, cemiplimab and PD-L1 inhibitors atezolizumab, avelumab, and durvalumab [ 63 ].

T cell activation occurs through the binding of the T cell receptor (TCR) to antigens present on the major histocompatibility complex (MHC) located on APC (e.g., tumor cell) surface. PD-1 is an inhibitory transmembrane protein expressed on the surface of T cells and other immune cells. PD-1 binds to its ligand PD-L1 on tumor cells and weakens T cell immune activity (weakened T effector cells in the figure). PD-1 and PD-L1 inhibitors elicit antitumor response by preventing PD-1 and PD-L1 checkpoints from binding to each other, leading to active T effector cells, cytokine release, and strong immune response.
With the success of checkpoint inhibitors in immunotherapy of advanced stage cancers, utilizing anti-PD-1 and anti-PD-L1 as tools for secondary cancer prevention in preneoplastic lesions has generated considerable interests [ 9 , 64 - 68 ]. For example, in a preclinical mouse model of oral premalignant lesions (OPLs), anti-PD-1 treatment significantly prevented malignant progression of OPLs. OPLs responded to PD-1 blockade with significant increases of CD8+ and CD4+ effector T cells in their microenvironment, induction of IFNγ and production of the T-cell effector granzyme B in tumor infiltrating cells, and induction of apoptosis in the epithelial cells of the OPLs, supporting the potential clinical application of immune checkpoint inhibitors in secondary prevention of OPL progression. In a recent case report, a patient with Muir-Torre syndrome, a variant of Lynch syndrome characterized by defective DNA mismatch repair and frequent gastrointestinal tumors and hyperplastic or neoplastic skin tumors, was treated with anti-PD-1 (pembrolizumab) immunotherapy as part of multi-modality treatment for his invasive bladder cancer. The patient previously had 136 cutaneous or visceral hyperplastic or neoplastic lesions within 19 years (mean 7.5 neoplasms/yr, range 2-20) prior to immunotherapy. The patient not only had a complete response of the bladder cancer, but also had an absence of new neoplasms during a 22-month follow-up period [ 37 ]. This study provides clinical support for using immune checkpoint inhibitors in cancer prevention settings.
CONCLUSIONS AND FUTURE DIRECTIONS
A number of chemopreventive agents have shown clear clinical efficacy in reducing cancer incidence among at-risk populations, including SERMs and AIs in preventing breast cancer and aspirin in preventing CRC and other cancers. Observational studies have shown statins and metformin could reduce cancer incidence, but more randomized controlled clinical trials must be performed to substantiate the clinical efficacy of statins and metformin in cancer prevention. Nevertheless, the intake of these chemopreventive agents has been hampered by possible side effects of long-term use. Identifying high-risk individuals with a positive risk–benefit ratio is critical to enhance the uptake of chemoprevention. In the long run, new, more effective chemopreventive agents with fewer side effects need to be developed before chemoprevention can be more widely accepted.
Immunoprevention has risen to prominence in cancer research because the immune system is a living, adaptable entity that can be enhanced by vaccines and tumor-specific antigens to prevent tumor onset or control tumor growth. Currently, the most successful form of immunoprevention is cancer vaccine for virally induced cancers. HPV and HBV vaccines have significantly reduced the rates of HPV and HBV infection and their associated cancers. On the other hand, vaccines preventing non-viral cancers are still at the early stage of development, but clinical trials have shown promising immunopreventive effects in targeting tumor-specific antigens, such as MUC1 and Her2 in high-risk populations. In the future, additional specific tumor antigens are desired. In addition, it may be necessary to develop multivalent vaccines against multiple tumor antigens because of the heterogeneity of premalignant lesions, analogous to multivalent vaccines against HPV subtypes. Another potential benefit of multivalent vaccines targeting several tumor antigens is to reduce the occurrence of immune escaped precancerous clones.
Preclinical studies and case reports have supported the utility of immune checkpoint inhibitors, such as anti-CTLA-4 and anti-PD-1, as a secondary prevention modality to prevent malignant progression of preneoplastic lesions. Side effects would be a major concern for the application of checkpoint inhibitors in cancer prevention settings. Developing novel checkpoint blockers, identifying new immunotherapeutic targets, and preselecting persons with favorable with benefit-risk ratio based on biomarkers of efficacy and toxicity can help move immunoprevention field forward. Immunoprevention is likely to take the central stage in future cancer prevention given that our immune system contains billions of fighters that can be induced, enhanced, and manipulated to prevent the onset and progression of cancer.
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Defining Patient Tolerability to Cancer Treatments Helps Reduce Negative Outcomes

For decades, cancer patients have participated in clinical trials of new therapies, and clinicians have assessed and recorded any adverse or toxic reactions to the treatments. Over the years, researchers have standardized the reporting of those side effects using Common Terminology Criteria for Adverse Events (CTCAE).
An Improved Prostate Cancer Biomarker Test May Help Men Avoid Unnecessary Biopsy
MISSION STATEMENT
The Division of Cancer Prevention furthers the mission of the National Cancer Institute by leading, supporting, and promoting rigorous, innovative research and training to prevent cancer and its consequences to improve the health of all people.
Read more about Our Research
Latest News
Cancer prevention clinical trials network funding opportunities open, hpv self-collection test blog, symptom seminar webinar, and more.
Notice of Funding Opportunities (NOFOs): Cancer Prevention Clinical Trials and More; Latest News; Upcoming Events
ULACNet Update - August 2024
In this issue we present: Living with HIV and Preventing Cervical Cancer, AQuIP Analysis, Screening for and Prevention of Anal Cancer as Standard of Care for People with HIV, and Auditing The ULACNet Program.
FDA Approves HPV Tests That Allow for Self-Collection in a Health Care Setting
On May 14, the Food and Drug Administration (FDA) expanded the approvals of two tests that detect cancer-causing types of human papillomavirus (HPV) in the cervix. Both tests are used as part of screening for cervical cancer…
Cancer Prevention Fellowship Program Applications Due Soon, Cancer Research Funding Opportunities, Early Career Scientist Seminar on Bladder Cancer Interception, and More
Latest News, Notice of Funding Opportunities (NOFOs): Cancer Research, Upcoming Events, Grant Submission News
Meetings and Events
Microbial host defense and effect on cancer initiation and progression.
- Wed, September 11, 2024: 1:00p.m. ET - Wed, September 11, 2024: 2:00p.m. ET
Environmental Exposure: HPV-related Head and Neck Cancer Webinar
- Thu, September 12, 2024: 1:00p.m. ET - Thu, September 12, 2024: 2:30p.m. ET
Translational Advances in Cancer Prevention Agent Development (TACPAD) – 3rd Biennial Virtual Meeting
- Mon, October 28, 2024: 11:00a.m. ET - Mon, October 28, 2024: 6:00p.m. ET
- Tue, October 29, 2024: 11:00a.m. ET - Tue, October 29, 2024: 6:00p.m. ET
- Wed, October 30, 2024: 11:00a.m. ET - Wed, October 30, 2024: 2:30p.m. ET
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Cancer Prevention
Home > Patients, Caregivers, and Advocates > About Cancer > Cancer Prevention
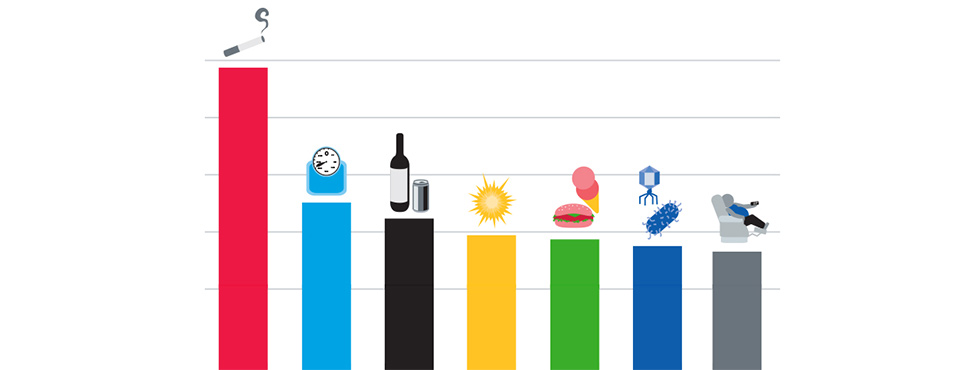
In the United States, about four in ten of the 1.9 million new cancer cases and nearly half of all cancer deaths are related to potentially preventable causes, including tobacco use, obesity, exposure to ultraviolet light, and vaccine-preventable infections with cancer-associated pathogens.
The AACR is committed to advancing the science of cancer prevention. We work with a wide range of partners in biomedical research to develop strategies and promising therapeutics to stop cancer before it starts.
Our ten peer-reviewed medical journals including Cancer Epidemiology, Biomarkers & Prevention and Cancer Prevention Research feature leading-edge research in the field.
Adopting healthy lifestyles that eliminate or reduce the risk of recognized causes of cancer, could decrease the number of people diagnosed with many forms of the disease. Moreover, healthy approaches to living can reduce the risk of cancer recurrences and improve outcomes during treatment.
Tobacco use including smoking has been shown to increase the risk for 17 types of cancer in addition to lung cancer. Eliminating tobacco use is the single most important step we can take to reduce the burden of cancer.
Poor diet, lack of physical activity, and obesity/overweight are known risk factors for many chronic diseases and conditions, and are estimated to account for about 20 percent of cancers in the United States.
The relationship between diet and cancer is complex. Because people consume a variety of foods that keep changing every day, the contribution of a specific type of food or food component in preventing a type of cancer is difficult to determine.
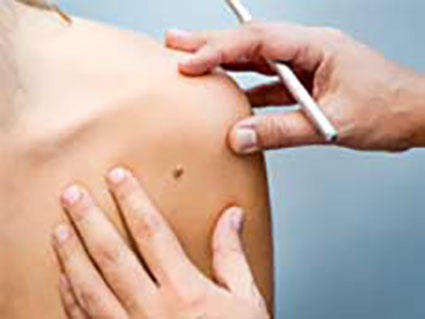
Exposure to ultraviolet (UV) light from the sun or indoor tanning is the predominant cause of all three main types of skin cancer – basal cell carcinoma, squamous cell carcinoma, and melanoma. Adopting sun-safe habits and avoiding the use of indoor tanning devices can decrease the incidence of skin cancer.
Infections with cancer-related pathogens account for about one in five cancer diagnoses worldwide, and taking measures to prevent and treat these infections can help prevent certain cancers.

Screening for colorectal cancer , the second leading cause of cancer death in the United States, by colonoscopy can both detect these cancers at an early stage, when successful treatment is more likely, and prevent them from developing in the first place. That’s because precancerous polyps found by colonoscopy can be removed during the procedure, thus preventing them from going on to become cancers.
If everyone followed colorectal cancer screening guidelines, at least 60 percent of colorectal cancer deaths in the United States could be avoided. Unfortunately, just 38 percent of adults in the United States are getting screened as recommended.
Similarly, Pap test screening for cervical cancer , which was diagnosed in more than 14,000 U.S. women in 2022 can both detect cancer at an early stage, when treatment outcomes tend to be better, and detect precancerous abnormalities, which can then be treated to prevent them developing into cancers.
Scientists are studying many different ways to prevent cancer, including:
- Methods to avoid or control factors known to cause cancer
- New ways to detect precancerous conditions sooner
- Chemoprevention, medicines to treat precancerous conditions or to keep cancer from ever developing.
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer.

- February is National Cancer Prevention Month
- Preventing Cancer: Identifying Risk Factors
- How Do Diet and Exercise Help Prevent Cancer?
Press Releases
- Click here to print the current content on this page.
- Click here to email the current page content.
- Click here to sign up for RSS.
- Click here to view the current page content in within a downloaded PDF document.

BOSTON, June 25, 2024 — The American Cancer Society, the National Cancer Institute, and Cancer Research UK are holding its first annual conference on cancer prevention research, June 25-27, in Boston. The event brings together scientists from discovery biology through translational and behavioral science to population and implementation research to create a thriving multidisciplinary cancer prevention research community. The goal is to help create a “new-look” for cancer prevention in the research community, and to showcase research to better understand cancer aetiology, risk factors, intervention development and implementation, and health inequalities in cancer prevention.
“This is an exciting opportunity for researchers across disciplines to engage in, learn about and discuss the latest concepts in cancer prevention research,” said Dr. William Dahut , chief scientific officer at the American Cancer Society. “The conference will be built on a philosophy of using advances in mechanistic understanding to inform more effective ways of preventing cancer.”
Conference co-chairs include John Burn of Newcastle University, Dr. Timothy Rebbeck of Dana-Farber Cancer Institute and Dr. Thea TIsty from the University of California, San Francisco. The conference agenda features a wide range of topics including:
- Deepening understanding of risk
- Precision prevention intervention development
- Health inequalities in cancer incidence and prevention
- Mechanistic insights informing prevention strategies
For more information on the cancer research prevention conference, click here .
About the American Cancer Society The American Cancer Society is a leading cancer-fighting organization with a vision to end cancer as we know it, for everyone. For more than 110 years, we have been improving the lives of people with cancer and their families as the only organization combating cancer through advocacy, research, and patient support. We are committed to ensuring everyone has an opportunity to prevent, detect, treat, and survive cancer. To learn more, visit cancer.org or call our 24/7 helpline at 1-800-227-2345. Connect with us on Facebook , X, and Instagram .
News Room Home
Media Relations Contacts
News Releases
Our Executive Leadership
Our Researchers
Social Media
News Alerts
What Causes Cancer?
VOICES of Black Women™ Media Resources
Select A Hope Lodge
Log in to our secure, personalized website to manage your care (formerly myMDAnderson).
Request an Appointment
If you are ready to make an appointment, select a button on the right. If you have questions about MD Anderson’s appointment process, our information page may be the best place to start.
- Donate Today

- Clinical Trials
- Our Doctors
Prevention & Personalized Risk Assessment
Research areas.
- Prevention & Personalized Risk Assessment
A major part of Making Cancer History® is preventing cancer from developing in the first place. We're constantly researching ways to reduce an individual's risk of getting cancer, and our discoveries are translated into advances in clinical care and recommendations to the community as a whole.
Cancer Prevention Center
Providing cancer risk assessment, screening and diagnostic services.
Community Health Research
Our researchers are leading community studies to learn more about what causes cancer and the best methods to prevent it. Below are active studies in which you can participate.
Active Living After Cancer
Improving the quality of life of cancer survivors through physical activity
C-MERIT STUDY
Studying the use of contrast-enhanced mammography (CEM) for detecting breast cancer
Families Aiming Toward Health (FAITH)
Aiming to reduce obesity and encourage a healthier lifestyle among African American adults and their families
Examining the relationship between HPV and cancer risk in men
MERIT Study
Developing the next generation of innovative approaches to improve early detection of breast cancer
PISCES Study
Implementing personalized treatments for smoking cessation
Prevención en Sus Manos
Improving access to cervical cancer screening in Texas
Smoking Cessation and Pregnancy Study
Increasing access to smoking cessation and smoke free home services for low-income pregnant women in Northeast Texas
TEXT2MOVE Study
Using a phone-based texting intervention to increase physical activity
Women on the Move
Testing if certain programs help women become more physically active
Be Well Communities™
Building on MD Anderson’s decades at the forefront of cancer prevention research, Be Well Communities™ uses best practices to deploy science-based cancer prevention strategies to communities with the greatest need.

