An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PLoS Comput Biol
- v.9(7); 2013 Jul


Ten Simple Rules for Writing a Literature Review
Marco pautasso.
1 Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France
2 Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,

The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .
Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
Funding Statement
This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Review of guidelines and literature for handling missing data in longitudinal clinical trials with a case study
Affiliation.
- 1 Clinical Biostatistics, Merck & Co., Inc., Rahway, NJ 07065, USA. [email protected]
- PMID: 17080924
- DOI: 10.1002/pst.189
Missing data in clinical trials are inevitable. We highlight the ICH guidelines and CPMP points to consider on missing data. Specifically, we outline how we should consider missing data issues when designing, planning and conducting studies to minimize missing data impact. We also go beyond the coverage of the above two documents, provide a more detailed review of the basic concepts of missing data and frequently used terminologies, and examples of the typical missing data mechanism, and discuss technical details and literature for several frequently used statistical methods and associated software. Finally, we provide a case study where the principles outlined in this paper are applied to one clinical program at protocol design, data analysis plan and other stages of a clinical trial.
PubMed Disclaimer
Similar articles
- Handling missing data issues in clinical trials for rheumatic diseases. Wong WK, Boscardin WJ, Postlethwaite AE, Furst DE. Wong WK, et al. Contemp Clin Trials. 2011 Jan;32(1):1-9. doi: 10.1016/j.cct.2010.09.001. Epub 2010 Sep 16. Contemp Clin Trials. 2011. PMID: 20840873 Review.
- Handling missing data in vaccine clinical trials for immunogenicity and safety evaluation. Li X, Wang WW, Liu GF, Chan IS. Li X, et al. J Biopharm Stat. 2011 Mar;21(2):294-310. doi: 10.1080/10543406.2011.550111. J Biopharm Stat. 2011. PMID: 21391003
- Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models. Ratitch B, O'Kelly M, Tosiello R. Ratitch B, et al. Pharm Stat. 2013 Nov-Dec;12(6):337-47. doi: 10.1002/pst.1549. Epub 2013 Jan 4. Pharm Stat. 2013. PMID: 23292975
- Missing data analysis: making it work in the real world. Graham JW. Graham JW. Annu Rev Psychol. 2009;60:549-76. doi: 10.1146/annurev.psych.58.110405.085530. Annu Rev Psychol. 2009. PMID: 18652544 Review.
- Analyzing longitudinal data with missing values. Enders CK. Enders CK. Rehabil Psychol. 2011 Nov;56(4):267-88. doi: 10.1037/a0025579. Epub 2011 Oct 3. Rehabil Psychol. 2011. PMID: 21967118 Review.
- Effectiveness of Early Postpartum Rectus Abdominis versus Transversus Abdominis Training in Patients with Diastasis of the Rectus Abdominis Muscles: A Pilot Randomized Controlled Trial. Simpson E, Hahne A. Simpson E, et al. Physiother Can. 2023 Nov 27;75(4):368-376. doi: 10.3138/ptc-2021-0111. eCollection 2023 Nov. Physiother Can. 2023. PMID: 38037580
- Return to Play After Surgical Treatment of High-Grade Acromioclavicular Joint Injuries in the Australian Football League. Borbas P, Warby S, Yalizis M, Smith M, Hoy G. Borbas P, et al. Orthop J Sports Med. 2022 Apr 6;10(4):23259671221085602. doi: 10.1177/23259671221085602. eCollection 2022 Apr. Orthop J Sports Med. 2022. PMID: 35400140 Free PMC article.
- Crohn's disease therapeutic dietary intervention (CD-TDI): study protocol for a randomised controlled trial. Raman M, Ma C, Taylor LM, Dieleman LA, Gkoutos GV, Vallance JK, McCoy KD, Lewis I, Jijon H, McKay DM, Mutch DM, Barkema HW, Gibson D, Rauch M, Ghosh S. Raman M, et al. BMJ Open Gastroenterol. 2022 Jan;9(1):e000841. doi: 10.1136/bmjgast-2021-000841. BMJ Open Gastroenterol. 2022. PMID: 35046093 Free PMC article.
- A latent class based imputation method under Bayesian quantile regression framework using asymmetric Laplace distribution for longitudinal medication usage data with intermittent missing values. Lee M, Rahbar MH, Gensler LS, Brown M, Weisman M, Reveille JD. Lee M, et al. J Biopharm Stat. 2020;30(1):160-177. doi: 10.1080/10543406.2019.1684306. Epub 2019 Nov 15. J Biopharm Stat. 2020. PMID: 31730441 Free PMC article.
- Handling Missing Data in the Modeling of Intensive Longitudinal Data. Ji L, Chow SM, Schermerhorn AC, Jacobson NC, Cummings EM. Ji L, et al. Struct Equ Modeling. 2018;25(5):715-736. doi: 10.1080/10705511.2017.1417046. Epub 2018 Feb 8. Struct Equ Modeling. 2018. PMID: 31303745 Free PMC article.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 06 September 2024
Neurological monitoring and management for adult extracorporeal membrane oxygenation patients: Extracorporeal Life Support Organization consensus guidelines
- Sung-Min Cho 1 , 2 ,
- Jaeho Hwang 1 ,
- Giovanni Chiarini 3 , 4 ,
- Marwa Amer 5 , 6 ,
- Marta V. Antonini 7 ,
- Nicholas Barrett 8 ,
- Jan Belohlavek 9 ,
- Daniel Brodie 10 ,
- Heidi J. Dalton 11 ,
- Rodrigo Diaz 12 ,
- Alyaa Elhazmi 5 , 6 ,
- Pouya Tahsili-Fahadan 1 , 13 ,
- Jonathon Fanning 14 ,
- John Fraser 14 ,
- Aparna Hoskote 15 ,
- Jae-Seung Jung 16 ,
- Christopher Lotz 17 ,
- Graeme MacLaren 18 ,
- Giles Peek 19 ,
- Angelo Polito 20 ,
- Jan Pudil 9 ,
- Lakshmi Raman 21 ,
- Kollengode Ramanathan 18 ,
- Dinis Dos Reis Miranda 22 ,
- Daniel Rob 9 ,
- Leonardo Salazar Rojas 23 ,
- Fabio Silvio Taccone 24 ,
- Glenn Whitman 2 ,
- Akram M. Zaaqoq 25 na1 &
- Roberto Lorusso 3 na1
Critical Care volume 28 , Article number: 296 ( 2024 ) Cite this article
Metrics details
Critical care of patients on extracorporeal membrane oxygenation (ECMO) with acute brain injury (ABI) is notable for a lack of high-quality clinical evidence. Here, we offer guidelines for neurological care (neurological monitoring and management) of adults during and after ECMO support.
These guidelines are based on clinical practice consensus recommendations and scientific statements. We convened an international multidisciplinary consensus panel including 30 clinician-scientists with expertise in ECMO from all chapters of the Extracorporeal Life Support Organization (ELSO). We used a modified Delphi process with three rounds of voting and asked panelists to assess the recommendation levels.
We identified five key clinical areas needing guidance: (1) neurological monitoring, (2) post-cannulation early physiological targets and ABI, (3) neurological therapy including medical and surgical intervention, (4) neurological prognostication, and (5) neurological follow-up and outcomes. The consensus produced 30 statements and recommendations regarding key clinical areas. We identified several knowledge gaps to shape future research efforts.
Conclusions
The impact of ABI on morbidity and mortality in ECMO patients is significant. Particularly, early detection and timely intervention are crucial for improving outcomes. These consensus recommendations and scientific statements serve to guide the neurological monitoring and prevention of ABI, and management strategy of ECMO-associated ABI.
Introduction
Extracorporeal membrane oxygenation (ECMO) is increasingly utilized, yet patients receiving ECMO support commonly experience major complications, including acute brain injury (ABI). ABI increases in-hospital mortality by a factor of 2–3 [ 1 , 2 ]. ABI is more common in venoarterial (VA) ECMO than venovenous (VV) ECMO, especially for those with extracorporeal cardiopulmonary resuscitation (ECPR) with 27–32% of ABI during ECMO support (Table 1 ) despite its survival benefit [ 3 , 4 ]. Although a protocolized neurological monitoring is shown to improve the detection of ABI, this is limited to a few ECMO centers [ 5 ]. The management of ECMO patients in the intensive care unit (ICU) is not standardized, and neurological monitoring and care vary significantly across ECMO centers, thus, the ICU management of patients with ABI during ECMO lacks high-quality evidence and recommendations.
As clinical experience accumulates and ECMO becomes more widely used, clinical guidelines and focused research on neurological monitoring and management of ABI are imperative to enhance ECMO patient care and improve early as well as long-term outcomes. This heterogeneity presents an opportunity to standardize and facilitate neurological care in ECMO [ 5 ].
To establish clinical guidelines on this topic, an international multidisciplinary panel of experts specialized in neurology, critical care, surgery, and other ECMO-related fields was assembled to provide clinical practice consensus recommendations and scientific statements in neurological monitoring and management of adult ECMO patients. These recommendations and statements have been promoted and endorsed by the Extracorporeal Life Support Organization (ELSO). We identified five key clinical areas needing recommendations: (1) neurological monitoring, (2) post-cannulation early physiological targets and their associations with ABI, (3) neurological therapy including medical and surgical intervention, (4) neurological prognostication, and (5) neurological follow-up and outcomes. Here, we present consensus recommendations based on the available evidence and related knowledge gaps warranting further investigations were also identified and summarized (Table 2 ).
Consensus guideline members
ELSO, an international non-profit consortium of healthcare institutions, researchers, and industry partners, developed this consensus statement. ELSO consists of 611 ECMO centers, with chapters in Europe, Asia–Pacific, North America, Latin America, Southwest Asia, and Africa.
An international multidisciplinary consensus panel of 30 experts, including neurologists, intensivists, surgeons, perfusionists, and other professionals in intensive care medicine with expertise or involvement in ECMO, from all ELSO chapters was assembled.
Each of the five-panel subgroups addressed a pre-selected clinical practice domain relevant to patients admitted to the ICU with ABI (ischemic stroke, ICH, or hypoxic-ischemic brain injury). Invited experts contributed to the guidelines through a three-phase process: (1) a literature search/review of neurological monitoring, management, and neurological ECMO outcomes, (2) summarizing the literature search/review, and (3) developing consensus guidelines using a modified Delphi method. The literature search and review performed comprehensively in PubMed on August 29, 2023 yielded up-to-date evidence on neurological monitoring and management strategies. Five key neurological areas needing recommendations were identified (see Introduction).
Guideline development
The selected articles were distributed to each subgroup. The subgroups summarized the findings and developed guidelines and recommendations for each subsection. Each subgroup nominated two leaders for cross-subgroup coordination. The consensus guideline members met regularly throughout the year in subgroup and whole-group settings to discuss their progress and reach a consensus on the finalized document. A modified Delphi process with three rounds of voting to assess the recommendation statements was implemented. Strong recommendation, weak recommendation, or no recommendation was defined when > 85%, 75–85%, and < 75% of panelists, respectively, agreed with a recommendation statement. Three rounds of voting and the authors’ comments about the expert consensus guideline appear in Supplemental Tables 1 – 3 . The guidelines and recommendations were summarized and presented as 5 sections: (1) neurological assessment and monitoring; (2) bedside management; (3) interventional neurology, neurosurgery, and neurocritical care; (4) neurological prognostication; and (5) long-term outcome and quality of life.
Neurological assessment and monitoring
Neurological examination.
Serial bedside examination remains the mainstay of neurological assessment in ECMO patients. However, neurological evaluation, especially early after ECMO cannulation, is frequently confounded by sedatives and paralytics, necessitating noninvasive multimodal neurological monitoring in patients with impaired consciousness. The data on ABI timing to ECMO cannulation/support are limited. Therefore, a baseline neurological assessment is recommended before and immediately after cannulation, followed by serial evaluations throughout ECMO support and after weaning. The ideal frequency of neurological examination is not yet established. Daily assessment by a neurologist/neurointensivist (if available) can improve neurological care. [ 5 , 7 ] More frequent bedside nursing assessment, every 1–4 h based on ABI risk, is reasonable. Particularly, assessing signs of life (such as gasping, pupillary light response, and increased consciousness) is integral to the clinical examination, as these signs observed before, during resuscitation, and while on ECMO support may be associated with improved neurological outcomes [ 8 ]. Historically, the absence of brainstem reflexes with fixed, dilated pupils before cannulation was equated to irreversible ABI and a contraindication to ECMO. However, during cardiopulmonary resuscitation (CPR), fixed and dilated pupils are frequently seen after epinephrine administration, and patients have achieved favorable outcomes despite these findings [ 9 ].
Serial neurological examination should include mental status assessment, brainstem reflexes (pupillary light response and oculocephalic, corneal, and cough/gag reflexes), and motor exam. Standardized scoring tools such as the Glasgow Coma Scale and the Confusion Assessment Method should be used. Assessing the motor response of extremities in neurological examinations is only helpful when analgo-sedation and paralytic is lightened or off. Therefore, neurological exam for spinal cord injury, a rare but devastating injury, is very challenging [ 10 ]. Sensory exams are mostly limited in ECMO patients.
Adequate analgo-sedation is essential to ECMO support and minimizes adverse events [ 11 ]. ECMO circuitry and common concomitant impaired liver or kidney function alter medication pharmacokinetics. Standardized sedation protocols with validated scoring systems, such as the Richmond Agitation Sedation Scale, are recommended. Overall, intermittent (as-needed) analgo-sedation is preferred over continuous infusion. Short-acting, non-benzodiazepine sedatives could be considered [ 11 ]. Daily reassessment of sedation goals, stepwise sedation weaning, and sedation interruptions can improve neurological exams and ABI diagnosis [ 11 ].
Neurological monitoring
Standardized neurological monitoring, clinical assessment, and a sedation cessation protocol may increase ABI detection and improve neurological outcomes [ 8 , 12 ]. In a single-center study (90% VA ECMO), autopsy shortly after ECMO decannulation showed that 68% of ECMO non-survivors had developed ABI [ 13 ]. In another cohort, 9 of 10 brains exhibited ABI at autopsy [ 14 ], suggesting that ABI incidence is likely higher than clinical detection. Early, accurate ABI detection with standardized neurological monitoring and early interventions is critical for mitigating ABI. Table 3 summarizes current neurological monitoring tools and their evidence (Supplemental Fig. 1 ), and Table 4 provides the consensus recommendations on neurological monitoring (Fig. 1 ). A concise review of sedation, disorders of consciousness and seizure is separately summarized in Supplemental File 1.