“Every community is unique,” says Ruth Rechis, Ph.D., director of Be Well Communities™ , MD Anderson’s place-based strategy for cancer prevention and control.
Building on MD Anderson’s decades at the forefront of cancer prevention research, Be Well Communities uses best practices to deploy science-based cancer prevention strategies to communities with the greatest need. It brings individuals and organizations together to promote wellness and to address modifiable risk factors for cancer.
“Focusing on a specific geographic area allows us to understand a community's history, culture, and assets as well as what the people from that community feel is needed to improve health overall,” Rechis says.
With the launch of Be Well™ Acres Homes in September 2021 , MD Anderson is focusing on a community in our own backyard – the historic Acres Homes neighborhood, located 8 miles northwest of downtown Houston. Before it was annexed by the City of Houston, Acres Homes was the largest unincorporated Black community in the South. Today, the population of approximately 58,000 people is 47% Black and 43% Hispanic. The area also has the highest referral rate to Harris Health System’s Lyndon B. Johnson Hospital for cancer care, where MD Anderson provides oncology services.
Empowering communities to champion healthy living
Be Well Acres Homes is the third Be Well Community, following initiatives successfully launched in Baytown and Pasadena in 2016. The Be Well Communities team uses a data-driven approach to select communities for investment based on capacity and need. Historically under-resourced communities with the infrastructure to carry out resident-led interventions are prioritized. Like Baytown and Pasadena, Acres Homes represents an area with high rates of residents who are living in poverty and/or without health insurance, and it has strong community organizations with engaged local leaders.
“Our goal is for the communities to lead these comprehensive community health initiatives long into the future,” Rechis says. “When you join our Be Well Communities’ steering committees, we ask you to fully commit to working together toward a common goal of improving wellness for the community overall. When the individuals who live in and work in the community are the ones who commit to action, that’s what will lead to long-term success.”
The short-term accomplishments of the Be Well Communities model are already clear: Since 2017, Be Well Communities has helped increase access to healthy eating and supported continuous food systems, distributing more than 4.5 million pounds of healthy food, including 2 million pounds in an emergency response capacity during the COVID-19 pandemic . More than 70,000 students have participated in health and physical activity programs, and more than 8,500 college students and staff have received access to sun safety and tobacco cessation resources.
In Pasadena, the community is sustaining 90% of the actions originally implemented by Be Well Communities. MD Anderson continues to serve as a member of the coalition, which the City of Pasadena Parks and Recreation department now leads. Be Well™ Baytown is now in its fifth year.
Short-term accountability, long-term health improvement
While Be Well Acres Homes celebrated its official launch in September, work to establish the steering committee and action plan began in 2019. The initiative involves intentional collaboration with Harris Health System, Memorial Hermann Community Benefit Corporation, The University of Texas Health Science Center at Houston (UTHealth) School of Public Health, and more than 30 community organizations, together with residents of Acres Homes.
“The Be Well Communities model has a long-term view on impact and a very short-term view on accountability,” says Michael Walsh Jr., executive director of Cancer Prevention and Control platform. “The coalition that MD Anderson has built over the past several years is designed to actively advance health equity and to sustain access to high-quality health and social services. We engage with our coalition partners on a daily and weekly basis to implement evidence-based actions for health, together.”
The Be Well Acres Homes action plan focuses on priorities identified by the community, in alignment with the City of Houston’s Complete Communities initiative. In the first year, 20 initial projects will focus on increasing opportunities for physical activity and healthy eating, including safe routes to schools, healthy food programs in food pantries, fitness programs and community gardens.
The backbone of Be Well Acres Homes’ support comes from MD Anderson’s Community Outreach and Engagement Fund, along with the Cancer Prevention and Control platform, which is part of the institution’s Moon Shots Program® . The Walmart Foundation is providing additional support through a $1 million grant to The University of Texas Foundation to support implementation of sustainable, neighborhood-based food security strategies.
“The Be Well Communities model goes beyond the scope of what a cancer center might typically do, and it addresses those factors we know can have a downstream effect on cancer risk,” Rechis says. “We’re thinking about how we work with communities to improve health over the long term. It’s exciting to see the enthusiasm building in Acres Homes, and we’re looking forward to growing the program with the community over the years to come.”
Request an appointment at MD Anderson online or by calling 1-877-632-6789.
Preventing Cancer in Texas and Beyond
One of MD Anderson’s goals is to build prevention and screening collaborations that will make an impact on health disparities, both in Texas and globally.
MD Anderson launches collaborative initiative to reduce breast cancer disparities in Houston area
The University of Texas MD Anderson Cancer Center today announced the launch of Texas Health Equity Alliance for Breast Cancer (THEAL), a community-wide health care initiative to reduce breast cancer disparities in the Houston area, particularly for Black women.
THEAL builds upon the expertise of MD Anderson’s Department of Health Disparities Research in addressing the determinants of cancer disparities while leveraging the cancer prevention and control platform’s experience in leading cross-sector collaboratives for community impact. Using successful models implemented in other cities, particularly in Chicago, the initiative seeks to lower the Black/white breast cancer mortality gap in Harris County by 15% over the next decade.
Read more about THEAL here.
Recent Global Partnerships
Md anderson expands global oncology efforts through new agreement with zambia ministry of health, md anderson and pan american health organization join forces to support cancer prevention and control in the americas, md anderson announces new collaboration in indonesia to reduce global cancer burden, learn more about cancer prevention topics, tobacco use, what's the best way to quit smoking.
Smoking is on the decline. But if you are one of the 28 million Americans still smoking, you probably know how hard it is to quit.
Does vaping cause lung cancer?
Vapes, or e-cigarettes, use electricity rather than fire to convert vape liquid with substances like tobacco, nicotine, CBD and THC, into a vapor that can be smoked or inhaled. We spoke with Edwin Ostrin, M.D., Ph.D., to learn more about how vaping impacts the lungs.
EndTobacco Program
Evidence-based actions for tobacco control including support for policy making and Texas’s only certified tobacco treatment training program.
Increasing doses of varenicline or nicotine replacement helps persistent smokers quit
Trial finds smokers who previously tried to quit and failed had greater cessation success if their treatment plan doses were increased or, in some cases, altered.
5 ways MD Anderson is researching the link between food and cancer
The relationship between what we eat and drink and our cancer risk is complex. It’s been the subject of research for decades. But could specific foods or nutrients impact our cancer risk – or even cancer treatment?
5 foods and drinks linked to cancer
Clinical dietitian Alyssa Tatum shares five food groups that have been linked to cancer and gives tips on how to approach your relationship with these foods going forward.
Do sugary drinks increase colorectal cancer risk?
The Yun Laboratory’s research explores how diet affects the intestine, with one project aiming to understand how sugary drinks can impact colorectal cancer development.Ahead, she shares more about her research into sugary beverages, including how we can apply these findings to our own diets.
Why is red meat bad for you?
From barbeque to brisket and sausage to steak, red meat is a mealtime and menu staple.Yet, for cancer prevention, it’s best to limit the amount of red meat you eat.
Is walking good exercise? 4 top questions answered
Simple as it may be, incorporating walking into an exercise routine can still lead to questions: Does every speed of walking count as exercise? How much should I walk each day? Do I need to do any other types of exercise, or is walking enough?
Move more and sit less to reduce your cancer risk
Alcohol Use
12 things to know about alcohol and cancer.
Drinking alcohol is linked to an increased risk for several cancers. Hearing this might lead to questions like ‘Is all alcohol linked to cancer?’ and ‘Do I need to stop drinking entirely?’ For answers, we called on Therese Bevers, M.D., medical director of MD Anderson’s Cancer Prevention Center, and Victoria Lee, a senior clinical dietitian.
What does drinking alcohol do to your body?
We spoke to physician Maher Karam-Hage, M.D., researcher Carrie Daniel-MacDougall, Ph.D., and senior clinical dietitian Victoria Lee to learn how alcohol impacts the body and the best way to proceed.
Sun Exposure
How does sunscreen work understanding uv protection.
Choosing a sunscreen isn’t as easy as it might seem. For help navigating the sometimes cloudy world of sunscreen, we spoke to Saira George, M.D., a dermatologist at MD Anderson in Sugar Land.
What happens when you get a sunburn
Sunburn causes skin sensations that many of us are all too familiar with: stinging, burning, blistering, peeling, and, of course, that hallmark redness.But why does sunburn happen? And are there any health risks that last after a sunburn has faded?
Cancer Screening
Hpv tests with self-collection: what to know.
In May 2024, the Food and Drug Administration (FDA) approved self-collection to test for HPV, the virus that causes cervical cancer. HPV, or the human papillomavirus, is a group of more than 100 viruses that can be passed from person to person.
Adoption and cancer risk: Should you get genetic testing?
A family history of cancer could put you at higher risk of developing that disease one day. But what if you were adopted as a child? How can you best assess your cancer risk as an adult?
Prostate specific antigen (PSA) levels by age: What to know
Prostate-specific antigen (PSA) is a protein made by the prostate. PSA levels can be measured in the bloodstream, and elevated PSA levels can sometimes be a symptom of prostate cancer. So, this antigen gives doctors a starting point to evaluate your risk of having that disease.
How to reduce your breast cancer risk
While there is no guaranteed way to prevent breast cancer, there are ways to reduce your risk. As a breast medical oncologist, my top advice is to practice breast awareness, follow screening guidelines and tell your doctor if you have a family history of cancer.
Featured Cancer Prevention Research Labs
Prevention research resources, related departments, behavioral science, clinical cancer prevention, epidemiology, health disparities research, health services research, cancer prevention and population sciences division, aim facility.
The Assessment, Intervention and Measurement core
Cancer Prevention and Control Platform
Translating prevention knowledge into practice
Prevent Cancer - National Cancer Plan
Imagine if most cancers never occurred. This is becoming more possible, as scientists estimate that we could prevent more than half of all cancers by better applying knowledge we already have. That means taking what we know about the risk factors for, causes of, and development of many cancers and using that information to better monitor and identify anything abnormal before it becomes cancer and to encourage behaviors that help minimize the risk of getting cancer.

The state of cancer prevention today
Many approaches to cancer prevention involve taking steps to reduce one’s risk of developing cancer, such as quitting smoking, wearing sunscreen, and keeping up to date with cancer screenings.
But changing human behavior is not easy. Getting more people to adopt and follow these preventive behaviors, especially among groups that have been medically underserved, could significantly lower the number of people being diagnosed with cancer.
Researchers also continue to study how to reduce cancer disparities, limit exposure to risk factors, and intercept cancer (identify and treat certain lesions before they become cancer), among other prevention approaches. However, there is still a great need for more research to understand what causes precancers and how they develop so that we can create effective prevention or interception methods for all cancers.
Strategies to advance cancer prevention
- Investigate what causes cancer and how precancers develop based on a person’s genetics, behavior, environment, and health history
- Create and test ways to promote preventive behaviors to individuals and communities
- Eliminate infections that lead to cancer
- Create vaccines that prevent cancer
- Develop approaches to limit exposure to toxins
- Increase focus on cancer prevention clinical trials
- Overcome health disparities at all levels and in all aspects of cancer prevention research
Stay informed—sign up for the National Cancer Plan Updates newsletter.
Examples of NCI-supported research to achieve this goal
- Cancer centers are building and offering programs to help people with cancer quit smoking through the Cancer Center Cessation Initiative .
- Clinical trials are testing two different vaccines to prevent or delay the onset of cancers associated with Lynch syndrome.
- The PREVENT program is supporting development of the best ideas in cancer prevention, with a focus on currently unmet needs.
Examples of activities across the government to achieve this goal
- The Environmental Protection Agency has a wide range of efforts to assess risk and reduce exposure to carcinogens , described on a new website.
- The National Aeronautics and Space Administration is working to identify medicines and dietary supplements that could help reduce the risk of cancer from radiation exposure.
- The Agricultural Science Center of Excellence for Nutrition and Diet for Better Health (ASCEND for Better Health) , launched by the U.S. Department of Agriculture (USDA), aims to accelerate research on diet-related chronic diseases, including cancer, and to translate research into solutions that improve public health and well-being. USDA also has virtual Cancer Moonshot Community Conversations available to watch on nutrition- and cancer-related topics.
- American Indian and Alaska Native people have a higher risk of getting several types of cancer compared with other race and ethnicity groups. The Indian Health Service shares cancer prevention and intervention webinars and downloadable posters, infographics, and fact sheets to increase awareness about the importance of getting early preventive cancer screening.
- The Department of Veterans Affairs is enhancing preventive services and cancer care for veterans by expanding access to genetic testing and lung and colorectal cancer screening.
Advertisement
Preventive Treatment with a CD73 Small Molecule Inhibitor Enhances Immune Surveillance in K-Ras Mutant Pancreatic Intraepithelial Neoplasia
Cancer Prev Res 2024;XX:XX–XX
L.N. Strickland, and W. Liu contributed equally to this article.
- Funder(s): National Cancer Institute (NCI)
- Award Id(s): R37CA237384
- Principal Award Recipient(s): F. McAllister
- Funder(s): Cancer Prevention and Research Institute of Texas (CPRIT)
- Award Id(s): RP200173
- Funder(s): Division of Cancer Prevention, National Cancer Institute (DCP, NCI)
- Award Id(s): 75N91019D00021-P00001-759102000002-1
- Principal Award Recipient(s): P.H. Brown , F. McAllister , J.M. Bailey-Lundberg
- Award Id(s): CA249924-02 , CA277161-01A1
- Principal Award Recipient(s): J.M. Bailey-Lundberg
- Award Id(s): R01CA27651
- Principal Award Recipient(s): Z. Zhao
- Funder(s): National Heart, Lung, and Blood Institute (NHLBI)
- Award Id(s): R01HL154720 , R01HL133900
- Principal Award Recipient(s): H.K. Eltzschig
- Funder(s): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- Award Id(s): R01DK122796
- Award Id(s): RP210045
- Principal Award Recipient(s): W. Liu
- Award Id(s): RP180734

- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
- Proof August 26 2024
- Accepted Manuscript August 5 2024
Lincoln N. Strickland , Wendao Liu , Usama Hussein , Nicolette Mardik , Xian Chen , Tingting Mills , Lana A. Vornik , Michelle I. Savage , Shizuko Sei , John Clifford , Holger K. Eltzschig , Powel H. Brown , Zhongming Zhao , Florencia McAllister , Jennifer M. Bailey-Lundberg; Preventive Treatment with a CD73 Small Molecule Inhibitor Enhances Immune Surveillance in K-Ras Mutant Pancreatic Intraepithelial Neoplasia. Cancer Prev Res (Phila) 2024; https://doi.org/10.1158/1940-6207.CAPR-24-0200
Download citation file:
- Ris (Zotero)
- Reference Manager
Immunoprevention is an emerging consideration for solid tumors, including pancreatic ductal adenocarcinoma (PDAC). We and others have shown that Kras mutations in genetic models of spontaneous pancreatic intraepithelial neoplasia (PanIN), which is a precursor to PDAC, results in CD73 expression in the neoplastic epithelium and some populations of infiltrating immune cells, including macrophages and CD8 T cells. CD73 is an ecto-enzyme that converts extracellular adenosine monophosphate to adenosine, a critical immune inhibitory molecule in PDAC. We hypothesized inhibition of CD73 would reduce the incidence of PanIN formation and alter the immune microenvironment. To test our hypothesis, we used the Kras G12D ; Pdx Cre1 ( KC ) genetically engineered mouse model and tested the utility of AB-680, a small molecule inhibitor targeting CD73, to inhibit PanIN progression. AB-680, or vehicle control, was administered using oral gavage delivery 3 days/week at 10 mg/kg, beginning when the mice were 2 months old and lasting 3 months. We euthanized the mice at 5 months old. In the KC model, we quantified significantly less pancreatitis, early and advanced PanIN, and quantified a significant increase in M1 macrophages in AB-680-treated mice. Single-cell RNA sequencing (scRNA-seq) of pancreata of AB-680-treated mice revealed increased infiltration of CD4 + T cells, CD8 + T cells, and mature B cells. The scRNA-seq analysis showed that CD73 inhibition reduced M2 macrophages, acinar, and PanIN cell populations. CD73 inhibition enhanced immune surveillance and expanded unique clonotypes of TCR and BCR, indicating that inhibition of CD73 augments adaptive immunity early in the neoplastic microenvironment.
Prevention Relevance: Previous studies found PanIN lesions in healthy pancreata. Not all progress to PDAC, suggesting a window for enhanced antitumor immunity through immunoprevention therapy. CD73 inhibition in our study prevents PanIN progression, reduces immune-suppressive macrophages and expands TCR and BCR unique clonotypes, highlighting an encouraging therapeutic avenue for high-risk individuals.
Article PDF first page preview
Supplementary data.
Supplementary Table S1: Gene expression changes used to generate gene ontology.
Supplementary Figure S1. Description of IHC quantification methods.
Supplementary Figure S2. AB-680 treatment significantly alters the gene expression profiles of each identified cluster.
Supplementary Figure S3. CD73 inhibitor treatment does not significantly change Granzyme B or B3GAT1 levels.
Supplementary Figure S4. AB-680 treatments results in distinct changes in gene expression in macrophages, dendritic cells and naïve T cells.
Supplementary Figure S5. Treatment with a CD73 small molecule inhibitor does not change AMP or inosine levels in pancreas tissue or serum.
Citing articles via
Email alerts.
- Online First
- Online ISSN 1940-6215
- Print ISSN 1940-6207
AACR Journals
- Blood Cancer Discovery
- Cancer Discovery
- Cancer Epidemiology, Biomarkers & Prevention
- Cancer Immunology Research
- Cancer Prevention Research
- Cancer Research
- Cancer Research Communications
- Clinical Cancer Research
- Molecular Cancer Research
- Molecular Cancer Therapeutics
- Information on Advertising & Reprints
- Information for Institutions/Librarians
- Privacy Policy
- Copyright © 2023 by the American Association for Cancer Research.
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Causes and Prevention
Cancer prevention is action taken to lower the risk of getting cancer. This can include maintaining a healthy lifestyle, avoiding exposure to known cancer-causing substances, and taking medicines or vaccines that can prevent cancer from developing.

Risk Factors for Cancer
Information about behaviors, exposures, and other factors that may influence the risk of cancer.
The Genetics of Cancer
Learn more about cancer as a genetic disease and how genetic changes that increase cancer risk can be inherited. This page also explains genetic testing and how certain genetic changes cause cancer.