Recommendations for neurological monitoring and neuroimaging on ECMO. ABI: acute brain injury; EEG: electroencephalography; rSO 2 : regional oxygen saturation; SSEP: somatosensory evoked potential; VA ECMO: venoarterial extracorporeal membrane oxygenation
Bedside management
Arterial oxygen.
The brain depends on aerobic glucose metabolism for energy, with an average cerebral consumption of 3.5 mL oxygen per 100 g of brain tissue per minute. Hyperoxemia (partial pressure of oxygen (PaO 2 ) > 100 or 120 mmHg: mild; > 300 mmHg: severe) and hypoxemia (PaO 2 < 60 or 70 mmHg) are associated with increased mortality in ICU patients, including subjects on ECMO [ 41 , 42 ].
Limited data exist on early (first 24 h) oxygen targets and neurological outcomes after VV ECMO cannulation. In a single-center observational cohort study, PaO 2 < 70 mmHg (hypoxemia) was associated with ABI, especially ICH [ 43 ]. There are no data on hyperoxemia as it is not often an issue clinically in VV ECMO patients.
In VA ECMO, when the heart recovers before lung recovery, cerebral hypoxemia (especially of the right side of the brain) may occur due to the “differential oxygenation” (also called “Harlequin Syndrome” or “North–South Syndrome”), which is monitored by arterial blood gases from right radial arterial line, especially for those supported with peripheral VA ECMO. Monitoring of cerebral oxygenation using NIRS may be useful in diagnosing differential oxygenation [ 15 ].
Severe hyperoxemia (PaO 2 > 300 mmHg) within 24 h after the cannulation may be associated with ABI and poor neurologic outcomes [ 4 , 42 , 44 ]. As optimal oxygenation targets are unknown, it is reasonable to avoid early (within 24 h) severe hyperoxemia and hypoxemia by manipulating the fraction of delivered oxygen from the ECMO sweep gas (Fig. 2 ). Given the high-quality data are limited, it is crucial to prospectively study the impact of hyperoxemia on ABI and neurological outcomes in VA ECMO as a multi-institutional study with protocolized neurological monitoring and diagnostic ABI adjudication. Importantly, further research is necessary to investigate the impact of hyperoxemia on each major VA ECMO cohort: postcardiotomy shock, ECPR, and post-acute myocardial infarction (AMI) as well as non-AMI cardiogenic shock.

Recommendations for bedside management on ECMO. ABG: arterial blood gas; BP: blood pressure; ECMO: extracorporeal membrane oxygenation; MAP: mean arterial pressure; PaCO 2 : partial pressure of carbon dioxide; PaO 2 : partial pressure of oxygen; VA: venoarterial; VV: venovenous
Arterial carbon dioxide
Severe acidosis and hypercapnia are common before ECMO cannulation, and both are rapidly corrected upon ECMO initiation by adjusting sweep gas flow across the oxygenator. Carbon dioxide is a potent cerebral vasodilator that increases cerebral blood flow[ 45 ] and neuronal metabolic demand [ 46 ]. Prolonged hypercapnia, common in pre-ECMO patients, may impair cerebral autoregulation, leading to high cerebral blood flow and a narrow regulatory pressure window [ 40 , 47 ]. While high partial pressure of carbon dioxide (PaCO 2 ) should be avoided, rapid correction of sustained high PaCO 2, particularly soon after ECMO initiation, sometimes leads to rapid hypocapnia; it may cause cerebral vasoconstriction and a decrease in cerebral oxygen delivery, resulting in cerebral ischemia [ 46 ]. Routine use of full-dose anticoagulation therapy at ECMO initiation and thereafter may cause hemorrhagic conversion of an ischemic injury.
In an ELSO registry analysis, a rapid early decrease in PaCO 2 was independently associated with an increased risk of ICH in ARDS patients with VV ECMO [ 48 ]. An ELSO retrospective study of 11,972 VV ECMO patients showed that those with ΔPaCO 2 > 50% in the peri-cannulation period were more likely to experience ABI (infarct and ICH) [ 49 ].
A higher ΔPaCO 2 in VA ECMO was associated with ICH in a single-center observational study [ 50 ]. However, an ELSO retrospective study of 3125 ECPR patients showed ΔPaCO 2 higher in ABI than non-ABI, but ΔPaCO 2 was not significantly associated with ABI [ 4 ]. These findings are limited by (a) a lack of sensitive, reliable, and readily available diagnostic markers of ABI, (b) retrospective observations, and (c) inconsistent arterial blood gas sampling. Further research with standardized neurological diagnostic/monitoring tools and granular arterial blood gas data is necessary. However, avoiding a large ΔPaCO 2 > 50% in the peri-cannulation period for both VA and VV ECMO is reasonable.
Temperature
Inducing hypothermia during ischemia prolongs the tolerance of organs to ischemia, improving neurological outcomes [ 51 ]. Thus, it could be reasonable to use hypothermia in VA ECMO patients where cerebral ischemic and hypoperfusion time is prolonged. This rationale is even more important in patients who have already suffered severe hypoxic-ischemic brain injury, as in ECPR. However, as demonstrated by a meta-analysis of 2643 ECPR patients (35 studies), data on this topic are severely heterogeneous and limited to low-quality evidence [ 52 ]. One randomized controlled trial on cardiogenic shock patients requiring VA ECMO compared moderate hypothermia (33–34 °C) versus normothermia (36–37 °C), showing no mortality difference at 30 days [ 53 ]. This study was limited by (1) insufficient sample size due to inaccurate effect size estimation based on non-ECMO studies, (2) lack of formal neurological assessment, and (3) primary outcome being mortality outcome at 30 days instead of neurological outcomes at 90 or 180 days. The basic and preclinical science on hypothermia in ischemia is strong, and VA ECMO patients have a high incidence of ABI and prolonged absent/low cerebral perfusion. Also, bleeding complications and coagulopathy were similar between those with hypothermia vs. without in a meta-analysis of ECPR patients [ 52 ]. A robust multicenter prospective observation cohort study is needed to test the effect of hypothermia strategically in each major VA ECMO cohort. There is no data on hypothermia in VV ECMO patients.
Blood pressure
No data exists on early and optimal blood pressure (BP) goals and ABI prevention, especially for stroke or hypoxic-ischemic brain injury, as the timing of ABI is not well-defined during the peri-cannulation period. After acute ischemic stroke, permissive hypertension (BP ≤ 220/120 mmHg) is recommended by the AHA[ 54 ]; it is reasonable to target mean arterial blood pressure (MAP) that can provide adequate cerebral perfusion in the setting of acute ischemic stroke.
Higher BPs lead to increased afterload, which may hinder myocardial recovery (VA ECMO only), particularly when the left ventricle is not vented. In the absence of high-quality data, allowing patients with acute ischemic stroke to autoregulate is reasonable if the heart can tolerate it. After ICH, lower BP (systolic BP < 140 mmHg and MAP < 90 mmHg) is preferred due to anticoagulation-associated ICH [ 55 ]. Cerebral autoregulation function in the setting of non-pulsatile blood flow and ABI is an active research area, and autoregulatory dysfunction may contribute to ABI in ECMO (Supplemental File 2) [ 56 ].
Low pulse pressure (< 20 mmHg) in the first 24 h of VA ECMO was associated with ABI [ 57 ]. However, data are weak regarding improving pulse pressure with inotropes, or left ventricle venting in ECMO [ 58 ]. Evidence on BP goals for optimal cerebral perfusion in ECMO patients is sparse. Yet, individualized BP management tailored to dynamic cerebral autoregulation function is likely needed in this complex population. However, evidence as well as related therapeutic actions in this regard are still limited and represent mandatory objectives for future research to enhance ECMO patient management and most likely ABI complications prevention and/or reduction. A summary of consensus recommendations and evidence appears in Table 5 and Supplemental Table 4 .
Interventional neurology, neurosurgery, and neurocritical care
ABI diagnosis in ECMO patients is based on comprehensive neurological assessment and brain imaging. Neurological assessment for acute stroke should include the Glasgow Coma Scale and the National Institutes of Health Stroke Scale. Non-contrast head CT is imperative to rule out ICH with acute neurological exam change. CT angiogram is needed to assess for large vessel occlusion.
Brain perfusion optimization
Managing intracranial pressure (ICP) and BP contributes to adequate brain perfusion in ABI patients. Elevating the head of the bed by 30 degrees might benefit patients with ABI and elevated ICP [ 61 ]. However, brain oxygenation and circulation improve in the supine position, benefiting perfusion-dependent patients with acute ischemic strokes. The head of the bed could be guided by monitoring surrogate markers of cerebral hemodynamics (i.e., transcranial Doppler ultrasound: cerebral blood flow velocity) and oxygenation (i.e., NIRS: regional saturation) [ 62 , 63 ]. If the heart can tolerate a higher BP, it’s reasonable to target a higher BP target (although individualized BP goal is recommended) to achieve adequate cerebral perfusion pressure, such as permissive hypertension for ischemic stroke. However, increased BP is associated with hematoma extension in ICH, so reducing BP (systolic BP < 140 mmHg) is reasonable, as ECMO patients are usually on full anticoagulation at the time of ICH.
Managing ischemic stroke
Tissue plasminogen activator (tpa).
Non-contrast head CT is imperative to rule out bleeding in acute neurological change, particularly during ECMO. tPA is a time-dependent intervention in acute ischemic stroke. Intravenous tPA in the setting of ECMO carries a high risk of bleeding, especially with systemic anticoagulation and platelet dysfunction. Given these risks, the use of tPA is generally not indicated in ECMO patients. Although there is limited literature specifically addressing this issue, the consensus among experts is to avoid tPA (Fig. 3 ).

Recommendations for interventional neurology, neurosurgery & neurocritical care on ECMO. CT: computed tomography; ECMO: extracorporeal membrane oxygenation; ICH: intracranial hemorrhage; ICP: intracranial pressure; PbtO 2 : brain tissue oxygenation; tPA: tissue plasminogen activator; VV: venovenous; VA: venoarterial
Mechanical thrombectomy
CT angiogram is needed to rule out large vessel occlusion, typically accompanied by a CT perfusion scan to assess salvageable penumbra. Mechanical thrombectomy should be pursued for patients with large vessel occlusion detected by CT angiogram (accompanied by a CT perfusion scan to assess salvageable penumbra), by consulting stroke specialists, as tPA is generally not recommended in ECMO [ 64 ].
Decompressive craniectomy
Decompressive craniectomy may be indicated in patients with space-occupying lesions with acute intracranial hypertension, such as hemispheric infarction with malignant edema. Hyperosmolar therapy is indicated for cerebral edema [ 1 ]. Systemic anticoagulation monitoring and resumption are necessary post-operatively. Successful craniectomy has been reported for patients on ECMO [ 65 ]. As evidence is limited, the risks versus benefits of such an intervention should be judiciously discussed in a multidisciplinary manner.
Managing ICH
There are two primary considerations in ICH management. First, preventing hematoma expansion by BP control and discontinuing systemic anticoagulation is recommended. The duration of systemic anticoagulation varies based on the mode of ECMO. VV ECMO may allow anticoagulation discontinuation until decannulation based on multiple reports of heparin-free VV ECMO with a heparin-coated circuit [ 66 ]. In contrast, holding systemic anticoagulation carries a higher risk of thromboembolism with VA ECMO, especially the ECMO circuit [ 67 , 68 ]. Early cessation without reversal and judicious resumption of anticoagulation with repeated neuroimaging appeared feasible in the cohort of patients with ECMO-associated ischemic stroke and ICH [ 37 ]. Second, surgical or minimally invasive surgery hematoma evacuation may be considered. There is limited data on neurosurgical interventions in ECMO[ 69 ] for patients with no other management options. Neurosurgery may be considered and utilized. Multidisciplinary discussion should be undertaken, involving neurosurgeons and neurologists in decision-making.
Intracranial pressure monitoring
While external ventricular drainage may be indicated in patients with ICH with intraventricular extension and hydrocephalus, ECMO is associated with coagulopathy and requires systemic anticoagulation. Therefore, external ventricular drain insertion is a high-risk procedure associated with intra- and post-procedural bleeding [ 69 ]. External ventricular drain may be considered in selected patients at risk of imminent death from intraventricular hemorrhage and hydrocephalus. Monitoring ICP or invasive brain tissue oxygenation may be used in patients at high risk of ICP. Invasive ICP and brain tissue oxygenation have not been shown to improve long-term outcomes and may increase the risk of parenchymal hemorrhage in ECMO patients.
Cerebral venous sinus thrombosis (CVST)
Diagnosis of CVST requires a high index of suspicion in patients with risk factors for thrombosis, including internal jugular vein cannulation. Particularly, large dual-lumen VV ECMO cannulas may be associated with ABI, possibly due to venous hypertension and cannula-related thrombosis [ 70 ]. Clinical diagnosis is challenging because of varying neurological manifestations, including non-specific symptoms such as headache, seizure, or encephalopathy [ 71 ]. The diagnosis is made with brain CT in ECMO. Systemic anticoagulation is the primary treatment; however, in deteriorating patients, endovascular mechanical thrombectomy in advanced centers may be considered [ 72 ]. Lumbar puncture or other spinal fluid drainage and acetazolamide may be considered for patients with increased ICP, along with anti-edema interventions (raising the head of the bed, hyperosmolar therapy, sedation/analgesia, etc.) [ 73 ]. In severe CVST cases with hemispheric cerebral edema, decompressive craniectomy may be considered. A summary of consensus recommendations and evidence is provided in Table 6 and Supplemental Table 5 .
Neurological prognostication
Neurological prognostication is imperative in patients supported by ECPR, in which severe hypoxic-ischemic brain injury may occur as a consequence of refractory cardiac arrest and/or due to inadequate ECMO flow and differential hypoxia. It provides families and caregivers critical information and guides treatment decisions based on the likelihood of a meaningful neurological recovery. As the data on neurological prognostication is limited [ 74 ], a comprehensive approach to prognostication is needed.
Clinical examination plays a pivotal role in prognostication. Practitioners should first rule out potential confounding factors, such as sedatives, significant electrolyte disturbances, and hypothermia, to prevent an overly pessimistic prognosis. Daily clinical/neurological assessments are recommended for patients undergoing targeted temperature management, with the most crucial evaluation conducted after rewarming [ 74 ]. Attention should be given to pupillary and corneal reflexes [ 75 , 76 ]. Clinicians must exercise caution to mitigate the “self-fulfilling prophecy” bias, which occurs when prognostic test results indicating poor outcomes influence treatment decisions [ 77 ].