Cancer Prevention Overview (PDQ®)
General information about cancer prevention and descriptions of the concepts used in cancer-specific prevention summaries.
Causes and Prevention Research
Find research articles on cancer causes and prevention, which may include news stories, clinical trials, blog posts, and descriptions of active studies.
Dine on DNA: Compounds from nucleic acids in food show anticancer effects
Specific nucleosides hinder replication of tumor cells in step toward cancer prevention.
When people eat, they ingest the nucleic acids that reside in all living things. The compounds in these acids could inhibit the growth of cancer cells, according to findings published in PLOS ONE by Osaka Metropolitan University Associate Professor Akiko Kojima-Yuasa of the Graduate School of Human Life and Ecology and colleagues.
Consuming nucleic acids found in food has been shown to boost the immune system and prevent some diseases. The nucleotides and nucleosides that result from digesting the acids are largely responsible for these beneficial effects.
Professor Kojima-Yuasa's team used compounds of nucleic acids derived from salmon milt DNA and torula yeast RNA and showed that chemical compounds like guanosine could prevent the proliferation of certain cancer cells in laboratory rats. The compounds stopped the cells from starting their replication phase.
"Our research provides a new perspective on the physiological functions of nucleic acids derived from food," Professor Kojima-Yuasa explained. "We hold hope that this will be a crucial step toward cancer prevention."
- Dietary Supplements and Minerals
- Immune System
- Human Biology
- Medical Topics
- Pharmacology
- Diseases and Conditions
- Prostate cancer
- Omega-3 fatty acid
- Biopharmaceutical
- Cervical cancer
Story Source:
Materials provided by Osaka Metropolitan University . Note: Content may be edited for style and length.
Journal Reference :
- Nahoko Shiomi, Mamia Furuta, Yutaro Sasaki, Isao Matsui-Yuasa, Keisuke Kiriyama, Mica Fujita, Keita Sutoh, Akiko Kojima-Yuasa. Suppression of Ehrlich ascites tumor cell proliferation via G1 arrest induced by dietary nucleic acid-derived nucleosides . PLOS ONE , 2024; 19 (7): e0305775 DOI: 10.1371/journal.pone.0305775
Cite This Page :
Explore More
- Ancient Sea Cow Attacked by Croc and Sharks
- Ancient Gene Gives Spiders Their Narrow Waist
- More Fish Species at Risk of Extinction
- Can Fungi Turn Food Waste Into Something Tasty?
- Gene Therapy Gets a Turbo Boost
- Atlantic and Arctic Current Mixing Crucial
- New Model to Enhance Extreme Rainfall Prediction
- Graphene Tech to Revolutionize Batteries
- Drug May Stop Migraines Before Headache Starts
- Catching and Destroying 'Forever Chemicals'
Trending Topics
Strange & offbeat.

Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Research We Fund: Extramural Discovery Science
- Discovery Boost Grants (DBG)
- Arizona (AZ) Discovery Boost Grants (DBG)
- Mission Boost Grants (MBG)
- Research Scholar Grants
- Postdoctoral Fellowships
- Clinician Scientist Development Grant (CSDG)
- ASTRO-ACS Clinician Scientist Development Grant (CSDG)
- Institutional Research Grants
- RFA: Extramural Discovery Science Accelerator Award
- RFA: Cancer Health Equity Research Centers
- RFA: Real-World Data Impact Award (RWIA)
- RFA: The Role of Health Policy and Health Insurance in Improving Access to and Performance of Cancer Prevention, Early Detection, and Treatment Services
- ACS Professor Award
- RFA: Metastasis in Melanoma and Other Cancers
- TheoryLab Collaborative (TLC) Grant
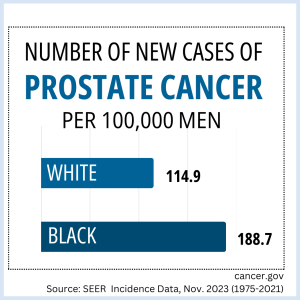
- IMPACT RFA to Support Research to Reduce Prostate Cancer Mortality

RFA: Leukemia Exploration and Prevention Grant Program (LEAP)
- Extramural Grants Department Staff Contacts
- Definition of Research Areas
- FAQs: ACS Extramural Discovery Science and Grant Application Submissions
- Extramural Priority Research Areas
- Our Grant Process
- American Cancer Society Research Events
- 2022 Jiler Conference - The Event
- 2022 Jiler Conference - The Feedback
- Biochemistry and Immunology of Cancer
- Cell Biology and Preclinical Cancer Research
- Clinical and Cancer Control Research
- Peer Review Committee for Experimental Therapeutics (ET)
- Peer Review Committee for DNA Mechanisms in Cancer (DMC)
- Peer Review Committee for Institutional Research Grants (IRG)
- Peer Review Committee for RNA Mechanisms in Cancer (RMC)
- Peer Review Committee for Tumor Biochemistry and Endocrinology (TBE)
- Peer Review Committee for Cancer Cell Biology (CCB)
- Peer Review Committee for Cancer Detection and Progression (CDP)
- Peer Review Committee for Healthcare Outcomes, Policy, and Systems Research (HOPS)
- Peer Review Committee for Mission Boost Grants
- Peer Review Committee for Immunology and Blood Cell Development (IBCD)
- Peer Review Committee for Treatment, Palliative Care, and Survivorship Research
- Discovery Scientific Council
- Peer Review Committee for Metastasis and Microenvironment (MM)
- Peer Review Committee for Etiology, Screening, and Early Detection (ESED)
- Peer Review Committee for Cancer Prevention and Health Promotion (CPHP)
- ACS Professor Peer Review Committee
- Community Research Partner Participation on Grant Peer Review Committees
- American Cancer Society Professors
- Nobel Laureates and the American Cancer Society

The formation of cancer is orchestrated by a confluence of external, cell-nonautonomous, factors and internal, cell-autonomous, factors. Discovering medical interventions that can disrupt this complex interplay of factors is challenging but necessary.
In the bone marrow, the acquisition of somatic mutations in leukemia-associated genes, resulting in clonal mosaicism, is termed clonal hematopoiesis (CH). CH is unequivocally associated with hematologic malignancies (HM) and, therefore, considered a precancerous condition.
While in the general population CH is common in the elderly (more than 10% in people aged 70 and older), the overall risk of malignant transformation is low.
However, in high-risk populations, like those with germline predispositions to HM, CH rates are much higher, and the risk of HM development is significantly greater.
One such high-risk population includes people with RUNX1 familial platelet disorder ( RUNX1 -FPD), a rare heritable disease caused by germline RUNX1 mutations. Individuals with RUNX1 -FPD have a 35 to 50% lifetime risk of HM with acute myeloid leukemia as the most common and second deadliest HM.
Despite the established link between RUNX1 -FPD and hematologic malignancies, there remains a significant gap in understanding the cell-nonautonomous factors that contribute to clonal evolution and malignant transformation, as well as the precise mechanistic pathways by which RUNX1 mutations promote cancer development. This knowledge gap impedes the development of targeted strategies for early intervention and prevention.
A deeper understanding of how extrinsic signals and the broader microenvironment interact with intrinsic RUNX1 mutations is critical to uncovering novel therapeutic targets. The RUNX1 -FPD community of families is in urgent need of cancer interception and prevention therapies.
LEAP Grant Program Overview
The RUNX1 Research Program and the American Cancer Society (ACS) have partnered to make a demonstrable leap forward in the pursuit of cancer interception and prevention treatments for patients with RUNX1 -FPD. The grant mechanism is called Leukemia Exploration and Prevention, or LEAP, to symbolize this overarching goal.
Grant proposals are investigator-initiated and pursue questions specifically designed to enable the discovery of cancer interception or prevention therapies for RUNX1 -FPD HMs.
Applicants for the LEAP grant program must propose a research project in one of the two areas of focus below:
The first area is focused on deepening our understanding of the mechanisms that cause each leukemogenic step, beginning with germline RUNX1 -mutated hematopoietic stem/progenitor cells (HSPCs) that acquire deleterious somatic mutations and expand over time, culminating in overt RUNX1 -mutated leukemia. Projects should consider experimental designs that address both cell-autonomous and cell-nonautonomous factors.
The second area of the LEAP grant program is committed to supporting high-risk projects with strong scientific rationale to pursue a cancer interception treatment. Projects could focus on preclinical studies and/or clinical trials designed to test innovative approaches to treatment. This can include repurposing existing regulatory-approved treatments or novel therapeutics. The intent here is to act on behalf of the urgency felt by the patient community.
Eligibility
You ARE eligible to submit a proposal if you:
- Work at a US academic institution or eligible non-profit
- Are a full-time independent investigator at any career stage
Grant Mechanisms
Applicants may only apply for one of the two funding mechanisms: the LEAP-Research Scholar Grant or the LEAP-Team Award.
LEAP-Research Scholar Grant
Intent: Supports an independent research project that aligns with one of the two LEAP program focus areas. Research Scholar Grants (RSGs) provide support for independent, self-directed researchers.
Term and Budget: Awards are for up to $215,000 per year for up to 4 years ($860,000 total direct costs) plus 10% allowable indirect costs.
LEAP-Team Award
Intent: Supports a team research project that aligns with one of the LEAP program research focus areas. The application must clearly articulate how the team will synergize to accelerate scientific advancement and clinical benefit.
Applicants must clearly demonstrate the assembly of an interdisciplinary team with diverse expertise, ensuring a comprehensive approach to address the research objectives. Each team should have 1 Lead PI and at least 1 Team Principal.
- Lead PI: Coordinating PI; assumes the authority and responsibility to direct the project.
- Team Principal: Directs a specific area of the scientific and technical work and leads a component of the research based on their area(s) of expertise. Full-time independent researchers at any career stage can serve as Team Principals.
The Lead PI and all Team Principals share authority for scientific leadership.
Term and Budget: Team awards are for up to $430,000 per year direct costs for up to 4 years ($1,720,000 direct costs), plus 10% allowable indirect costs.
A formal review of each LEAP grant will occur after 2 years. At this time, the project’s progress will be assessed, and PIs will have the opportunity to adjust the project’s specific aims if necessary for the remaining 2 years of the LEAP grant.
Anticipated Funding: Funds are available for 3 LEAP-RSG awards or 1 LEAP-RSG award and 1 LEAP-Team award.
LEAP RFA Release: August 28, 2024
Application Deadline: October 29, 2024
Anticipated Grant Award Notification: June 2025
Anticipated Grant Start Date: September 2025
Program Contact: Paul Campbell, PhD, paul.campbell@cancer.org
Applications must be submitted through ProposalCentral
Grant Application Instructions
Grant Policies
Help us end cancer as we know it, for everyone.

- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 26, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Synthetic immunology: Approaching a turning point in the treatment and prevention of disease
by Heidelberg University
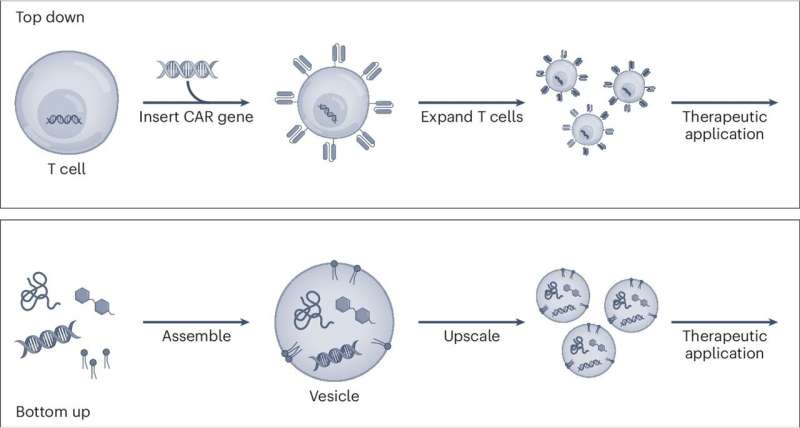
Synthetic immunology, an innovative field of research that could lead to fundamentally new approaches and methods in the treatment of infectious diseases and cancer, is the topic of a new article published in Nature Nanotechnology .
Heidelberg researchers Prof. Dr. Kerstin Göpfrich, Prof. Dr. Michael Platten, Prof. Dr. Friedrich Frischknecht, and Prof. Dr. Oliver T. Fackler describe a so-called bottom-up approach that uses the toolbox of nanotechnology and synthetic biology to construct systems from molecular building blocks and specifically equip them with immune functions.
The experts in the fields of synthetic biology, neuroimmunology, parasitology, and virology conduct research at Heidelberg University, the Heidelberg and Mannheim University Hospitals, the German Cancer Research Center, and the Max Planck Institute for Medical Research.
In the new bottom-up approach in synthetic immunology, complex immune functions are no longer created by modifying existing cells or molecules, but rather originate from molecular building blocks like nanoscale components or artificial cells assembled into complex systems.
The process is based on technologies and techniques from synthetic biology such as the design of proteins and peptides, polymer synthesis as well as DNA/RNA modification and DNA/RNA origami—the three-dimensional "folds" of DNA or RNA strands—to produce functional nanostructures.
As the authors discuss in their article, the new bottom-up approach in synthetic immunology will allow unparalleled precision and control in shaping immune functions . By manufacturing immune components from the ground up, immune responses can be custom-engineered with great specificity and efficiency.
This could lead to groundbreaking approaches in the development of new therapies and vaccines that circumvent the constraints of traditional approaches, such as unwanted side effects or time-limited efficacy.
The Heidelberg researchers believe that the bottom-up approach not only promises improved therapeutic approaches and methods but can also push the future boundaries of what is possible in the treatment of complex illnesses.
The further development of this research field could lead to the development of completely synthetic immune effectors that, according to the vision, could then prevent and treat diseases.
"We are facing a turning point in the treatment and prevention of infectious diseases and cancer. The synergies between synthetic biology and immunology open up a number of possibilities that could one day create a completely new basis for how we deal with disease," states Prof. Göpfrich.
Explore further
Feedback to editors

Managing early stages of abortion care at home after 12 weeks is safe and reduces time spent in hospital, study finds
8 hours ago

Billions worldwide consume inadequate levels of micronutrients critical to human health, new study finds

Blocking the longevity gene S6K1 extends lifespan in mice by reducing inflammation
10 hours ago

Machine learning predicts which patients will continue taking opioids after hand surgery

Study describes a new molecular pathway involved in the control of reproduction
11 hours ago

Machine learning helps identify rheumatoid arthritis subtypes

Immune protection against tuberculosis reinfection driven by cells that dampen lung inflammation, shows study

Girls with mental health conditions have lower HPV vaccination coverage
12 hours ago

Mankai plant found to reduce post-meal sugar levels in diabetics

Unique chicken line advances research on autoimmune disease that affects humans
Related stories.
Peptide boronic acids offer new prospects for synthetic immunology
Aug 13, 2024
Another step toward synthetic cells
Jun 21, 2022

Engineered peptides open new avenue for immunotherapy drug development
Apr 19, 2024

Researchers develop 'founding document' on synthetic cell development
May 3, 2024
Programming synthetic exosomes to optimize wound healing
Sep 6, 2021
DNA nanotube rings: Research team develops important building block for artificial cells
Mar 25, 2024
Recommended for you

Smart mask monitors breath for signs of health
13 hours ago

Gene therapy gets a turbo boost from researchers
16 hours ago

Combo immunotherapy produces distinct waves of cancer-fighting T cells with each dose

Scientists discover how the body's killer cells attack cancer
17 hours ago

VR headsets could be life changing for people with intellectual disability
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 June 2024
Why do patients with cancer die?
- Adrienne Boire ORCID: orcid.org/0000-0002-9029-1248 1 na1 ,
- Katy Burke 2 na1 ,
- Thomas R. Cox ORCID: orcid.org/0000-0001-9294-1745 3 , 4 na1 ,
- Theresa Guise 5 na1 ,
- Mariam Jamal-Hanjani 6 , 7 , 8 na1 ,
- Tobias Janowitz ORCID: orcid.org/0000-0002-7820-3727 9 , 10 na1 ,
- Rosandra Kaplan 11 na1 ,
- Rebecca Lee ORCID: orcid.org/0000-0003-2540-2009 12 , 13 na1 ,
- Charles Swanton ORCID: orcid.org/0000-0002-4299-3018 7 , 8 , 14 na1 ,
- Matthew G. Vander Heiden ORCID: orcid.org/0000-0002-6702-4192 15 , 16 na1 &
- Erik Sahai ORCID: orcid.org/0000-0002-3932-5086 12 na1
Nature Reviews Cancer volume 24 , pages 578–589 ( 2024 ) Cite this article
20k Accesses
1 Citations
523 Altmetric
Metrics details
- Cancer models
- Cancer therapy
Cancer is a major cause of global mortality, both in affluent countries and increasingly in developing nations. Many patients with cancer experience reduced life expectancy and have metastatic disease at the time of death. However, the more precise causes of mortality and patient deterioration before death remain poorly understood. This scarcity of information, particularly the lack of mechanistic insights, presents a challenge for the development of novel treatment strategies to improve the quality of, and potentially extend, life for patients with late-stage cancer. In addition, earlier deployment of existing strategies to prolong quality of life is highly desirable. In this Roadmap, we review the proximal causes of mortality in patients with cancer and discuss current knowledge about the interconnections between mechanisms that contribute to mortality, before finally proposing new and improved avenues for data collection, research and the development of treatment strategies that may improve quality of life for patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
The changing landscape of cancer in the USA — opportunities for advancing prevention and treatment

Causes of death among people living with metastatic cancer
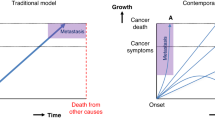
Deceptive measures of progress in the NHS long-term plan for cancer: case-based vs. population-based measures
Dillekås, H., Rogers, M. S. & Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 8 , 5574–5576 (2019).
Article PubMed PubMed Central Google Scholar
Seyfried, T. N. & Huysentruyt, L. C. On the origin of cancer metastasis. Crit. Rev. Oncog. 18 , 43–73 (2013).
Schnurman, Z. et al. Causes of death in patients with brain metastases. Neurosurgery 93 , 986–993 (2023).
Article PubMed Google Scholar
Gallardo-Valverde, J. M. et al. Obstruction in patients with colorectal cancer increases morbidity and mortality in association with altered nutritional status. Nutr. Cancer 53 , 169–176 (2005).
Swanton, C. et al. Embracing cancer complexity: hallmarks of systemic disease. Cell 187 , 1589–1616 (2024).
Article CAS PubMed Google Scholar
Wheatley-Price, P., Blackhall, F. & Thatcher, N. The influence of sex in non-small cell lung cancer. Onkologie 32 , 547–548 (2009).
Abu-Sbeih, H. et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J. Clin. Oncol. 38 , 576 (2020).
Neugut, A. I. et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J. Clin. Oncol. 24 , 2368–2375 (2006).
Sullivan, D. R. et al. Association of early palliative care use with survival and place of death among patients with advanced lung cancer receiving care in the Veterans Health Administration. JAMA Oncol. 5 , 1702–1709 (2019).
Sallnow, L. et al. Report of the Lancet Commission on the value of death: bringing death back into life. Lancet 399 , 837–884 (2022).
Abdel-Karim, I. A., Sammel, R. B. & Prange, M. A. Causes of death at autopsy in an inpatient hospice program. J. Palliat. Med. 10 , 894–898 (2007).
Pautex, S. et al. Anatomopathological causes of death in patients with advanced cancer: association with the use of anticoagulation and antibiotics at the end of life. J. Palliat. Med. 16 , 669–674 (2013).
Khorana, A. A., Francis, C. W., Culakova, E., Kuderer, N. M. & Lyman, G. H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 5 , 632–634 (2007).
Levi, M. & Scully, M. How I treat disseminated intravascular coagulation. Blood 131 , 845–854 (2018).
Cines, D. B., Liebman, H. & Stasi, R. Pathobiology of secondary immune thrombocytopenia. Semin. Hematol. 46 , S2 (2009).
Ghanavat, M. et al. Thrombocytopenia in solid tumors: prognostic significance. Oncol. Rev. 13 , 43–48 (2019).
Article CAS Google Scholar
Anker, M. S. et al. Advanced cancer is also a heart failure syndrome: a hypothesis. J. Cachexia Sarcopenia Muscle 12 , 533 (2021).
Asdahl, P. H. et al. Cardiovascular events in cancer patients with bone metastases — a Danish population-based cohort study of 23,113 patients. Cancer Med. 10 , 4885–4895 (2021).
Sinn, D. H. et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS ONE 10 , e0124434 (2015).
Zisman, A. et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J. Urol. 169 , 909–916 (2003).
Suárez, C. et al. Carotid blowout syndrome: modern trends in management. Cancer Manag. Res. 10 , 5617 (2018).
Lin, A. L. & Avila, E. K. Neurologic emergencies in the cancer patient: diagnosis and management. J. Intensive Care Med. 32 , 99 (2017).
Gamburg, E. S. et al. The prognostic significance of midline shift at presentation on survival in patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 48 , 1359–1362 (2000).
Mokri, B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 56 , 1746–1748 (2001).
Mastall, M. et al. Survival of brain tumour patients with epilepsy. Brain 144 , 3322–3327 (2021).
Steindl, A. et al. Neurological symptom burden impacts survival prognosis in patients with newly diagnosed non-small cell lung cancer brain metastases. Cancer 126 , 4341–4352 (2020).
Girard, N. et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am. J. Surg. Pathol. 33 , 1752–1764 (2009).
Lee, P. et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 69 , 328–333 (2007).
Kookoolis, A. S., Puchalski, J. T., Murphy, T. E., Araujo, K. L. & Pisani, M. A. Mortality of hospitalized patients with pleural effusions. J. Pulm. Respir. Med. 4 , 184 (2014).
PubMed PubMed Central Google Scholar
Cousins, S. E., Tempest, E. & Feuer, D. J. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst. Rev . https://doi.org/10.1002/14651858.CD002764 (2016).
Baker, M. L. et al. Mortality after acute kidney injury and acute interstitial nephritis in patients prescribed immune checkpoint inhibitor therapy. J. Immunother. Cancer 10 , e004421 (2022).
Bhave, P., Buckle, A., Sandhu, S. & Sood, S. Mortality due to immunotherapy related hepatitis. J. Hepatol. 69 , 976–978 (2018).
Lameire, N. H., Flombaum, C. D., Moreau, D. & Ronco, C. Acute renal failure in cancer patients. Ann. Med. 37 , 13–25 (2005).
Ries, F. & Klastersky, J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am. J. Kidney Dis. 8 , 368–379 (1986).
Wong, J. L. & Evans, S. E. Bacterial pneumonia in patients with cancer: novel risk factors and management. Clin. Chest Med. 38 , 263–277 (2017).
Lee, L. Y. W. et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395 , 1919–1926 (2020).
Article CAS PubMed PubMed Central Google Scholar
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584 , 430–436 (2020). This study used a platform of 17.4 million pseudo-anonymized health-care records to determine risk factors for COVID-19.
Pelosof, L. C. & Gerber, D. E. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin. Proc. 85 , 838–854 (2010).
Donovan, P. J. et al. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J. Clin. Endocrinol. Metab. 100 , 2024–2029 (2015).
Burtis, W. J. et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N. Engl. J. Med. 322 , 1106–1112 (1990). First study to show that patients with cancer-associated hypercalcaemia had elevated concentrations of plasma parathyroid hormone-related protein .
Ellison, D. H. & Berl, T. The syndrome of inappropriate antidiuresis. N. Engl. J. Med. 356 , 2064–2072 (2007).
Okabayashi, T. et al. Diagnosis and management of insulinoma. World J. Gastroenterol. 19 , 829–837 (2013).
Giometto, B. et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch. Neurol. 67 , 330–335 (2010).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4 , 1721 (2018).
Feng, S. et al. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J. Thorac. Oncol. 12 , 1626–1635 (2017).
Coustal, C. et al. Prognosis of immune checkpoint inhibitors-induced myocarditis: a case series. J. Immunother. Cancer 11 , e004792 (2023).
Kuderer, N. M., Dale, D. C., Crawford, J., Cosler, L. E. & Lyman, G. H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106 , 2258–2266 (2006).
Agarwal, M. A. et al. Ventricular arrhythmia in cancer patients: mechanisms, treatment strategies and future avenues. Arrhythm. Electrophysiol. Rev . 12 , e16 (2023).
Zafar, A. et al. The incidence, risk factors, and outcomes with 5-fluorouracil-associated coronary vasospasm. JACC CardioOncol. 3 , 101–109 (2021).
Polk, A. et al. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 6 , e012798 (2016).
Safdar, A., Bodey, G. & Armstrong, D. Infections in patients with cancer: overview. Princip. Pract. Cancer Infect. Dis. https://doi.org/10.1007/978-1-60761-644-3_1 (2011).
Foster, D. S., Jones, R. E., Ransom, R. C., Longaker, M. T. & Norton, J. A. The evolving relationship of wound healing and tumor stroma. JCI Insight 3 , e99911 (2018).
Park, S. J. & Bejar, R. Clonal hematopoiesis in cancer. Exp. Hematol. 83 , 105 (2020).
Liebman, H. A. Thrombocytopenia in cancer patients. Thromb. Res. https://doi.org/10.1016/S0049-3848(14)50011-4 (2014).
Chakraborty, R. et al. Characterisation and prognostic impact of immunoparesis in relapsed multiple myeloma. Br. J. Haematol. 189 , 1074–1082 (2020).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26 , 1125–1134 (2020).
Munn, D. H. & Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39 , 1–6 (2016).
Kochar, R. & Banerjee, S. Infections of the biliary tract. Gastrointest. Endosc. Clin. N. Am. 23 , 199–218 (2013).
Valvani, A., Martin, A., Devarajan, A. & Chandy, D. Postobstructive pneumonia in lung cancer. Ann. Transl. Med. 7 , 357–357 (2019).
Rolston, K. V. I. Infections in cancer patients with solid tumors: a review. Infect. Dis. Ther. 6 , 69–83 (2017).
Wu, X. et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLoS One 13 , e0205729 (2018).
The clinicopathological and prognostic role of thrombocytosis in patients with cancer: a meta-analysis. Oncol. Lett . 13 , 5002–5008 (2017).
Kasthuri, R. S., Taubman, M. B. & Mackman, N. Role of tissue factor in cancer. J. Clin. Oncol. 27 , 4834 (2009).
Wade, J. C. Viral infections in patients with hematological malignancies. Hematology 2006 , 368–374 (2006).
Article Google Scholar
Ersvaer, E., Liseth, K., Skavland, J., Gjertsen, B. T. & Bruserud, Ø. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 11 , 1–12 (2010).
Kuter, D. J. Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies. Haematologica 107 , 1243 (2022).
Rodgers, G. M. et al. Cancer- and chemotherapy-induced anemia. J. Natl Compr. Canc. Netw. 10 , 628–653 (2012).
Nesher, L. & Rolston, K. V. I. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 42 , 5–13 (2014).
Blijlevens, N. M. A., Logan, R. M. & Netea, M. G. Mucositis: from febrile neutropenia to febrile mucositis. J. Antimicrob. Chemother. 63 , i36–i40 (2009).
Petrelli, F. et al. Association of steroid use with survival in solid tumours. Eur. J. Cancer 141 , 105–114 (2020).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52 , 1219–1226 (2020). This study identified the molecular characteristics of clonal haematopoiesis that increased risk of therapy-related myeloid neoplasms, with different characteristics associated with different treatment exposures.
Bhatia, R. et al. Do cancer and cancer treatments accelerate aging? Curr. Oncol. Rep. 24 , 1401 (2022).
Eisenstein, T. K. The role of opioid receptors in immune system function. Front. Immunol. 10 , 485158 (2019).
Böll, B. et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 100 , 239 (2021).
Ruiz-Giardin, J. M. et al. Blood stream infections associated with central and peripheral venous catheters. BMC Infect. Dis. 19 , 1–9 (2019).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124 , 188–195 (2014).
Brahmer, J. R. et al. Safety profile of pembrolizumab monotherapy based on an aggregate safety evaluation of 8937 patients. Eur. J. Cancer 199 , 113530 (2024). Analysis of the toxicity profile of anti-PD1 therapy in more than 8,000 patients.
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381 , 1535–1546 (2019).
Vozy, A. et al. Increased reporting of fatal hepatitis associated with immune checkpoint inhibitors. Eur. J. Cancer 123 , 112–115 (2019).
Palaskas, N., Lopez-Mattei, J., Durand, J. B., Iliescu, C. & Deswal, A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J. Am. Heart Assoc. 9 , e013757 (2020).
Janssen, J. B. E. et al. Immune checkpoint inhibitor-related Guillain–Barré syndrome: a case series and review of the literature. J. Immunother. 44 , 276–282 (2021).
Camelliti, S. et al. Mechanisms of hyperprogressive disease after immune checkpoint inhibitor therapy: what we (don’t) know. J. Exp. Clin. Cancer Res. 39 , 236 (2020).
Kitamura, W. et al. Bone marrow microenvironment disruption and sustained inflammation with prolonged haematologic toxicity after CAR T-cell therapy. Br. J. Haematol. 202 , 294–307 (2023).
Seano, G. et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 3 , 230 (2019).
Madhusoodanan, S., Ting, M. B., Farah, T. & Ugur, U. Psychiatric aspects of brain tumors: a review. World J. Psychiatry 5 , 273 (2015).
Gerstenecker, A. et al. Cognition in patients with newly diagnosed brain metastasis: profiles and implications. J. Neurooncol. 120 , 179 (2014).
Krishna, S. et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 617 , 599–607 (2023). This study demonstrated that high-grade gliomas remodel neural circuits in the human brain, which promotes tumour progression and impairs cognition.
Taylor, K. R. et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature 623 , 366–374 (2023). This study showed that brain-derived neurotrophic factor (BDNF)–tropomyosin-related kinase B (TRKB) signalling promotes malignant synaptic plasticity and augments tumour progression.
Hanahan, D. & Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 41 , 573–580 (2023).
Ahles, T. A. & Root, J. C. Cognitive effects of cancer and cancer treatments. Annu. Rev. Clin. Psychol . 14 , 425–451 (2018).
Allexandre, D. et al. EEG correlates of central origin of cancer-related fatigue. Neural Plast. 2020 , 8812984 (2020).
Büttner-Teleagă, A., Kim, Y. T., Osel, T. & Richter, K. Sleep disorders in cancer — a systematic review. Int. J. Environ. Res. Public Health 18 , 11696 (2021).
Walsh, D. & Nelson, K. A. Autonomic nervous system dysfunction in advanced cancer. Support. Care Cancer 10 , 523–528 (2002).
Ghandour, F. et al. Presenting psychiatric and neurological symptoms and signs of brain tumors before diagnosis: a systematic review. Brain Sci. 11 , 1–20 (2021).
Akechi, T. et al. Somatic symptoms for diagnosing major depression in cancer patients. Psychosomatics 44 , 244–248 (2003).
Nho, J. H., Kim, S. R. & Kwon, Y. S. Depression and appetite: predictors of malnutrition in gynecologic cancer. Support. Care Cancer 22 , 3081–3088 (2014).
Thaker, P. H. et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12 , 939–944 (2006). This study linked chronic behavioural stress to higher levels of tissue catecholamines and tumour angiogenesis, resulting in greater tumor burden and invasion in ovarian cancer.
Chang, A. et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med . 15 , eadf1147 (2023).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341 , 1236361 (2013). This study showed that the formation of autonomic nerve fibres in the prostate gland regulates prostate cancer development and dissemination in mouse models.
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 4 , 17105 (2018).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12 , 489–495 (2011). International consensus definitions of cancer cachexia.
Bossi, P., Delrio, P., Mascheroni, A. & Zanetti, M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients 13 , 1980 (2021).
Farkas, J. et al. Cachexia as a major public health problem: frequent, costly, and deadly. J. Cachexia Sarcopenia Muscle 4 , 173–178 (2013).
Dennison, E. M., Sayer, A. A. & Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 13 , 340–347 (2017).
Farasat, M. et al. Long-term cardiac arrhythmia and chronotropic evaluation in patients with severe anorexia nervosa (LACE-AN): a pilot study. J. Cardiovasc. Electrophysiol. 31 , 432–439 (2020).
Mehler, P. S., Anderson, K., Bauschka, M., Cost, J. & Farooq, A. Emergency room presentations of people with anorexia nervosa. J. Eat. Disord. 11 , 16 (2023).
Ferrer, M. et al. Cachexia: a systemic consequence of progressive, unresolved disease. Cell 186 , 1824–1845 (2023).
Bourke, C. D., Berkley, J. A. & Prendergast, A. J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 37 , 386–398 (2016).
Tisdale, M. J. Biology of cachexia. J. Natl Cancer Inst. 89 , 1763–1773 (1997).
Babic, A. et al. Adipose tissue and skeletal muscle wasting precede clinical diagnosis of pancreatic cancer. Nat. Commun. 14 , 4754 (2023).
Waning, D. L. et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21 , 1262 (2015). This study showed that bone metastases cause TGFβ to be released from the bone marrow, resulting in leakage of calcium from skeletal muscle cells contributing to muscle weakness.
Greco, S. H. et al. TGF-β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS ONE 10 , e0132786 (2015).
Johnen, H. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13 , 1333–1340 (2007). This study showed that GDF15 was elevated in patients with cancer-associated weight loss and that this was a central regulator of appetite and therefore a potential therapeutic target.
Al-Sawaf, O. et al. Body composition and lung cancer-associated cachexia in TRACERx. Nat. Med. 29 , 846–858 (2023). This study showed an association among lower skeletal muscle area, subcutaneous adipose tissue and visceral adipose tissue and decreased survival in patients with non-small-cell lung cancer and these were associated with higher levels of circulating GDF15.
Ahmed, D. S., Isnard, S., Lin, J., Routy, B. & Routy, J. P. GDF15/GFRAL pathway as a metabolic signature for cachexia in patients with cancer. J. Cancer 12 , 1125–1132 (2021).
Rebbapragada, A., Benchabane, H., Wrana, J. L., Celeste, A. J. & Attisano, L. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 23 , 7230 (2003).
Queiroz, A. L. et al. Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer. Nat. Commun. 13 , 1–17 (2022).
Loumaye, A. et al. Role of activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 100 , 2030–2038 (2015).
Barton, B. E. & Murphy, T. F. Cancer cachexia is mediated in part by the induction of IL-6-like cytokines from the spleen. Cytokine 16 , 251–257 (2001).
Webster, J. M., Kempen, L. J. A. P., Hardy, R. S. & Langen, R. C. J. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 11 , 597675 (2020).
Strassmann, G., Masui, Y., Chizzonite, R. & Fong, M. Mechanisms of experimental cancer cachexia local involvement of 11-1 in colon-26 tumor. J. Immunol. 150 , 2341–2345 (1993).
Stovroff, M. C., Fraker, D. L., Swedenborg, J. A. & Norton, J. A. Cachectin/tumor necrosis factor: a possible mediator of cancer anorexia in the rat. Cancer Res. 48 , 4567–4572 (1988).
CAS PubMed Google Scholar
Wyke, S. M. & Tisdale, M. J. NF-κB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin–proteasome system in skeletal muscle. Br. J. Cancer 92 , 711 (2005).
Cai, D. et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119 , 285–298 (2004). This study showed that activation of NF-κB, through muscle-specific transgenic expression of activated inhibitor of NF-κB kinase subunit β (IKKβ), causes profound muscle wasting in mice.
Patel, H. J. & Patel, B. M. TNF-α and cancer cachexia: molecular insights and clinical implications. Life Sci. 170 , 56–63 (2017).
Mergenthaler, P., Lindauer, U., Dienel, G. A. & Meisel, A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36 , 587 (2013).
Sillos, E. M. et al. Lactic acidosis: a metabolic complication of hematologic malignancies case report and review of the literature. Cancer 92 , 2237–46 (2000).
Rampello, E., Fricia, T. & Malaguarnera, M. The management of tumor lysis syndrome. Nat. Clin. Pract. Oncol. 3 , 438–447 (2006).
Delano, M. J. & Moldawer, L. L. The origins of cachexia in acute and chronic inflammatory diseases. Nutr. Clin. Pract. 21 , 68–81 (2006).
Lombardi, A., Villa, S., Castelli, V., Bandera, A. & Gori, A. T-cell exhaustion in Mycobacterium tuberculosis and nontuberculous mycobacteria infection: pathophysiology and therapeutic perspectives. Microorganisms 9 , 2460 (2021).
Moldawer, L. L. & Sattler, F. R. Human immunodeficiency virus-associated wasting and mechanisms of cachexia associated with inflammation. Semin. Oncol. 25 , 73–81 (1998).
von Kobbe, C. Targeting senescent cells: approaches, opportunities, challenges. Aging 11 , 12844 (2019).
Shafqat, S., Chicas, E. A., Shafqat, A. & Hashmi, S. K. The Achilles’ heel of cancer survivors: fundamentals of accelerated cellular senescence. J. Clin. Invest. 132 , e158452 (2022).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22 , 340–355 (2022).
Terry, W., Olson, L. G., Ravenscroft, P., Wilss, L. & Boulton-Lewis, G. Hospice patients’ views on research in palliative care. Intern. Med. J. 36 , 406–413 (2006).
White, C. & Hardy, J. What do palliative care patients and their relatives think about research in palliative care? A systematic review. Support. Care Cancer 18 , 905–911 (2010).
Foster, B., Bagci, U., Mansoor, A., Xu, Z. & Mollura, D. J. A review on segmentation of positron emission tomography images. Comput. Biol. Med. 50 , 76–96 (2014).
Bera, K., Braman, N., Gupta, A., Velcheti, V. & Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 19 , 132–146 (2022).
Kaczanowska, S. et al. Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer Cell 42 , 35–51.e8 (2024).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152 , 244–248 (2016).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25 , 487–495 (2019).
Gyawali, B., Hey, S. P. & Kesselheim, A. S. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. eClinicalMedicine 21 , 100332 (2020).
Hong, W. et al. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl Acad. Sci. USA 112 , E5351–E5360 (2015).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15 , 234–248 (2018).
Bowden, M. B. et al. Demographic and clinical factors associated with suicide in gastric cancer in the United States. J. Gastrointest. Oncol. 8 , 897–901 (2017).
Zaorsky, N. G. et al. Suicide among cancer patients. Nat. Commun. 10 , 1–7 (2019).
CAS Google Scholar
Hu, X. et al. Suicide risk among individuals diagnosed with cancer in the US, 2000 – 2016 . JAMA Netw. Open 6 , e2251863 (2023).
Google Scholar
Abdel-Rahman, O. Socioeconomic predictors of suicide risk among cancer patients in the United States: a population-based study. Cancer Epidemiol. 63 , 101601 (2019).
Pinquart, M. & Duberstein, P. R. Depression and cancer mortality: a meta-analysis. Psychol. Med. 40 , 1797–1810 (2010).