A comprehensive prognostication strategy should include electrophysiological tests, the evaluation of biomarkers of ABI, and neuroimaging (Table 7 ). Notwithstanding, new modalities are under investigation and will hopefully provide additional clues in such a setting regarding early and enhanced detection of ABI as well as prognostication in ECMO patients [ 78 , 79 ]. An unfavorable neurological outcome in patients without ECMO and cardiac arrest is strongly suggested by at least two indicators of severe ABI. These include the absence of pupillary and corneal reflexes at ≥ 72 h, bilateral lack of N20 cortical waves in somatosensory evoked potentials (SSEP) at ≥ 24 h, highly malignant EEG patterns at > 24 h, neuron-specific enolase levels exceeding 60 μg/L at 48 h or 72 h, status myoclonus ≤ 72 h, and extensive diffuse anoxic injury observed on brain CT/MRI [ 74 , 80 ]. This approach has not been validated in ECMO patients and has limited evidence [ 30 ].
Neuron-specific enolase values are often higher in ECMO patients due to ongoing hemolysis [ 30 , 85 ]. The most accurate neuron-specific enolase threshold for predicting an unfavorable neurological outcome in ECPR remains unknown, possibly exceeding 100 μg/L. There are sparse data on ECMO patients regarding other biomarkers, such as neurofilament light chain or tau. A combination of clinical, biomarker, electrophysiological, and neuroimaging assessment may effectively predict a neurological outcome within the first week following cardiac arrest [ 81 ]. However, limited data exist for this approach in ECMO patients; further research is needed to validate its utility. A summary of consensus recommendations and evidence is provided in Table 7 .
Other neurological diseases
Neurological prognostication in other ABI (non-hypoxic-ischemic brain injury) with ECMO is challenging and relies on less robust data than cardiac arrest. In the context of stroke (ischemic and hemorrhagic), clinical factors impacting outcomes include neurological exam, age, functionality (i.e., modified Rankin Scale), size, and stroke location. For example, age and the location of intracerebral hemorrhage may contribute to neurological prognosis [ 86 ]. However, decisions regarding withdrawal of life-sustaining therapy should be highly individualized with multidisciplinary discussions and considered patient preferences, as data on ECMO patients are sparse.
ICH while the patient is anticoagulated during ECMO carries extremely high mortality and morbidity, as shown in large ELSO registry-based investigations [ 87 , 88 ]. However, these studies did not account for withdrawing life-sustaining therapy in ECMO. Without data, no recommendations for neurological prognostication in ECMO patients can be made.
Brain death on ECMO
A systematic review reported that an apnea test could be included in brain-death criteria in ECMO patients by reducing sweep gas flow or adding exogenous carbon dioxide [ 89 ]. When an apnea test is challenging due to hemodynamic/cardiopulmonary instability, a cerebral angiogram or nuclear scan (radionuclide brain scan) is preferred [ 89 ]. We provide recommendations on apnea tests in ECMO patients (Supplemental Fig. 2 ).
Goals of care discussion
Goals of care and end-of-life discussions are often culturally influenced or determined. Therefore, it is difficult to propose international guidelines for such. No patient-level research guides communicating with families or managing ECMO discontinuation [ 82 ]. Families of ECMO patients experience significant anxiety, depression, and post-traumatic stress disorder long after hospital discharge [ 83 ]. Frequent family conversations/meetings should focus on informed consent, early goal-setting with timelines and re-evaluation, clear communication, and emotional support with compassion [ 82 ]. Ethics should be discussed openly, including whether to continue or discontinue care and resource allocation issues [ 82 ]. Routine use of ethics consultation within 72 h of cannulation, if the resource is available, can mitigate ethical conflicts by setting clear expectations [ 84 ]. Withdrawal from ECMO should be a structured process involving preparatory family meetings and clinical aspects, including symptom management, technical circuit management, and bereavement support, containing family and staff support.[ 90 ].
Long-term outcome and quality of life
Sparse information exists on long-term outcomes. Long-term MRI found cerebral infarction or hemorrhage in 37–52% of adult ECMO survivors [ 59 , 60 ]. Cognitive impairment and neuroradiologic findings were associated [ 59 , 60 ]. ECMO patients often suffer long-term psychiatric disorders, including organic mental disorders, obsessive–compulsive disorders, and post-traumatic stress disorders [ 91 ]. The incidence of neuroradiologic findings was significantly higher in VA ECMO patients than VV ECMO patients [ 59 ]. Given the high frequency, a routine, long-term, structured, standardized follow-up program is recommended for all ECMO centers. Such programs should encompass disease-specific care for underlying and acquired conditions, focusing on neurological and psychiatric disorders. Program design depends on the availability of institutional and international resources. ECMO centers should adapt follow-up programs their specific patient populations and resources while adhering to the recommendations outlined in Table 8 .
Neurological outcomes and quality of life
Assessing ECMO survivors’ quality of life is crucial to understanding the overall impact of ECMO. It is preferable to use internationally recognized, validated tests at standardized intervals. Establishing uniform measures of cognitive function in ECMO patients may clarify outcomes in future studies. Therefore, all patients should have their modified Rankin Scale assessed at discharge and during each follow-up. Additional detailed assessments may be performed based on local practices and patient conditions (e.g., Glasgow Outcome Scale Extended, Montreal Cognitive Assessment). A summary of consensus recommendations and evidence is provided in Table 8 and Supplemental Fig. 3 .
The impact of ABI on morbidity and mortality in ECMO patients is high, and early ABI detection and timely intervention may improve outcomes. Therefore, standardized neurological monitoring and neurological expertise are recommended for ECMO patients. These consensus recommendations and scientific statements serve to guide the neurological monitoring and prevention of ABI, and management strategy of ECMO-associated ABI These recommendations strongly benefit from multidisciplinary care, where it is available, to maximize the chances of favorable long-term outcomes and a good quality of life. Further research on predisposing factors, prevention, neuroimaging and management are ongoing or further required in an attempt to reduce or prevent such dreadful adverse events in ECMO patients.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- Acute brain injury
Acute myocardial infarction
Computed tomography
Extracorporeal membrane oxygenation
Extracorporeal cardiopulmonary resuscitation
Electroencephalogram
Extracorporeal Life Support Organization
Intracranial hemorrhage
Intracranial pressure
Intensive care unit
Magnetic resonance imaging
Near-infrared spectroscopy
Partial pressure of carbon dioxide
Partial pressure of oxygen
Regional oxygen saturation
Somatosensory evoked potential
Tissue plasminogen activator
Venoarterial
Cho SM, Farrokh S, Whitman G, Bleck TP, Geocadin RG. Neurocritical care for extracorporeal membrane oxygenation patients. Crit Care Med. 2019;47(12):1773–81.
Article PubMed Google Scholar
Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, Vizzardi E, Rycus PT, Muellenbach R, Mueller T, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. 2017;45(8):1389–97.
Ubben JFH, Heuts S, Delnoij TSR, Suverein MM, van de Koolwijk AF, van der Horst ICC, Maessen JG, Bartos J, Kavalkova P, Rob D, et al. Extracorporeal cardiopulmonary resuscitation for refractory OHCA: lessons from three randomized controlled trials-the trialists’ view. Eur Heart J Acute Cardiovasc Care. 2023;12(8):540–7.
Article PubMed PubMed Central Google Scholar
Shou BL, Ong CS, Premraj L, Brown P, Tonna JE, Dalton HJ, Kim BS, Keller SP, Whitman GJR, Cho SM. Arterial oxygen and carbon dioxide tension and acute brain injury in extracorporeal cardiopulmonary resuscitation patients: analysis of the extracorporeal life support organization registry. J Heart Lung Transplant. 2023;42(4):503–11.
Ong CS, Etchill E, Dong J, Shou BL, Shelley L, Giuliano K, Al-Kawaz M, Ritzl EK, Geocadin RG, Kim BS, et al. Neuromonitoring detects brain injury in patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg. 2023;165(6):2104–10.
Shoskes A, Migdady I, Rice C, et al. Brain injury is more common in venoarterial extracorporeal membrane oxygenation than venovenous extracorporeal membrane oxygenation: a systematic review and meta-analysis. Crit Care Med. 2020;48(12):1799–808. https://doi.org/10.1097/CCM.0000000000004618 .
Article CAS PubMed Google Scholar
Cho SM, Ziai W, Mayasi Y, Gusdon AM, Creed J, Sharrock M, Stephens RS, Choi CW, Ritzl EK, Suarez J, et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J. 2020;66(4):388–93.
Debaty G, Lamhaut L, Aubert R, Nicol M, Sanchez C, Chavanon O, Bouzat P, Durand M, Vanzetto G, Hutin A, et al. Prognostic value of signs of life throughout cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest. Resuscitation. 2021;162:163–70.
Desai M, Wang J, Zakaria A, Dinescu D, Bogar L, Singh R, Dalton H, Osborn E. Fixed and dilated pupils, not a contraindication for extracorporeal support: a case series. Perfusion. 2020;35(8):814–8.
Le Guennec L, Shor N, Levy B, Lebreton G, Leprince P, Combes A, Dormont D, Luyt CE. Spinal cord infarction during venoarterial-extracorporeal membrane oxygenation support. J Artif Organs. 2020;23(4):388–93.
Crow J, Lindsley J, Cho SM, Wang J, Lantry JH 3rd, Kim BS, Tahsili-Fahadan P. Analgosedation in critically Ill adults receiving extracorporeal membrane oxygenation support. ASAIO J. 2022;68(12):1419–27.
Article CAS PubMed PubMed Central Google Scholar
Cho SM, Ziai W, Geocadin R, Choi CW, Whitman G. Arterial-sided oxygenator clot and TCD emboli in VA-ECMO. Ann Thorac Surg. 2018;107(1):326–7.
Cho S-M, Geocadin RG, Caturegli G, Chan V, White B, Dodd-o J, Kim BS, Sussman M, Choi CW, Whitman G, et al. Understanding characteristics of acute brain injury in adult ECMO: an autopsy study. Crit Care Med. 2020;94:1650.
Google Scholar
Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68(12):1543–9.
Zhao D, Shou BL, Caturegli G, et al. Trends on near-infrared spectroscopy associated with acute brain injury in venoarterial extracorporeal membrane oxygenation. ASAIO J. 2023;69(12):1083–9. https://doi.org/10.1097/MAT.0000000000002032 .
Pozzebon S, Blandino Ortiz A, Franchi F, et al. Cerebral near-infrared spectroscopy in adult patients undergoing veno-arterial extracorporeal membrane oxygenation. Neurocrit Care. 2018;29(1):94–104. https://doi.org/10.1007/s12028-018-0512-1 .
Bertini P, Marabotti A, Paternoster G, et al. Regional cerebral oxygen saturation to predict favorable outcome in extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2023;37(7):1265–72. https://doi.org/10.1053/j.jvca.2023.01.007 .
Joram N, Beqiri E, Pezzato S, et al. Continuous monitoring of cerebral autoregulation in children supported by extracorporeal membrane oxygenation: a pilot study. Neurocrit Care. 2021;34(3):935–45. https://doi.org/10.1007/s12028-020-01111-1 .
Tian F, Farhat A, Morriss MC, et al. Cerebral hemodynamic profile in ischemic and hemorrhagic brain injury acquired during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020;21(10):879–85. https://doi.org/10.1097/PCC.0000000000002438 .
Caturegli G, Zhang LQ, Mayasi Y, et al. Characterization of cerebral hemodynamics with TCD in patients undergoing VA-ECMO and VV-ECMO: a prospective observational study. Neurocrit Care. 2023;38(2):407–13. https://doi.org/10.1007/s12028-022-01653-6 .
Kavi T, Esch M, Rinsky B, Rosengart A, Lahiri S, Lyden PD. Transcranial doppler changes in patients treated with extracorporeal membrane oxygenation. J Stroke Cerebrovasc Dis. 2016;25(12):2882–5. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.07.050 .
Caturegli G, Kapoor S, Ponomarev V, et al. Transcranial Doppler microemboli and acute brain injury in extracorporeal membrane oxygenation: a prospective observational study. JTCVS Tech. 2022;15:111–22. https://doi.org/10.1016/j.xjtc.2022.07.026 .
Oddo M, Taccone FS, Petrosino M, et al. The Neurological Pupil index for outcome prognostication in people with acute brain injury (ORANGE): a prospective, observational, multicentre cohort study. Lancet Neurol. 2023;22(10):925–33. https://doi.org/10.1016/S1474-4422(23)00271-5 .
Miroz JP, Ben-Hamouda N, Bernini A, et al. Neurological pupil index for early prognostication after venoarterial extracorporeal membrane oxygenation. Chest. 2020;157(5):1167–74. https://doi.org/10.1016/j.chest.2019.11.037 .
Magalhaes E, Reuter J, Wanono R, et al. Early EEG for prognostication under venoarterial extracorporeal membrane oxygenation. Neurocrit Care. 2020;33(3):688–94. https://doi.org/10.1007/s12028-020-01066-3 .
Amorim E, Firme MS, Zheng WL, et al. High incidence of epileptiform activity in adults undergoing extracorporeal membrane oxygenation. Clin Neurophysiol. 2022;140:4–11. https://doi.org/10.1016/j.clinph.2022.04.018 .
Sinnah F, Dalloz MA, Magalhaes E, et al. Early electroencephalography findings in cardiogenic shock patients treated by venoarterial extracorporeal membrane oxygenation. Crit Care Med. 2018;46(5):e389–94. https://doi.org/10.1097/CCM.0000000000003010 .
Peluso L, Rechichi S, Franchi F, et al. Electroencephalographic features in patients undergoing extracorporeal membrane oxygenation. Crit Care. 2020;24(1):629. https://doi.org/10.1186/s13054-020-03353-z .
Cho SM, Choi CW, Whitman G, et al. Neurophysiological findings and brain injury pattern in patients on ECMO. Clin EEG Neurosci. 2021;52(6):462–9. https://doi.org/10.1177/1550059419892757 .
Ben-Hamouda N, Ltaief Z, Kirsch M, et al. Neuroprognostication under ecmo after cardiac arrest: Are classical tools still performant? Neurocrit Care. 2022;37(1):293–301. https://doi.org/10.1007/s12028-022-01516-0 .
Kim YO, Ko RE, Chung CR, et al. Prognostic value of early intermittent electroencephalography in patients after extracorporeal cardiopulmonary resuscitation. J Clin Med. 2020;9(6):1745. https://doi.org/10.3390/jcm9061745 .
Lidegran MK, Mosskin M, Ringertz HG, Frenckner BP, Linden VB. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: clinical benefits in diagnosis and treatment. Acad Radiol. 2007;14(1):62–71. https://doi.org/10.1016/j.acra.2006.10.004 .