Fitzgerald, P. et al. The relationship between depression and physical symptom burden in advanced cancer. BMJ Support. Palliat. Care 5 , 381–388 (2015).
Chida, Y., Hamer, M., Wardle, J. & Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 5 , 466–475 (2008).
He, X. Y. et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell 42 , 474–486.e12 (2024). This study found that chronic stress shifts the normal circadian rhythm of neutrophils resulting in increased neutrophil extracellular trap (NET) formation via glucocorticoid release, resulting in a metastasis-promoting microenvironment.
Fann, J. R., Ell, K. & Sharpe, M. Integrating psychosocial care into cancer services. J. Clin. Oncol. 30 , 1178–1186 (2012).
Jacobsen, P. B. & Wagner, L. I. A new quality standard: the integration of psychosocial care into routine cancer care. J. Clin. Oncol. 30 , 1154–1159 (2012).
Gorin, S. S. et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J. Clin. Oncol. 30 , 539–547 (2012).
Li, M. et al. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psychooncology 26 , 573–587 (2017).
Bova, G. S. et al. Optimal molecular profiling of tissue and tissue components: defining the best processing and microdissection methods for biomedical applications. Mol. Biotechnol. 29 , 119–152 (2005).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520 , 353–357 (2015). This study found that metastasis-to-metastasis spread was common in prostate cancer evolution and that lesions affecting tumour suppressor genes occurred as single events, whereas mutations in genes involved in androgen receptor signalling commonly involved multiple, convergent events in different metastases.
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173 , 581–594.e12 (2018). This study examined evolutionary trajectories of 100 renal cancers and found that metastasis competence was driven by chromosome complexity, not by driver mutation load, and that loss of 9p and 14q was a common driver.
Spain, L. et al. Late-stage metastatic melanoma emerges through a diversity of evolutionary pathways. Cancer Discov. 13 , 1364–1385 (2023). This study examined evolutionary trajectories of melanoma metastasis and observed frequent whole-genome doubling and widespread loss of heterozygosity, often involving antigen-presentation machinery.
Download references
Acknowledgements
A.B. is funded by National Institutes of Health/National Cancer Institute P30 CA008748 and R01-CA245499. K.B. is employed by the UK National Health Service. T.R.C. acknowledges funding support from the National Health and Medical Research Council (NHMRC) Ideas (2000937), Project (1129766, 1140125), Development (2013881) and Fellowship (1158590) schemes, a Cancer Institute NSW Career Development Fellowship (CDF171105), Cancer Council NSW project support (RG19-09, RG23-11) and Susan G. Komen for the Cure (CCR17483294). T.G. is funded by the Cancer Prevention and Research Institute of Texas Grant 00011633. M.J.-H. has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETs and NIHR. T.J. acknowledges funding from Cancer Grand Challenges (NIH: 1OT2CA278690-01; CRUK: CGCATF-2021/100019), the Mark Foundation for Cancer Research (20-028-EDV), the Osprey Foundation, Fortune Footwear, Cold Spring Harbour Laboratory (CSHL) and developmental funds from CSHL Cancer Center Support Grant 5P30CA045508. R.K. is funded by the Intramural Research Program, the National Cancer Institute, NIH Clinical Center and the National Institutes of Health (NIH NCI ZIABC011332-06 and NIH NCI ZIABC011334-10). R.L. is supported by a Wellcome Early Career Investigator Award (225724/Z/22/Z). E.S. is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (CC2040), the UK Medical Research Council (CC2040) and the Wellcome Trust (CC2040) and the European Research Council (ERC Advanced Grant CAN_ORGANISE, Grant agreement number 101019366). E.S. reports personal grants from Mark Foundation and the European Research Council. C.S. is a Royal Society Napier Research Professor (RSRP\R\210001). His work is supported by the Francis Crick Institute that receives its core funding from Cancer Research UK (CC2041), the UK Medical Research Council (CC2041) and the Wellcome Trust (CC2041) and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (ERC Advanced Grant PROTEUS Grant agreement number 835297). M.G.V.H. reports support from the Lustgarten Foundation, the MIT Center for Precision Cancer Medicine, the Ludwig Center at MIT and NIH grants R35 CA242379 and P30 CA1405141.
Author information
These authors contributed equally: Adrienne Boire, Katy Burke, Thomas R. Cox, Theresa Guise, Mariam Jamal-Hanjani, Tobias Janowitz, Rosandra Kaplan, Rebecca Lee, Charles Swanton, Matthew G. Vander Heiden, Erik Sahai.
Authors and Affiliations
Memorial Sloan Kettering Cancer Center, New York, NY, USA
Adrienne Boire
University College London Hospitals NHS Foundation Trust and Central and North West London NHS Foundation Trust Palliative Care Team, London, UK
Cancer Ecosystems Program, The Garvan Institute of Medical Research and The Kinghorn Cancer Centre, Darlinghurst, New South Wales, Australia
Thomas R. Cox
School of Clinical Medicine, St Vincent’s Healthcare Clinical Campus, UNSW Medicine and Health, UNSW Sydney, Sydney, New South Wales, Australia
Department of Endocrine Neoplasia and Hormonal Disorders, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Theresa Guise
Cancer Metastasis Laboratory, University College London Cancer Institute, London, UK
Mariam Jamal-Hanjani
Department of Oncology, University College London Hospitals, London, UK
Mariam Jamal-Hanjani & Charles Swanton
Cancer Research UK Lung Centre of Excellence, University College London Cancer Institute, London, UK
Cold Spring Harbour Laboratory, Cold Spring Harbour, New York, NY, USA
Tobias Janowitz
Northwell Health Cancer Institute, New York, NY, USA
Paediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Rosandra Kaplan
Tumour Cell Biology Laboratory, The Francis Crick Institute, London, UK
Rebecca Lee & Erik Sahai
Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK
Rebecca Lee
Cancer Evolution and Genome Instability Laboratory, The Francis Crick Institute, London, UK
Charles Swanton
Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA, USA
Matthew G. Vander Heiden
Dana-Farber Cancer Institute, Boston, MA, USA
You can also search for this author in PubMed Google Scholar
Contributions
All authors researched data for the article. A.B., K.B., T.R.C., T.G., T.J., C.S., M.G.V.H, R.K., M.J.-H. and E.S. contributed substantially to discussion of the content. T.C., R.L. and E.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Correspondence to Thomas R. Cox or Erik Sahai .
Ethics declarations
Competing interests.
A.B. is an inventor on pending patents 63/449,817, 63/052,139 as well as awarded patents 11,305,014 and 10,413,522; all issued to the Sloan Kettering Institute. She has received personal fees from Apelis Pharmaceuticals and serves as an unpaid member of the Evren Technologies SAB. K.B., T.R.C., T.G., T.J. and R.K. declare no competing interests. M.J.-H. reports support from Achilles Therapeutics Scientific Advisory Board and Steering Committee, Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster and Bristol Myers Squibb outside the submitted work. R.L. reports personal fees from Pierre Fabre and has research funding from BMS, Astra Zeneca and Pierre Fabre outside the submitted work. E.S. reports grants from Novartis, Merck Sharp Dohme, AstraZeneca and personal fees from Phenomic outside the submitted work. C.S. reports grants and personal fees from Bristol Myers Squibb, AstraZeneca, Boehringer-Ingelheim, Roche-Ventana, personal fees from Pfizer, grants from Ono Pharmaceutical, Personalis, grants, personal fees and other support from GRAIL, other support from AstraZeneca and GRAIL, personal fees and other support from Achilles Therapeutics, Bicycle Therapeutics, personal fees from Genentech, Medixci, China Innovation Centre of Roche (CiCoR) formerly Roche Innovation Centre, Metabomed, Relay Therapeutics, Saga Diagnostics, Sarah Canon Research Institute, Amgen, GlaxoSmithKline, Illumina, MSD, Novartis, other support from Apogen Biotechnologies and Epic Bioscience outside the submitted work; in addition, C.S. has a patent for PCT/US2017/028013 licensed to Natera Inc., UCL Business, a patent for PCT/EP2016/059401 licensed to Cancer Research Technology, a patent for PCT/EP2016/071471 issued to Cancer Research Technology, a patent for PCT/GB2018/051912 pending, a patent for PCT/GB2018/052004 issued to Francis Crick Institute, University College London, Cancer Research Technology Ltd, a patent for PCT/GB2020/050221 issued to Francis Crick Institute, University College London, a patent for PCT/EP2022/077987 pending to Cancer Research Technology, a patent for PCT/GB2017/053289 licensed, a patent for PCT/EP2022/077987 pending to Francis Crick Institute, a patent for PCT/EP2023/059039 pending to Francis Crick Institute and a patent for PCT/GB2018/051892 pending to Francis Crick Institute. C.S. is Co-chief Investigator of the NHS Galleri trial funded by GRAIL. He is Chief Investigator for the AstraZeneca MeRmaiD I and II clinical trials and Chair of the Steering Committee. C.S. is cofounder of Achilles Therapeutics and holds stock options. M.G.V.H. is a scientific adviser for Agios Pharmaceuticals, iTeos Therapeutics, Sage Therapeutics, Faeth Therapeutics, Droia Ventures and Auron Therapeutics on topics unrelated to the presented work.
Peer review
Peer review information.
Nature Reviews Cancer thanks Vickie Baracos, Clare M. Isacke, who co-reviewed with Amanda Fitzpatrick and Erica Sloan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An autoimmune encephalitis characterized by complex neuropsychiatric features and the presence of immunoglobulin G (IgG) antibodies against the NR1 subunit of the NMDA receptors in the central nervous system.
Partial collapse or incomplete inflation of the lung.
Pressure-induced movement of brain tissue.
An ageing-associated process in which haematopoiesis becomes dominated by one or a small number of genetically distinct stem or progenitor cells. Clonal haematopoiesis is linked to an increased risk of haematological malignancies.
Inability of the heart to pump blood properly.
Constriction of the arteries that supply blood to the heart.
(CRH). One of the major factors that drives the response of the body to stress.
(DIC). A rare but serious condition in which abnormal blood clotting occurs throughout the blood vessels of the body.
Inflammation of the brain.
An abnormal connection that forms between two body parts, such as an organ or blood vessel and another often unrelated structure in close proximity.
A rare disorder in which the immune system of a body attacks the nerves, which can lead to paralysis.
The stopping of flow of blood, typically associated with the bodies response to prevent and stop bleeding.
A build-up of fluid within the cavities of the brain.
Elevated calcium levels in the blood, often caused by overactive parathyroid glands. Hypercalcaemia is linked to kidney stones, weakened bones, altered digestion and potentially altered cardiac and brain function.
(HPD). Rapid tumour progression sometimes observed during immune checkpoint inhibitor treatment.
The condition that occurs when the level of sodium in the blood is low.
Harm, which is often unavoidable, caused by cancer treatments.
The marked suppression of polyclonal immunoglobulins in the body.
(LEMS). A neuromuscular junction disorder affecting communication between nerves and muscles, which manifests as a result of a paraneoplastic syndrome or a primary autoimmune disorder. Many cases are associated with small-cell lung cancer.
When cancer cells spread to the tissue layers covering the brain and spinal cord (the leptomeninges).
Also known as pulmonary oedema is a condition caused by excess fluid in the lungs. This fluid collects in the alveoli compromising function and making it difficult to breathe.
The observation of displacement of brain tissue across the centre line of the brain, suggestive of uneven intracranial pressure.
Decreased blood flow to the myocardium, commonly called a heart attack.
Inflammation specifically of the middle layer of the heart wall.
A group of rare disorders that occur when the immune system reacts to changes in the body triggered by the presence of a neoplasm.
A dense network of nerves that transmit information from the brain (efferent neurons) to the periphery and conversely transmit information from the periphery to the brain (afferent neurons). A component of the peripheral nervous system is the autonomic nervous system.
A build-up of fluid between the tissues that line the lungs and the chest wall.
A condition characterized by loss of skeletal muscle mass and function.
The lodging of a circulating blood clot within a vessel leading to obstruction. Thromboembolisms may occur in veins (venous thromboembolism) and arteries (arterial thromboembolism).
A key component of the pathway regulating blood clotting, specifically the receptor and cofactor for factor VII/VIIa.
A syndrome occurs when tumour cells release their contents into the bloodstream, either spontaneously or more typically, in response to therapeutic intervention.
Devices worn on the body, typically in the form of accessories or clothing, that incorporate advanced electronics and technology to monitor, track or enhance various aspects of human life. Examples include smartwatches and fitness trackers.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Boire, A., Burke, K., Cox, T.R. et al. Why do patients with cancer die?. Nat Rev Cancer 24 , 578–589 (2024). https://doi.org/10.1038/s41568-024-00708-4
Download citation
Accepted : 15 May 2024
Published : 19 June 2024
Issue Date : August 2024
DOI : https://doi.org/10.1038/s41568-024-00708-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The road less travelled.
Nature Reviews Cancer (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- Open access
- Published: 21 August 2024
Association between gynecologic cancer and Alzheimer’s disease: a bidirectional mendelian randomization study
- Di Cao 1 , 2 na1 ,
- Shaobo Zhang 3 na1 ,
- Yini Zhang 1 , 2 ,
- Ming Shao 4 , 5 ,
- Qiguang Yang 6 &
- Ping Wang 1 , 2
BMC Cancer volume 24 , Article number: 1032 ( 2024 ) Cite this article
280 Accesses
7 Altmetric
Metrics details
Alzheimer’s disease (AD) manifests with a higher rate of occurrence in women. Previous epidemiological studies have suggested a potential association between AD and gynecological cancers, but the causal relationship between them remains unclear. This study aims to explore the causal link between 12 types of gynecological cancers and AD using a bidirectional Mendelian randomization (MR) approach.
We obtained genetic correlation tools for AD using data from the most extensive genome-wide association study. Genetic correlation data for 12 types of gynecological cancers were also sourced from the Finnish Biobank. These cancers include breast cancer (BC), cervical adenocarcinoma (CA), cervical squamous cell carcinoma (CSCC), cervical cancer (CC), endometrial cancer (EC), ovarian endometrioid carcinoma (OEC), ovarian cancer (OC), ovarian serous carcinoma (OSC), breast carcinoma in situ (BCIS), cervical carcinoma in situ (CCIS), endometrial carcinoma in situ (ECIS), and vulvar carcinoma in situ (VCIS). We used the inverse-variance weighted (IVW) model for causal analysis and conducted horizontal pleiotropy tests, heterogeneity tests, MR-PRESSO tests, and leave-one-out analyses to ensure the robustness of our results. We also applied replication analysis and meta-analysis to further validate our experimental results.
The study found that EC ( P _IVW =0.037, OR [95% CI] = 1.032 [1.002, 1.064]) and CCIS ( P _IVW = 0.046, OR [95% CI] = 1.032 [1.011, 1.064]) increase the risk of AD, whereas OC was negatively correlated with AD ( P _IVW = 0.016, OR [95% CI] = 0.974[0.954, 0.995]). In reverse MR analysis, AD increased the risk of CC ( P _IVW = 0.039, OR [95% CI] = 1.395 [1.017, 1.914]) and VCIS ( P _IVW = 0.041, OR [95% CI] = 1.761 [1.027, 2.021]), but was negatively correlated with OEC ( P _IVW = 0.034, OR [95% CI] = 0.634 [0.417, 0.966]). Sensitivity analysis results demonstrated robustness. These findings were further substantiated through replication and meta-analyses.
Conclusions
Our MR study supports a causal relationship between AD and gynecological cancers. This encourages further research into the incidence of gynecological cancers in female Alzheimer’s patients and the active prevention of AD.
Peer Review reports
Gynecological cancer refers to cancers that originate in the female reproductive system [ 1 ]. Since the incidence of breast cancer in women is higher than in men, this article classifies breast cancer as a form of gynecological cancer, a perspective shared by some research studies [ 2 , 3 ]. Breast cancer, cervical cancer, ovarian cancer, and endometrial cancer, as common malignancies among women, have seen a gradual increase in incidence rates in recent years, with the age of onset trending towards younger populations [ 4 ]. According to the latest global cancer statistics from 2020, the incidence rate of breast cancer in women has surpassed that of lung cancer, making it the most common cancer worldwide [ 5 ]. In 2020, there were 2.3 million new cases of breast cancer globally, accounting for 11.7% of all cancer cases, with breast cancer deaths comprising 6.9% of all cancer-related deaths. New cases of cervical cancer were 0.60 million, representing 3.1% of all cancer cases; endometrial cancer had 0.42 million new issues, accounting for 2.2%; and ovarian cancer had nearly 0.31 million new cases, making up 1.6% of all topics [ 6 ]. These figures indicate a substantial economic burden on society [ 7 ]. For gynecological cancers, particularly recurrent and advanced stages, traditional standard treatments often leave much to be desired [ 8 ]. While developing new treatment strategies is crucial, preventing these diseases is also important and is increasingly recognized as a priority by the public.
Alzheimer’s Disease [AD] is a common neurodegenerative condition in the geriatric population [ 9 ], with studies finding a higher prevalence in women [ 10 , 11 , 12 ]. Epidemiological evidence supports an inverse relationship between the incidence of cancer and AD [ 13 , 14 ] – a diagnosis of cancer reduces the risk of developing AD, and vice versa [ 15 ]. However, does this apply to all cancers? Research indicates that women with breast cancer have a significantly increased risk of early-onset Alzheimer’s and related dementias [ADRD] [ 16 ]. Additionally, older breast cancer survivors exhibiting age-related phenotypes and genotypes may face increased risks of cognitive decline [ 17 ]. Studies have also found that breast cancer patients carrying the APOE4 allele experience declines in memory, attention, and learning abilities for an extended period post-treatment [ 18 ]. In a study involving over six million women, 36,131 breast cancer patients and 3019 cervical cancer patients were found to have early-onset ADRD [ 19 ]. Moreover, AD patients tend to be diagnosed with gynecological cancers at a later stage, when the disease is more severe, often missing the optimal treatment window [ 20 ]. Due to the limitations of clinical cancer research and the communication challenges posed by cognitive impairments in AD patients, establishing a causal relationship between the two conditions is challenging.
Like randomized controlled trials, Mendelian randomization [MR] studies involve the random allocation of genes during embryonic development, similar to the random assignment of interventions at the start of a trial [ 21 ]. This method reduces the impact of confounding factors. It overcomes common causality issues in observational epidemiological studies, avoiding the high costs, ethical concerns, feasibility, and experimental environment issues associated with the randomized controlled trial [ 22 , 23 ]. Hence, this study designed a bidirectional MR study using single nucleotide polymorphisms [SNPs] as instrumental variables [IVs] to explore the bidirectional causal effects between gynecological tumors and AD, offering new insights into preventing and treating AD and cancer.
Study design
To explore the causal relationship between gynecologic cancer and AD, the present study conducted a bidirectional MR analysis. Figure 1 illustrates our study’s methodology and process. The selected genetic IVs need to satisfy three assumptions of MR analysis [ 24 ]. First, genetic variations are assumed to be closely related to the exposure event. Second, there is no relation between gynecologic cancer, AD, and confounding factors. Finally, genetic variations are assumed to directly influence disease outcomes through exposure factors, excluding other pathways. We derived our genetic instruments for exposure and outcome from publicly available genome-wide association study [GWAS] summary statistics. As all ethical aspects have been addressed in the original research, our study requires no additional ethical approval. This study follows the STROBE-MR writing guidelines.