Cvetkovic M, Chiarini G, Belliato M, et al. International survey of neuromonitoring and neurodevelopmental outcome in children and adults supported on extracorporeal membrane oxygenation in Europe. Perfusion. 2023;38(2):245–60. https://doi.org/10.1177/02676591211042563 .
Malfertheiner MV, Koch A, Fisser C, et al. Incidence of early intra-cranial bleeding and ischaemia in adult veno-arterial extracorporeal membrane oxygenation and extracorporeal cardiopulmonary resuscitation patients: a retrospective analysis of risk factors. Perfusion. 2020;35:8–17. https://doi.org/10.1177/0267659120907438 .
Lockie CJA, Gillon SA, Barrett NA, et al. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med. 2017;45(10):1642–9. https://doi.org/10.1097/CCM.0000000000002579 .
Zotzmann V, Rilinger J, Lang CN, et al. Early full-body computed tomography in patients after extracorporeal cardiopulmonary resuscitation (eCPR). Resuscitation. 2020;146:149–54. https://doi.org/10.1016/j.resuscitation.2019.11.024 .
Prokupets R, Kannapadi N, Chang H, et al. Management of anticoagulation therapy in ECMO-associated ischemic stroke and intracranial hemorrhage. Innovations (Phila). 2023;18(1):49–57. https://doi.org/10.1177/15569845221141702 .
Cho SM, Wilcox C, Keller S, et al. Assessing the SAfety and FEasibility of bedside portable low-field brain Magnetic Resonance Imaging in patients on ECMO (SAFE-MRI ECMO study): study protocol and first case series experience. Crit Care. 2022;26(1):119. https://doi.org/10.1186/s13054-022-03990-6 .
Lorusso R, Taccone FS, Belliato M, et al. Brain monitoring in adult and pediatric ECMO patients: the importance of early and late assessments. Minerva Anestesiol. 2017;83(10):1061–74. https://doi.org/10.23736/S0375-9393.17.11911-5 .
Khanduja S, Kim J, Kang JK, et al. Hypoxic-ischemic brain injury in ecmo: pathophysiology, neuromonitoring, and therapeutic opportunities. Cells. 2023;12(11):1546. https://doi.org/10.3390/cells12111546 .
de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, Bosman RJ, de Waal RA, Wesselink R, de Keizer NF. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12(6):R156.
Al-Kawaz MN, Canner J, Caturegli G, Kannapadi N, Balucani C, Shelley L, Kim BS, Choi CW, Geocadin RG, Whitman G, et al. Duration of hyperoxia and neurologic outcomes in patients undergoing extracorporeal membrane oxygenation. Crit Care Med. 2021;49(10):e968–77.
Akbar AF, Shou BL, Feng CY, Zhao DX, Kim BS, Whitman G, Bush EL, Cho SM, Investigators H. Lower oxygen tension and intracranial hemorrhage in veno-venous extracorporeal membrane oxygenation. Lung. 2023;201(3):315–20.
Cho SM, Canner J, Chiarini G, Calligy K, Caturegli G, Rycus P, Barbaro RP, Tonna J, Lorusso R, Kilic A, et al. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2020;48(10):e897–905.
Harper AM, Bell RA. The effect of metabolic acidosis and alkalosis on the blood flow through the cerebral cortex. J Neurol Neurosurg Psychiatry. 1963;26(4):341–4.
Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48(6):1011–23.
Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology. 2015;122(1):196–205.
Deng B, Ying J, Mu D. Subtypes and mechanistic advances of extracorporeal membrane oxygenation-related acute brain injury. Brain Sci. 2023;13(8):1165.
Cavayas YA, Munshi L, Del Sorbo L, Fan E. The early change in Pa. Am J Respir Crit Care Med. 2020;201(12):1525–35.
Shou BL, Ong CS, Zhou AL, Al-Kawaz MN, Etchill E, Giuliano K, Dong J, Bush E, Kim BS, Choi CW, et al. Arterial carbon dioxide and acute brain injury in venoarterial extracorporeal membrane oxygenation. ASAIO J. 2022;42(4):503–11.
van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130(Pt 12):3063–74.
Huang M, Shoskes A, Migdady I, Amin M, Hasan L, Price C, Uchino K, Choi CW, Hernandez AV, Cho SM. Does targeted temperature management improve neurological outcome in extracorporeal cardiopulmonary resuscitation (ECPR)? J Intensive Care Med. 2022;37(2):157–67.
Levy B, Girerd N, Amour J, Besnier E, Nesseler N, Helms J, Delmas C, Sonneville R, Guidon C, Rozec B, et al. Effect of moderate hypothermia vs normothermia on 30-day mortality in patients with cardiogenic shock receiving venoarterial extracorporeal membrane oxygenation: a randomized clinical trial. JAMA. 2022;327(5):442–53.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49(3):e46–110.
Minhas JS, Moullaali TJ, Rinkel GJE, Anderson CS. Blood pressure management after intracerebral and subarachnoid hemorrhage: the knowns and known unknowns. Stroke. 2022;53(4):1065–73.
Kazmi SO, Sivakumar S, Karakitsos D, Alharthy A, Lazaridis C. Cerebral pathophysiology in extracorporeal membrane oxygenation: pitfalls in daily clinical management. Crit Care Res Pract. 2018;2018:3237810.
PubMed PubMed Central Google Scholar
Shou BL, Wilcox C, Florissi I, Kalra A, Caturegli G, Zhang LQ, Bush E, Kim B, Keller SP, Whitman GJR, et al. Early low pulse pressure in VA-ECMO is associated with acute brain injury. Neurocrit Care. 2023;38(3):612–21.
Lee SI, Lim YS, Park CH, Choi WS, Choi CH. Importance of pulse pressure after extracorporeal cardiopulmonary resuscitation. J Card Surg. 2021;36(8):2743–50.
Risnes I, Wagner K, Nome T, et al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2006;81(4):1401–6. https://doi.org/10.1016/j.athoracsur.2005.10.008 .
von Bahr V, Kalzen H, Hultman J, et al. Long-term cognitive outcome and brain imaging in adults after extracorporeal membrane oxygenation. Crit Care Med. 2018;46(5):e351–8. https://doi.org/10.1097/CCM.0000000000002992 .
Article Google Scholar
Ropper AH, O’Rourke D, Kennedy SK. Head position, intracranial pressure, and compliance. Neurology. 1982;32(11):1288–91.
Blanco P, Abdo-Cuza A. Transcranial Doppler ultrasound in neurocritical care. J Ultrasound. 2018;21(1):1–16.
Miller C, Armonda R. Participants in the International Multi-disciplinary Consensus conference on Multimodality Monitoring of cerebral blood flow and ischemia in the critically ill. Neurocrit Care. 2014;21(2):1–26.
Raha O, Hall C, Malik A, D’Anna L, Lobotesis K, Kwan J, Banerjee S. Advances in mechanical thrombectomy for acute ischaemic stroke. BMJ Med. 2023;2(1): e000407.
Friesenecker BE, Peer R, Rieder J, Lirk P, Knotzer H, Hasibeder WR, Mayr AJ, Dunser MW. Craniotomy during ECMO in a severely traumatized patient. Acta Neurochir (Wien). 2005;147(9):993–6.
Ryu KM, Chang SW. Heparin-free extracorporeal membrane oxygenation in a patient with severe pulmonary contusions and bronchial disruption. Clin Exp Emerg Med. 2018;5(3):204–7.