Flowchart of the bidirectional Mendelian randomization study. Abbreviations MR, Mendelian randomization; SNP, Single nucleotide polymorphism; GWAS, genome-wide association study; LD, linkage disequilibrium
Data sources
The 12 selected common cancers in women for this study, including breast cancer (BC), cervical adenocarcinoma (CA), cervical squamous cell carcinoma (CSCC), cervical cancer (CC), endometrial cancer (EC), ovarian endometrioid carcinoma (OEC), ovarian cancer (OC), ovarian serous carcinoma (OSC), breast carcinoma in situ (BCIS), cervical carcinoma in situ (CCIS), endometrial carcinoma in situ (ECIS), and vulvar carcinoma in situ (VCIS), were analyzed using IVs from the Finnish Cancer Registry (R9) in the Finnish Biobank. The Finnish Biobank was established in the early 20th century and contains data related to healthcare, genetics, familial inheritance, demographics, education, employment, and other aspects, offering high research value and practicality [ 25 ]. The AD whole-genome dataset is derived from the European Alzheimer’s Disease Biobank (EADB) Alliance, which consists of 39,106 samples and 46,828 controls, with a sample size of 487,511 and 20,921,626 SNPs [ 26 ]. All participants are of European ancestry. More details can be found in Table 1 .
Selection and evaluation of IVs
In the forward MR analysis, gynecologic cancer is considered the exposure factor, and AD is the outcome event. The IVs associated with gynecologic cancer should demonstrate genome-wide significance ( P < 5e-06). Initially, we selected genetic variations adhering to the criterion of P < 5e-08. However, under these stringent conditions, no available SNPs were available for cancers such as CA and CSCC. In the reverse MR analysis, AD is treated as the exposure and gynecologic cancer as the outcome, with AD-related IVs meeting the P < 5e-08 threshold. In the bidirectional MR analysis, to obtain independent SNPs, the linkage disequilibrium (LD) parameters (R^2) should be < 0.001 and kb = 10,000. The F-statistic represents the strength of the MR analysis, with a value greater than 10 indicating statistical robustness [ 27 ]. The formula for calculating the F-statistic is R^2(N-2)/(1-R^2) [ 28 ]. It is essential to exclude SNPs associated with confounding factors. We utilized Phenoscanner (version 2, accessed on October 30, 2023) to eliminate SNPs linked to potential confounders. The filtered SNPs will serve as the IVs for our study.
MR analysis
MR studies investigating the relationship between exposure and outcome primarily use the inverse-variance weighted (IVW) method because it can obtain a robust result without pleiotropy [ 29 ]. MR-Egger, Simple Mode, weighted median (WM), and Weighted Mode methods are used as supplementary methods to assess the robustness of the primary analysis.
We conducted various sensitivity analyses to ensure the robustness of the outcomes obtained from the bi-directional MR. Sensitivity analysis includes horizontal pleiotropy test, heterogeneity test, MR-PRESSO test, and leave-one-out analysis. The horizontal pleiotropy test is performed by MR-Egger regression. If a significant intercept term is found in MR-Egger analysis, it indicates the presence of horizontal pleiotropy [ 30 , 31 ]. Cochran’s Q test is used to assess the heterogeneity of SNPs. If Cochran’s Q statistic is statistically significant ( P ≤ 0.05), it suggests considerable heterogeneity in the analysis results. MR-PRESSO test using MR pleiotropy residual sum and outlier test is used to detect outliers [ 32 ]. If outliers are detected, they will be removed, and the remaining IVs will be reanalyzed. Leave-one-out analysis is used to evaluate whether a single SNP determines significant results [ 33 ]. The risk association between gynecologic cancer and AD is expressed as an odds ratio [OR] and 95% confidence interval (CI). If P ≤ 0.05, it provides evidence for a possible causal relationship. We used the Steiger test to perform directionality testing to avoid biases caused by reverse causation. Analysis was conducted using R 4.3.1, utilizing several packages, including TwoSampleMR, ggplot2, and MRPRESSO.
Confirmatory analysis and meta-analysis
To ensure the reliability of our study results, we conducted a replication validation using an additional AD GWAS dataset from the GWAS Catalog, with accession number GCST007320 [ 34 ]. This dataset includes 71,880 cases and 383,378 controls, all of European ancestry. We applied this AD dataset to conduct a bidirectional MR analysis with 12 types of gynecological cancers. The selection process of IVs, the MR analysis standards, and sensitivity testing methods were consistent with the initial analysis. We performed a meta-analysis to combine the IVW results from the replication and initial analyses that showed causal associations. The choice of effect model was based on the heterogeneity of the results. When heterogeneity was not significant, a fixed-effect model was used; otherwise, a random-effect model was applied [ 35 ]. The meta-analysis was performed using the meta package and Review Manager 5.3.
Causal effect of gynecologic cancer on AD
In the forward MR analysis, we included a total of 78 independent SNPs associated with BC, 2 independent SNPs associated with CA, 4 independent SNPs associated with CSCC, 6 independent SNPs associated with CC, 11 independent SNPs associated with EC, 5 independent SNPs associated with OEC, 6 independent SNPs associated with OC, 11 independent SNPs associated with OSC, seven independent SNPs associated with BCIS, 12 independent SNPs associated with CCIS, one independent SNP associated with ECIS, and 3 independent SNPs associated with VCIS (Supplementary Table 1 ). Importantly, all IVs exhibited F-statistics well above 10, ranging from 256.345 to 34274.379, which indicates a low risk of bias and supports fulfilling the strong instrumental assumptions required for MR (Supplementary Table 3 ).
The IVW method showed that when AD was the outcome factor, EC could increase the risk of AD ( P _IVW = 0.037, OR [95% CI] = 1.032 [1.002, 1.064]), while OC could suppress the risk of AD ( P _IVW = 0.016, OR [95% CI] = 0.974 [0.954, 0.995]), and CCIS could promote the risk of AD ( P _IVW = 0.046, OR [95% CI] = 1.032 [1.011, 1.064]) (Fig. 2 [A]). The calculation results of MR Egger, WM, Simple mode, and Weighted mode were consistent with the direction of the IVW results, indicating the robustness and reliability of the primary analysis methods. There was no causal relationship between other common tumors in women and AD.

Forest plots depicting the causal estimates between gynecological cancer and AD. ( A ) Forward MR analysis forest plot, with gynecological cancer as the exposure event and AD as the outcome event. ( B ) Reverse MR analysis forest plot, with AD as the exposure event and gynecological cancer as the outcome event. Abbreviations N SNPs, number of SNPs; OR, odds ratio; CI, confidence interval; AD, Alzheimer’s disease; BC, breast cancer; CA, cervical adenocarcinoma; CSCC, cervical squamous cell carcinoma; CC, cervical cancer; EC, endometrial cancer; OEC, ovarian endometrioid carcinoma; OC, ovarian cancer, OSC, ovarian serous carcinoma; BCIS, breast carcinoma in situ; CCIS, cervical carcinoma in situ; ECIS, endometrial carcinoma in situ; VCIS, vulvar carcinoma in situ; IVW, Inverse-variance weighted
Causal effect of AD on gynecologic cancer
In the reverse MR analysis, focusing on gynecologic cancer as the outcome, we observed a definitive causal link between AD and conditions such as CC, VCIS, and OEC. Specifically, AD appears to elevate the risk for CC ( P _IVW = 0.039, OR [95% CI] = 1.395 [1.017, 1.914]) and VCIS ( P _IVW = 0.041, OR [95% CI] = 1.761 [1.027, 2.021]). At the same time, it conversely reduces the risk for OEC ( P _IVW = 0.034, OR [95% CI] = 0.634 [0.417, 0.966]), as illustrated in (Fig. 2 [B]) and detailed in Supplementary Table 2 . Notably, no causal links were found between AD and other common tumors in females. The consistency of these findings across various analytical methods, aligning with the direction of the IVW results, underscores their reliability. Furthermore, all IVs exhibit an F-statistic significantly above 10 (Supplementary Tables 4 to 15 ), suggesting a minimal influence of weak instrument bias on the MR analysis.
Sensitivity analyses results
In addition, sensitivity analysis using the leave-one-out method in both forward and reverse MR analyses showed no evidence of directional pleiotropy (Fig. 3 ). The funnel plot revealed no evidence of asymmetry, indicating a lower risk of directional pleiotropy. MR-Egger regression testing with all P -values > 0.05 showed no directional pleiotropy between gynecologic cancer and AD. Cochran’s Q statistic showed no significant heterogeneity among instrumental SNP effects ( P > 0.05). Furthermore, MR-PRESSO results demonstrated no statistically substantial outliers or influential points ( P > 0.05), suggesting no significant interference or bias was found when evaluating the relationship between exposure and outcome (Table 2 ). The Supplementary Figs. 1 – 23 present scatter plots, funnel plots, leave-one-out sensitivity analyses and forest plots.

The leave-one-out plot of SNPs associated with gynecological cancer and AD. ( A ) Forward MR leave-one-out sensitivity analysis for the ‘EC’ on ‘Alzheimer’s disease’. ( B ) Forward MR leave-one-out sensitivity analysis for the ‘CCIS’ on ‘Alzheimer’s disease’. ( C ) Forward MR leave-one-out sensitivity analysis for the ‘OC’ on ‘Alzheimer’s disease’. ( D ) Reverse MR leave-one-out sensitivity analysis for the ‘Alzheimer’s disease’ on ‘CC’. ( E ) Reverse MR leave-one-out sensitivity analysis for the ‘Alzheimer’s disease’ on ‘VCIS’. ( F ) Reverse MR leave-one-out sensitivity analysis for the ‘Alzheimer’s disease’ on ‘OEC’. Abbreviations CC, cervical cancer; EC, endometrial cancer; OEC, ovarian endometrioid carcinoma; OC, ovarian cancer, CCIS, cervical carcinoma in situ; VCIS, vulvar carcinoma in situ
Validation analysis and meta-analysis
After applying another GWAS data for AD (accession number GCST007320) and conducting bidirectional MR analysis, we observed trends similar to those found in the preliminary analysis. Specifically, EC ( P _IVW = 0.030, OR [95% CI] = 1.013 [1.011,1.016]) and CCIS ( P _IVW = 0.001, OR [95% CI] = 1.007 [1.006,1.008]) were found to possibly increase the risk of AD, while OC ( P _IVW = 0.045, OR [95% CI] = 0.997 [0.995,0.999]) could reduce the risk of AD. Reverse MR analysis indicated that AD might increase the disease risk for CC ( P _IVW = 0.038, OR [95% CI] = 2.257 [1.592,3.199]) and VCIS ( P _IVW = 0.008, OR [95% CI] = 2.210 [2.047,2.386]), and decrease the onset risk for OEC ( P _IVW = 0.015, OR [95% CI] = 0.798 [0.735,0.866]). Sensitivity analysis showed no irregularities. Additionally, meta-analysis of the OR results from two IVW instances further reinforced this impression. Meta-analyzing the OR results from two IVW rounds further confirmed this impression. Details in Fig. 4 and Supplementary Tables 16 to 17 . The scatter plots, funnel plots, leave-one-out sensitivity analysis, and forest plots for the replication MR analyses can be found in Supplementary Figs. 24 – 46 .

Meta-analysis of causal associations ( P _IVW < 0.05) between gynecological cancer and AD. ( A ) Meta-analysis of OR [95% CI] for EC with AD as the outcome. ( B ) Meta-analysis of OR [95% CI] for OC with AD as the outcome. ( C ) Meta-analysis of OR [95% CI] for CCIS with AD as the outcome. ( D ) Meta-analysis of OR [95% CI] for CC with AD as the exposure. ( E ) Meta-analysis of OR [95% CI] for VICS with AD as the exposure. ( F ) Meta-analysis of OR [95% CI] for OEC with AD as the exposure. Abbreviations CC, cervical cancer; EC, endometrial cancer; OEC, ovarian endometrioid carcinoma; OC, ovarian cancer, CCIS, cervical carcinoma in situ; VCIS, vulvar carcinoma in situ; 95% CI, 95% confidence interval; OR, odds ratio
In today’s society, as women’s roles and importance in social life grow, so does the incidence of common tumors in women, influenced by their unique physiological makeup and hormone levels. In addition, epidemiological data shows that AD, with a tendency to affect women [ 36 ], presents significant challenges to society and families. Currently, there is a lack of solid scientific evidence linking AD with common cancers in women. We have initiated a bidirectional MR study, employing comprehensive GWAS summary statistics, to investigate potential causal links between common female cancers and AD.
Specifically, our research, utilizing forward MR analysis, suggests that individuals with EC and CCIS may be at a higher risk of developing AD. In comparison, those with OC may have a lower risk. Other common female cancers, like BC, seem not to impact the risk of developing AD. Although current research has shown that cancer survivors have a reduced incidence of AD [ 37 ], there are also studies demonstrating a connection between EC and AD. One study found that the expression of SERPINA3 in EC is associated with disease progression, poor differentiation, high malignancy, and advanced stages of cancer [ 38 ], especially in cells expressing negative estrogen receptors (ER). Increased expression of SERPINA3 was observed in these ER-negative cells. Suppressing the presentation of the SERPINA3 gene can inhibit the proliferation of cancer cells. However, SERPINA3 also plays a crucial role in the development of AD [ 39 ], with elevated levels of SERPINA3 protein found in the blood, brain [including the hippocampus], and cerebrospinal fluid of AD patients [ 40 ]. Analysis has shown that one of the components of amyloid plaques in AD is the SERPINA3 protein and an increase in the levels of SERPINA3 protein in the cerebrospinal fluid may be indicative of mild cognitive impairment in the progression of AD [ 41 ]. Additionally, EC and AD are interconnected through multiple common pathways, such as the mTOR signaling network [ 42 ] and G-protein-coupled receptors (GPCRs) [ 43 ], which play significant roles in the pathology of both diseases. THOP1 (Thimet oligopeptidase), a neuropeptide processing enzyme, was observed to have significantly increased in AD brain tissue as a neuroprotective response to Aβ toxicity [ 44 , 45 ]. However, in a comparative transcriptome analysis, researchers observed that the expression of the THOP1 gene was significantly downregulated as EC progressed to its late stages, weakening its neuroprotective effect on AD [ 46 ].
CCIS, also known as grade 3 cervical intraepithelial neoplasia [ 47 ], had approximately 30–50% of cases potentially progressing to cancer [ 48 ]. CCIS is mainly associated with human papillomavirus (HPV) infection [ 49 ]. Our research findings indicated that CCIS might increase the risk of developing AD. A preliminary connection between the two diseases was identified through a detailed comparison of their pathology, etiology, and biological mechanisms. HPV may play a latent role in AD development, especially concerning inflammation and oxidative stress. Research using a systems biology approach has discovered that HPV interacts with several crucial genes linked to AD, like EGFR, APOE, APP, and CASP8 [ 50 ]. Research further indicated that HPV could disrupt the mucosal barrier and modify immune reactions, leading to the dissemination of invasive yeast into the brain, initiating inflammatory cytokines, and thus facilitating the generation of Aβ protein, indirectly leading to AD [ 51 ]. Additionally, machine learning studies have identified HPV-71 (OR = 3.56, P = 0.02) as a potential risk factor for AD [ 52 ]. This indicates the requirement for more comprehensive research to investigate the association between CCIS and AD and explain their underlying biological mechanisms.
Furthermore, this study found that OC may increase the risk of AD, a link that could be associated with the multifunctional protein BAG3. BAG3 is involved in the regulation of various cellular processes, such as apoptosis, development, and selective autophagy [ 53 ]. It has a significant impact on the development of both OC and AD. In OC, BAG3 enhances the invasive capabilities of tumor cells by interacting with matrix metalloproteinase-2, a calcium-dependent peptidase involved in extracellular matrix remodeling [ 54 ]. It also promotes cancer cell proliferation by interacting with the 3’-untranslated region of Skp2 mRNA, countering the suppressive effects of miR-21-5p on Skp2 expression, thereby bolstering the survival capacity of tumor cells [ 55 ]. Simultaneously, although miR-340 inhibits the survival and promotes the apoptosis of OC cells by downregulating BAG3, the overexpression of BAG3 effectively counteracts these effects and further accelerates tumor development by activating the PI3K/AKT signaling pathway [ 56 ]. However, in patients with AD, BAG3 plays a neuroprotective role. Research demonstrated that specifically removing BMAL1, a protein involved in circadian rhythms, from a mouse model activated astrocytes and stimulated BAG3 expression. The increased expression of BAG3 allowed astrocytes to more efficiently consume αSyn and tau, diminishing their activity in AD models, thereby assisting in managing the balance of neurotoxic proteins during AD progression [ 57 ]. Furthermore, research revealed that increasing BAG3 expression, under proteasome inhibition, promoted the degradation of tau in neurons and decreased phosphorylated tau levels [ 58 ]. In clinical research, a large cross-sectional study found that patients diagnosed with OC had a lower risk of developing AD upon discharge (multivariate OR [95% CI] = 0.35 [0.30–0.41]) [ 59 ], indicating a strong negative correlation. OC treatment often involves oophorectomy, and a Danish prospective study found that dementia incidence increased by 18% following bilateral oophorectomy, while it decreased by 13% after unilateral oophorectomy [ 60 ]. This could be related to the everyday use of hormone replacement therapy (HRT) post-oophorectomy, and epidemiological studies have found that estrogen has a protective effect against AD [ 61 , 62 , 63 ]. This may be a reason why OC survivors are less likely to develop AD.
Our reverse MR study results revealed that AD may heighten the risk for CC and VCIS while possibly lowering the risk for OEC. CC, as one of the common malignancies leading to female mortality [ 64 ], is generally preventable through early screening and treatment. However, the probability of dementia patients undergoing the Papanicolaou smear test (PST) for CC prevention is lower than that of the general population [ 65 ]. Simultaneously, epidemiological studies reveal a higher incidence of VCIS and OEC in middle-aged and elderly individuals, with a predominance of middle-aged women [ 66 , 67 ]. Considering that a majority of older women may exhibit increased tolerance to diseases due to factors such as age, lifestyle convenience, and cognitive decline, there is a reduction in regular health check-ups and cancer screenings. Timely CC screenings can effectively prevent such diseases. The incidence of CC was highly associated with high-risk HPV infections, which also caused abnormal proliferation of vulvar cells, increasing the risk of carcinogenesis [ 68 ]. Vaccination against HPV effectively reduced the risk of VICS and CC [ 69 ]. Following a diagnosis of AD, the risk of misdiagnosis or delayed diagnosis when encountering other conditions such as CC, VCIS, and OEC is heightened due to the decline in cognitive function and expressive abilities.
In recent years, increasing research has identified AD as primarily an autoimmune disease occurring within the brain [ 70 ]. The immune system in AD patients has undergone various changes; a study based on the Healthy Aging in Neighborhoods of Diversity across the Life Span study found a significant correlation between the rate of decline in the immune system and the rate of cognitive decline, with poorer immune function associated with worse cognitive abilities [ 71 ]. The immune system is closely linked to the incidence of CC and VCIS, and immune suppression has been established as a risk factor for CC [ 72 ]. HPV infection is one of the primary causes of many gynecological cancers; it may evade host immune surveillance, leading to CC and VCIS [ 73 , 74 ]. Consequently, the compromised immune system function facilitates persistent HPV infections, likely a critical factor in AD patients’ higher risk of developing CC and VCIS. Female AD patients often experience reduced estrogen levels [ 75 , 76 ], and excessive estrogen secretion is a common cause of OEC [ 77 ]; therefore, AD patients may reduce their risk of OEC through the estrogen pathway. Mutations in the PTEN gene are involved in the development of both AD and OEC [ 78 ]; PTEN, a tumor suppressor gene, regulates the proliferation and differentiation of neural stem cells in the nervous system, affecting neural regeneration [ 79 ]. In AD patients, PTEN often shows a decrease and distribution change [ 80 ]. PTEN deletion is also a common driving factor for OEC [ 81 , 82 ], with patients carrying a PTEN expression deficit experiencing worse outcomes [ 83 ]. Therefore, further research is needed to verify whether AD provides a protective effect against OEC. Our findings suggest that future considerations could include enhanced screening and prevention of CC and VCIS among AD patients.
Our bidirectional MR study results have circumvented the issues of reverse causality and confounding biases encountered in traditional observational studies [ 84 ]. Simultaneously, it has also overcome the inconvenience associated with clinical observations of cancer and cognitive impairment patients. This study marks the first attempt to explore the causal relationship between AD patients and gynecological cancer, providing a novel research perspective on the prevention of both diseases. CC and CCIS represent two types of cancer occurring in the cervix at different stages, OC is a general term for malignant ovarian tumors, and OEC represents a subtype of these tumors. Due to the heterogeneity of cancer, which leads to diversity and variability at different stages and types, our study aimed to include as many current classifications of gynecological cancers as possible. Our results also showed that the causal relationship between AD and tumors of different natures and degrees, even those occurring in the same location, varies. However, our study does have certain limitations. In the reverse MR analysis, our conclusions indicate that AD patients are at risk of developing CC and VCIS, while there is a negative correlation between AD and OEC incidence. Nevertheless, there is a lack of direct clinical observation studies to confirm this standpoint. Additionally, the IVs for gynecological cancer were uniformly sourced from the Finnish database to ensure data consistency. Although the results from all methodologies exhibit a degree of robustness, the limitation lies in the relatively small sample size and the limited number of available IVs in our study. A replication MR analysis using an additional set of AD’s GWAS data further validated the reliability of our study findings. Furthermore, our analysis primarily focuses on the European population, necessitating caution when generalizing the research findings to other populations.
In conclusion, our study findings support the hypothesis of a causal relationship between AD and certain gynecological cancers. However, to validate this study’s results, we recommend including a more extensive dataset from gynecological cancer GWAS and incorporating additional genetic IVs. We encourage more researchers to investigate the relationship between female AD patients and the incidence of gynecological cancer and to continue in-depth research in this field.
Data availability
Data is provided within the manuscript or supplementary information files. The data for 12 gynecological cancers is sourced from the FinnGen database, and the dataset link is https://figshare.com/articles/dataset/Gynecological_cancer_application_data/24980757 . The GWAS data for Alzheimer’s disease is sourced from IEU open GWAS and GWAS catalog, and the relevant dataset can be obtained from the following link: https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90027158/ , https://ftp.ebi.ac.uk/pub/databases/gwas/summary_statistics/GCST007001-GCST008000/GCST007320/ .
Abbreviations
- Alzheimer’s disease
- Mendelian randomization
Breast cancer
Cervical adenocarcinoma
Cervical squamous cell carcinoma
Cervical cancer
Endometrial cancer
Ovarian endometrioid carcinoma
Ovarian cancer
Ovarian serous carcinoma
Breast carcinoma in situ
Cervical carcinoma in situ
Endometrial carcinoma in situ
Vulvar carcinoma in situ
Inverse-variance weighted
Single nucleotide polymorphism
Instrumental variables
- Genome-wide association study
European Alzheimer’s Disease Biobank
Linkage disequilibrium
Weighted median
Confidence interval
Estrogen receptors
G-protein-coupled receptors
Human papillomavirus
Hormone replacement therapy
Papanicolaou smear test
Ledford LRC, Lockwood S. Scope and epidemiology of gynecologic cancers: an overview. Semin Oncol Nurs. 2019;35(2):147–50.
Article PubMed Google Scholar
Kim M, Suh DH, Lee KH, Eom KY, Toftdahl NG, Mirza MR, et al. Major clinical research advances in gynecologic cancer in 2018. J Gynecol Oncol. 2019;30(2):e18.
Article PubMed PubMed Central Google Scholar
Kim M, Suh DH, Lee KH, Eom KY, Lee JY, Lee YY, et al. Major clinical research advances in gynecologic cancer in 2019. J Gynecol Oncol. 2020;31(3):e48.
Article CAS PubMed PubMed Central Google Scholar
Waldmann A, Eisemann N, Katalinic A. Epidemiology of malignant cervical, Corpus Uteri and Ovarian tumours - Current Data and Epidemiological trends. Geburtshilfe Frauenheilkd. 2013;73(2):123–9.
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast Edinb Scotl. 2022;66:15–23.
Article Google Scholar
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Stewart SL, Lakhani N, Brown PM, Larkin OA, Moore AR, Hayes NS. Gynecologic cancer prevention and control in the National Comprehensive Cancer Control Program: progress, current activities, and future directions. J Womens Health 2002. 2013;22(8):651–7.
Keyvani V, Riahi E, Yousefi M, Esmaeili SA, Shafabakhsh R, Moradi Hasan-Abad A, et al. Gynecologic cancer, cancer stem cells, and possible targeted therapies. Front Pharmacol. 2022;13:823572.
Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol Ther. 2022;11(2):553–69.
Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet Lond Engl. 2013;381(9882):2016–23.
Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurol Barc Spain. 2017;32(8):523–32.
CAS Google Scholar
Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement J Alzheimers Assoc. 2018;14(9):1171–83.
Sherzai AZ, Parasram M, Haider JM, Sherzai D. Alzheimer disease and cancer: a national inpatient sample analysis. Alzheimer Dis Assoc Disord. 2020;34(2):122.
Article CAS PubMed Google Scholar
Ospina-Romero M, Glymour MM, Hayes-Larson E, Mayeda ER, Graff RE, Brenowitz WD, et al. Association between alzheimer disease and cancer with evaluation of study biases. JAMA Netw Open. 2020;3(11):e2025515.
Dong Z, Xu M, Sun X, Wang X. Mendelian randomization and transcriptomic analysis reveal an inverse causal relationship between Alzheimer’s disease and cancer. J Transl Med. 2023;21(1):527.
Du XL, Song L, Schulz PE, Xu H, Chan W. Risk of developing alzheimer’s disease and related dementias in association with cardiovascular disease, stroke, hypertension, and diabetes in a large cohort of women with breast cancer and with up to 26 years of follow-up. J Alzheimers Dis. 2022;87(1):415–32.
Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC, et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol. 2018;36(32):3211–22.
Article CAS PubMed Central Google Scholar
Lehrer S, Rheinstein PH. Breast Cancer, Alzheimer’s Disease, and APOE4 allele in the UK Biobank Cohort. J Alzheimers Dis Rep. 2021;5(1):49–53.
Xu WY, Raver E, Jung J, Li Y, Thai G, Lee S. Rural-urban disparities in preventive breast and cervical cancer screening among women with early-onset dementia. BMC Womens Health. 2023;23(1):255.
Gorin SS, Heck JE, Albert S, Hershman D. Treatment for breast cancer in patients with Alzheimer’s disease. J Am Geriatr Soc. 2005;53(11):1897–904.
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Burgess S, Thompson SG. Bias in causal estimates from mendelian randomization studies with weak instruments. Stat Med. 2011;30(11):1312–23.
Ziegler A, Mwambi H, König IR. Mendelian randomization versus path models: making Causal inferences in genetic epidemiology. Hum Hered. 2015;79(3–4):194–204.
Emdin CA, Khera AV, Kathiresan S, Mendelian Randomization. JAMA. 2017;318(19):1925–6.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18.
Bellenguez C, Küçükali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54(4):412–36.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52.
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223–42.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, EPIC- InterAct Consortium. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiol Camb Mass. 2017;28(1):30–42.
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing alzheimer’s disease risk. Nat Genet. 2019;51(3):404–13.
Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–20.
Santiago JA, Quinn JP, Potashkin JA. Sex-specific transcriptional rewiring in the brain of alzheimer’s disease patients. Front Aging Neurosci. 2022;14:1009368.
Lanni C, Masi M, Racchi M, Govoni S. Cancer and Alzheimer’s disease inverse relationship: an age-associated diverging derailment of shared pathways. Mol Psychiatry. 2021;26(1):280–95.
de Mezer M, Rogaliński J, Przewoźny S, Chojnicki M, Niepolski L, Sobieska M, et al. SERPINA3: stimulator or inhibitor of pathological changes. Biomedicines. 2023;11(1):156.
Kamboh MI, Minster RL, Kenney M, Ozturk A, Desai PP, Kammerer CM, et al. Alpha-1-antichymotrypsin (ACT or SERPINA3) polymorphism may affect age-at-onset and disease duration of Alzheimer’s disease. Neurobiol Aging. 2006;27(10):1435–9.
Han Y, Jia J, Jia XF, Qin W, Wang S. Combination of plasma biomarkers and clinical data for the detection of sporadic Alzheimer’s disease. Neurosci Lett. 2012;516(2):232–6.
Wang Y, Sun Y, Wang Y, Jia S, Qiao Y, Zhou Z, et al. Identification of novel diagnostic panel for mild cognitive impairment and Alzheimer’s disease: findings based on urine proteomics and machine learning. Alzheimers Res Ther. 2023;15(1):191.
Rosner M, Fuchs C, Siegel N, Valli A, Hengstschläger M. New insights into the role of the tuberous sclerosis genes in leukemia. Leuk Res. 2009;33(7):883–5.
Abou-Elhamd AS, Kalamegam G, Ahmed F, Assidi M, Alrefaei AF, Pushparaj PN, et al. Unraveling the catha edulis extract effects on the cellular and molecular signaling in SKOV3 cells. Front Pharmacol. 2021;12:666885.
Pollio G, Hoozemans JJM, Andersen CA, Roncarati R, Rosi MC, van Haastert ES, et al. Increased expression of the oligopeptidase THOP1 is a neuroprotective response to abeta toxicity. Neurobiol Dis. 2008;31(1):145–58.
Shi Y, Liu H, Yang C, Xu K, Cai Y, Wang Z, et al. Transcriptomic analyses for identification and prioritization of genes associated with alzheimer’s disease in humans. Front Bioeng Biotechnol. 2020;8:31.
Cho-Clark MJ, Sukumar G, Vidal NM, Raiciulescu S, Oyola MG, Olsen C, et al. Comparative transcriptome analysis between patient and endometrial cancer cell lines to determine common signaling pathways and markers linked to cancer progression. Oncotarget. 2021;12(26):2500–13.
Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet Lond Engl. 2021;398(10316):2084–92.
McCredie MRE, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–34.
Nishio M, To Y, Maehama T, Aono Y, Otani J, Hikasa H, et al. Endogenous YAP1 activation drives immediate onset of cervical carcinoma in situ in mice. Cancer Sci. 2020;111(10):3576–87.
Talwar P, Gupta R, Kushwaha S, Agarwal R, Saso L, Kukreti S, et al. Viral induced oxidative and inflammatory response in alzheimer’s disease pathogenesis with identification of potential drug candidates: a systematic review using systems biology approach. Curr Neuropharmacol. 2019;17(4):352–65.
Block J. Alzheimer’s disease might depend on enabling pathogens which do not necessarily cross the blood-brain barrier. Med Hypotheses. 2019;125:129–36.
Tejeda M, Farrell J, Zhu C, Wetzler L, Lunetta KL, Bush WS, et al. DNA from multiple viral species is associated with alzheimer’s disease risk. Alzheimers Dement. 2023;20(1):253–65.
Stürner E, Behl C. The role of the multifunctional BAG3 protein in cellular protein quality control and in disease. Front Mol Neurosci. 2017;10:177.
Suzuki M, Iwasaki M, Sugio A, Hishiya A, Tanaka R, Endo T, et al. BAG3 (BCL2-associated athanogene 3) interacts with MMP-2 to positively regulate invasion by ovarian carcinoma cells. Cancer Lett. 2011;303(1):65–71.
Yan J, Liu C, Jiang JY, Liu H, Li C, Li XY, et al. BAG3 promotes proliferation of ovarian cancer cells via post-transcriptional regulation of Skp2 expression. Biochim Biophys Acta Mol Cell Res. 2017;1864(10):1668–78.
Qu F, Wang X. microRNA-340 induces apoptosis by downregulation of BAG3 in ovarian cancer SKOV3 cells. Pharm. 2017;72(8):482–6.
Sheehan PW, Nadarajah CJ, Kanan MF, Patterson JN, Novotny B, Lawrence JH, et al. An astrocyte BMAL1-BAG3 axis protects against alpha-synuclein and tau pathology. Neuron. 2023;111(15):2383–e23987.
Lei Z, Brizzee C, Johnson GVW. BAG3 facilitates the clearance of endogenous tau in primary neurons. Neurobiol Aging. 2015;36(1):241–8.
Sherzai AZ, Parasram M, Haider JM, Sherzai D. Alzheimer Disease and Cancer: A National Inpatient Sample Analysis. Alzheimer Dis Assoc Disord. 2020;34(2):122–7.
Uldbjerg CS, Wilson LF, Koch T, Christensen J, Dehlendorff C, Priskorn L, et al. Oophorectomy and rate of dementia: a prospective cohort study. Menopause N Y N. 2022;29(5):514–22.
Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet Lond Engl. 1996;348(9025):429–32.
Article CAS Google Scholar
Pike CJ. Sex and the development of Alzheimer’s disease. J Neurosci Res. 2017;95(1–2):671–80.
Honjo H, Kikuchi N, Hosoda T, Kariya K, Kinoshita Y, Iwasa K, et al. Alzheimer’s disease and estrogen. J Steroid Biochem Mol Biol. 2001;76(1–5):227–30.
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer incidence, mortality, years of Life Lost, Years lived with disability, and disability-adjusted life-years for 32 Cancer groups, 1990 to 2015: a systematic analysis for the global burden of Disease Study. JAMA Oncol. 2017;3(4):524–48.
Article PubMed Central Google Scholar
Chen CY, Kung PT, Chiu LT, Tsai WC. Comparison of Cervical Cancer Screening used between individuals with disabilities and individuals without disabilities. Healthc Basel Switz. 2023;11(10):1363.
Google Scholar
Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018–22.
Moro F, Magoga G, Pasciuto T, Mascilini F, Moruzzi MC, Fischerova D, et al. Imaging in gynecological disease (13): clinical and ultrasound characteristics of endometrioid ovarian cancer. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2018;52(4):535–43.
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27.
Berenson AB, Chang M, Hawk ET, Ramondetta LM, Hoang T. Vulvar cancer incidence in the United States and its relationship to human papillomavirus vaccinations, 2001–2018. Cancer Prev Res Phila Pa. 2022;15(11):777–84.
Jevtic S, Sengar AS, Salter MW, McLaurin J. The role of the immune system in alzheimer disease: etiology and treatment. Ageing Res Rev. 2017;40:84–94.
Beydoun MA, Shaked D, Tajuddin SM, Weiss J, Evans MK, Zonderman AB. Accelerated epigenetic age and cognitive decline among urban-dwelling adults. Neurology. 2020;94(6):e613–25.
Dugué PA, Rebolj M, Garred P, Lynge E. Immunosuppression and risk of cervical cancer. Expert Rev Anticancer Ther. 2013;13(1):29–42.
Hardikar S, Johnson LG, Malkki M, Petersdorf EW, Galloway DA, Schwartz SM, et al. A population-based case-control study of genetic variation in cytokine genes associated with risk of cervical and vulvar cancers. Gynecol Oncol. 2015;139(1):90–6.
Vanajothi R, Srikanth N, Vijayakumar R, Palanisamy M, Bhavaniramya S, Premkumar K. HPV-mediated cervical cancer: a systematic review on immunological basis, molecular biology, and immune evasion mechanisms. Curr Drug Targets. 2022;23(8):782–801.
Yang H, Oh CK, Amal H, Wishnok JS, Lewis S, Schahrer E, et al. Mechanistic insight into female predominance in alzheimer’s disease based on aberrant protein S-nitrosylation of C3. Sci Adv. 2022;8(50):eade0764.
Scheyer O, Rahman A, Hristov H, Berkowitz C, Isaacson RS, Brinton RD, et al. Female sex and alzheimer’s risk: the menopause connection. J Prev Alzheimers Dis. 2018;5(4):225–30.
CAS PubMed PubMed Central Google Scholar
Ness RB. Endometriosis and ovarian cancer: thoughts on shared pathophysiology. Am J Obstet Gynecol. 2003;189(1):280–94.
Chen S, Li Y, Qian L, Deng S, Liu L, Xiao W, et al. A review of the clinical characteristics and novel molecular subtypes of endometrioid ovarian cancer. Front Oncol. 2021;11:668151.
Li Y, Ma R, Hao X. Therapeutic role of PTEN in tissue regeneration for management of neurological disorders: stem cell behaviors to an in-depth review. Cell Death Dis. 2024;15(4):268.
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of akt/PKB, increased phosphorylation of akt substrates and loss and altered distribution of akt and PTEN are features of alzheimer’s disease pathology. J Neurochem. 2005;93(1):105–17.
Martins FC, Couturier DL, Paterson A, Karnezis AN, Chow C, Nazeran TM, et al. Clinical and pathological associations of PTEN expression in ovarian cancer: a multicentre study from the ovarian tumour tissue analysis consortium. Br J Cancer. 2020;123(5):793–802.
Pejovic T, Cathcart AM, Alwaqfi R, Brooks MN, Kelsall R, Nezhat FR. Genetic links between endometriosis and endometriosis-associated ovarian cancer-a narrative review (endometriosis-associated cancer). Life Basel Switz. 2024;14(6):704.
de Nonneville A, Kalbacher E, Cannone F, Guille A, Adelaïde J, Finetti P et al. Endometrioid ovarian carcinoma landscape: pathological and molecular characterization. Mol Oncol. 2024.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98.
Download references
Acknowledgements
We are grateful for the public data on Alzheimer’s disease and gynecological cancers provided by the Finnish Biobank and the European Alzheimer’s Disease Biobank.
The study was funded by the Qihuang scholar in the National Support Program for Leading Talents of Traditional Chinese Medicine ([2018] No. 12), the Key Research and Development Project of the Jilin Provincial Department of Science and Technology (Grant No. 20220203153SF) and the Standardization Project of Traditional Chinese Medicine Management in Jilin Province (Grant zybz-2023-027).
Author information
Di Cao and Shaobo Zhang contributed equally to this work and should be considered co-first authors.
Authors and Affiliations
Hubei University of Chinese Medicine, Wuhan, Hubei, 430065, China
Di Cao, Yini Zhang & Ping Wang
Engineering Research Center of TCM Protection Technology and New Product Development for the Elderly Brain Health, Ministry of Education, Wuhan, Hubei, 430065, China
Changchun University of Chinese Medicine, Changchun, Jilin, 130000, China
Shaobo Zhang
Department of Environmental Genomics, Jiangsu Key Laboratory of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, 210000, China
Department of Genetic Toxicology, The Key Laboratory of Modern Toxicology of Ministry of Education, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, 210000, China
The Second Affiliated Hospital of Changchun University of Chinese Medicine, Changchun Hospital of Chinese Medicine, Changchun, Jilin, 130000, China
Qiguang Yang
You can also search for this author in PubMed Google Scholar
Contributions
DC and SZ conceived and designed the study. DC, YZ and MS performed the MR analyses. SZ and PW aided in data analyses. QY and PW assisted in interpreting results and writing the manuscript. All authors revised and approved the final manuscript.
Corresponding author
Correspondence to Ping Wang .
Ethics declarations
Ethical approval.
The analyses were based on publicly available data approved by relevant review boards. All studies contributing data to these analyses had the appropriate institutional review board approval from each country under the Declaration of Helsinki, and all participants provided informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cao, D., Zhang, S., Zhang, Y. et al. Association between gynecologic cancer and Alzheimer’s disease: a bidirectional mendelian randomization study. BMC Cancer 24 , 1032 (2024). https://doi.org/10.1186/s12885-024-12787-5
Download citation
Received : 02 January 2024
Accepted : 08 August 2024
Published : 21 August 2024
DOI : https://doi.org/10.1186/s12885-024-12787-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gynecological cancer
- Causal relationship
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
COMMENTS
Cancer Prevention Research publishes articles on cancer prevention, preclinical, clinical, and translational research, with special attention to molecular discoveries. The journal covers topics such as chemoprevention, biomarkers, artificial intelligence, and south-south collaboration.
Learn about the latest findings and trends in cancer prevention research from the National Cancer Institute (NCI). Explore topics such as multivitamins, alcohol, HPV, tobacco, climate change, and more.
Learn how NCI supports research to prevent cancer by reducing exposure to carcinogens, promoting healthy behaviors, and developing vaccines and drugs. Find out the latest research findings and the importance of cancer prevention for reducing the burden of cancer.
Cancer prevention encompasses a broad spectrum of strategies designed to lower the chance of developing cancer and reduce the morbidity of established cancer. There are three levels of cancer prevention. ... Immunoprevention has risen to prominence in cancer research because the immune system is a living, adaptable entity that can be enhanced ...
Microbial Host Defense and Effect on Cancer Initiation and Progression. Date (s): Wed, September 11, 2024: 1:00p.m. ET - 2:00p.m. ET. Location: Virtual via ZoomGov. The Division of Cancer Prevention (DCP) conducts and supports research to determine a person's risk of cancer and to find ways to reduce the risk. This.
Cancer Prevention Research publishes original studies, reviews, and commentaries in the fields of cancer prevention and interception. Learn about the journal's scope, editorial board, publication fees, and submission guidelines.
Short answer - yes! For over a decade, researchers have investigated the effect of AICR's Cancer Prevention Recommendations on cancer risk and other health outcomes. Studies have shown that following AICR's diet and lifestyle recommendations lower cancer risk, risk of other chronic diseases and help people live a longer and healthier life.
The Connect for Cancer Prevention Study is a research study from the National Cancer Institute, part of the National Institutes of Health. Connect is a study to better understand the causes of cancer and how to prevent it.
Cancer prevention includes treatments, procedures and lifestyle changes that aim to reduce the risk that cancer will develop in an individual. Some cancer prevention strategies are well known and ...
Division of Cancer Prevention, National Cancer Institute, Chemopreventive Agent Development Research Group, National Cancer Institute, Executive Plaza North 2056, 6130 Executive Boulevard, MSC ...
Your donation to the American Association for Cancer Research helps our more than 58,000 members worldwide drive progress against cancer. The AACR is the first and largest cancer research organization. Our mission is to prevent and cure cancer through research, education, communication, collaboration, funding, and advocacy.
The AACR is committed to advancing the science of cancer prevention. We work with a wide range of partners in biomedical research to develop strategies and promising therapeutics to stop cancer before it starts. Our ten peer-reviewed medical journals including Cancer Epidemiology, Biomarkers & Prevention and Cancer Prevention Research feature ...
The NCI Cancer Prevention Clinic (CPC) conducts research in cancer prevention, early detection and screening for groups at high risk of cancer. Housed in the NCI Center for Cancer Research, the clinic combines expertise from the Division of Cancer Prevention (DCP) with that of intramural investigators from the Center for Cancer Research (CCR) and the Division of Cancer Epidemiology and ...
The Cancer Prevention and Early Detection Facts & Figures, 2023-2024 is an educational companion for "Updated Review of Major Cancer Risk Factors and Screening Test Use in the United States, with a Focus on Changes During the COVID-19 Pandemic," a scientific paper published in Cancer Epidemiology, Biomarkers, and Prevention .
BOSTON, June 25, 2024 — The American Cancer Society, the National Cancer Institute, and Cancer Research UK are holding its first annual conference on cancer prevention research, June 25-27, in Boston. The event brings together scientists from discovery biology through translational and behavioral science to population and implementation research to create a thriving multidisciplinary cancer ...
AICR's resources can help you navigate questions about nutrition and lifestyle, and empower you to advocate for your health. AICR is committed to putting what we know about cancer prevention into action. To help you live healthier, we've taken the latest research and made. to support cancer research in honor or in memory of a loved one.
Cancer prevention research has seemingly never been a major priority of health research until recently. And in spite of the renewed interest, ...
Building on MD Anderson's decades at the forefront of cancer prevention research, Be Well Communities uses best practices to deploy science-based cancer prevention strategies to communities with the greatest need. It brings individuals and organizations together to promote wellness and to address modifiable risk factors for cancer.
CPS-3: Past, Present, and Future. In December 2013, the American Cancer Society (ACS) launched Cancer Prevention Study-3 (CPS-3) when ACS completed its initial recruitment. Who we invited: Our Cancer Prevention and Survivorship Team invited men and women between the ages of 30 and 65 who had no personal history of cancer to participate in the ...
How the Cancer Prevention Studies Save Lives. The American Cancer Society (ACS) Cancer Prevention Studies (CPS) help researchers identify cancer risk factors by allowing them to study large groups of people over long periods of time. ACS first began conducting long-term prospective studies (also called follow-up studies) in the 1950s.
Check In With Your Health. The choices we make each day can help reduce our risk of cancer. AICR's new Cancer Health Check will help you learn more about your. choices and how you can stack the odds in your favor. Start. AICR has led the charge in cancer prevention and survivorship for over 30 years. Stay in the loop on the latest AICR cancer ...
The state of cancer prevention today. Many approaches to cancer prevention involve taking steps to reduce one's risk of developing cancer, such as quitting smoking, wearing sunscreen, and keeping up to date with cancer screenings. ... (USDA), aims to accelerate research on diet-related chronic diseases, including cancer, and to translate ...
Abstract. Immunoprevention is an emerging consideration for solid tumors, including pancreatic ductal adenocarcinoma (PDAC). We and others have shown that Kras mutations in genetic models of spontaneous pancreatic intraepithelial neoplasia (PanIN), which is a precursor to PDAC, results in CD73 expression in the neoplastic epithelium and some populations of infiltrating immune cells, including ...
Learn about the factors that influence the risk of cancer and how to lower it. Find research articles, summaries, and resources on cancer prevention and genetics.
A research team has found that compounds in the nucleic acids derived from food can inhibit the growth of certain cancer cells. When people eat, they ingest the nucleic acids that reside in all ...
Using a combination of two protein-mapping methods, Scripps Research scientists uncover novel proteins that could be targeted by future cancer drugs. August 26, 2024. LA JOLLA, CA—Fighting cancer effectively often involves stopping cancer cells from multiplying, which requires understanding proteins that the cells rely on to survive. Protein ...
The RUNX1 Research Program and the American Cancer Society (ACS) have partnered to make a demonstrable leap forward in the pursuit of cancer interception and prevention treatments for patients with RUNX1-FPD. The grant mechanism is called Leukemia Exploration and Prevention, or LEAP, to symbolize this overarching goal.
Synthetic immunology, an innovative field of research that could lead to fundamentally new approaches and methods in the treatment of infectious diseases and cancer, is the topic of a new article ...
T.G. is funded by the Cancer Prevention and Research Institute of Texas Grant 00011633. M.J.-H. has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer ...
Gynecological cancer refers to cancers that originate in the female reproductive system [].Since the incidence of breast cancer in women is higher than in men, this article classifies breast cancer as a form of gynecological cancer, a perspective shared by some research studies [2, 3].Breast cancer, cervical cancer, ovarian cancer, and endometrial cancer, as common malignancies among women ...