Lamarche Y, Chow B, Bedard A, Johal N, Kaan A, Humphries KH, Cheung A. Thromboembolic events in patients on extracorporeal membrane oxygenation without anticoagulation. Innovations (Phila). 2010;5(6):424–9.
Fina D, Matteucci M, Jiritano F, Meani P, Lo Coco V, Kowalewski M, Maessen J, Guazzi M, Ballotta A, Ranucci M, Lorusso R. Extracorporeal membrane oxygenation without therapeutic anticoagulation in adults: a systematic review of the current literature. Int J Artif Organs. 2020;43(9):570–8.
Fletcher-Sandersjoo A, Thelin EP, Bartek J Jr, Elmi-Terander A, Broman M, Bellander BM. Management of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation (ECMO): an observational cohort study. PLoS ONE. 2017;12(12): e0190365.
Mazzeffi M, Kon Z, Menaker J, Johnson DM, Parise O, Gelsomino S, Lorusso R, Herr D. Large dual-lumen extracorporeal membrane oxygenation cannulas are associated with more intracranial hemorrhage. ASAIO J. 2019;65(7):674–7.
Idiculla PS, Gurala D, Palanisamy M, Vijayakumar R, Dhandapani S, Nagarajan E. Cerebral venous thrombosis: a comprehensive review. Eur Neurol. 2020;83(4):369–79.
Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY, Stroke C, American Heart Association, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–92.
Einhaupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, Masuhr F. European federation of neurological S: EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17(10):1229–35.
Nolan JP, Sandroni C, Bottiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM, et al. European resuscitation council and european society of intensive care medicine guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–69.
Nutma S, Ruijter BJ, Beishuizen A, Tromp SC, Scholten E, Horn J, van den Bergh WM, van Kranen-Mastenbroek VH, Thomeer EC, Moudrous W, et al. Myoclonus in comatose patients with electrographic status epilepticus after cardiac arrest: corresponding EEG patterns, effects of treatment and outcomes. Resuscitation. 2023;186: 109745.
Peluso L, Oddo M, Minini A, Citerio G, Horn J, Di Bernardini E, Rundgren M, Cariou A, Payen JF, Storm C, et al. Neurological pupil index and its association with other prognostic tools after cardiac arrest: a post hoc analysis. Resuscitation. 2022;179:259–66.
Elmer J, Kurz MC, Coppler PJ, Steinberg A, DeMasi S, De-Arteaga M, Simon N, Zadorozhny VI, Flickinger KL, Callaway CW, et al. Time to awakening and self-fulfilling prophecies after cardiac arrest. Crit Care Med. 2023;51(4):503–12.
Cho SM, Khanduja S, Kim J, Kang JK, Briscoe J, Arlinghaus LR, Dinh K, Kim BS, Sair HI, Wandji AN, Moreno E, Torres G, Gavito-Higuera J, Choi HA, Pitts J, Gusdon AM, Whitman GJ. detection of acute brain injury in intensive care unit patients on ECMO support using ultra-low-field portable mri: a retrospective analysis compared to head CT. Diagnostics (Basel). 2024;14(6):606.
Cho SM, Khanduja S, Wilcox C, Dinh K, Kim J, Kang JK, Chinedozi ID, Darby Z, Acton M, Rando H, Briscoe J, Bush E, Sair HI, Pitts J, Arlinghaus LR, Wandji AN, Moreno E, Torres G, Akkanti B, Gavito-Higuera J, Keller S, Choi HA, Kim BS, Gusdon A, Whitman GJ. Clinical use of bedside portable low-field brain magnetic resonance imaging in patients on ECMO: The results from multicenter SAFE MRI ECMO study. Res Sq [Preprint] . 2024
Rajajee V, Muehlschlegel S, Wartenberg KE, Alexander SA, Busl KM, Chou SHY, Creutzfeldt CJ, Fontaine GV, Fried H, Hocker SE, et al. Guidelines for neuroprognostication in comatose adult survivors of cardiac arrest. Neurocrit Care. 2023;38(3):533–63.
Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of good neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2022;48(4):389–413. https://doi.org/10.1007/s00134-022-06618-z .
Moynihan KM, Dorste A, Siegel BD, Rabinowitz EJ, McReynolds A, October TW. Decision-making, ethics, and end-of-life care in pediatric extracorporeal membrane oxygenation: a comprehensive narrative review. Pediatr Crit Care Med. 2021;22(9):806–12. https://doi.org/10.1097/PCC.0000000000002766 .
Onrust M, Lansink-Hartgring AO, van der Meulen I, Luttik ML, de Jong J, Dieperink W. Coping strategies, anxiety and depressive symptoms in family members of patients treated with extracorporeal membrane oxygenation: a prospective cohort study. Heart Lung Mar. 2022;52:146–51. https://doi.org/10.1016/j.hrtlng.2022.01.002 .
Wirpsa MJ, Carabini LM, Neely KJ, Kroll C, Wocial LD. Mitigating ethical conflict and moral distress in the care of patients on ECMO: impact of an automatic ethics consultation protocol. J Med Ethics. 2021. https://doi.org/10.1136/medethics-2020-106881 .
Petermichl W, Philipp A, Hiller KA, Foltan M, Floerchinger B, Graf B, Lunz D. Reliability of prognostic biomarkers after prehospital extracorporeal cardiopulmonary resuscitation with target temperature management. Scand J Trauma Resusc Emerg Med. 2021;29(1):147.
Gregorio T, Pipa S, Cavaleiro P, Atanasio G, Albuquerque I, Castro Chaves P, Azevedo L. Original intracerebral hemorrhage score for the prediction of short-term mortality in cerebral hemorrhage: systematic review and meta-analysis. Crit Care Med. 2019;47(6):857–64.
Cho SM, Canner J, Caturegli G, Choi CW, Etchill E, Giuliano K, Chiarini G, Calligy K, Rycus P, Lorusso R, et al. Risk factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: analysis of data from the extracorporeal life support organization registry. Crit Care Med. 2021;49(1):91–101.
Hwang J, Kalra A, Shou BL, Whitman G, Wilcox C, Brodie D, Zaaqoq AM, Lorusso R, Uchino K, Cho SM. Epidemiology of ischemic stroke and hemorrhagic stroke in venoarterial extracorporeal membrane oxygenation. Crit Care. 2023;27(1):433.
Lie SA, Hwang NC. Challenges of brain death and apnea testing in adult patients on extracorporeal corporeal membrane oxygenation-a review. J Cardiothorac Vasc Anesth. 2019;33(8):2266–72.
Machado DS, Garros D, Montuno L, Avery LK, Kittelson S, Peek G, Moynihan KM. Finishing well: compassionate extracorporeal membrane oxygenation discontinuation. J Pain Symptom Manage. 2022;63(5):e553–62.
Kalra A, Kang JK, Khanduja S, Menta AK, Ahmad SA, Liu O, Rodriguez E, Spann M, Hernandez AV, Brodie D, et al. Long-term neuropsychiatric, neurocognitive, and functional outcomes of patients receiving ECMO. Neurology. 2024;102(3): e208081.
Risnes I, Heldal A, Wagner K, et al. Psychiatric outcome after severe cardio-respiratory failure treated with extracorporeal membrane oxygenation: a case-series. Psychosomatics. 2013;54(5):418–27. https://doi.org/10.1016/j.psym.2013.02.008 .
Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics. 2018;36(6):675–97. https://doi.org/10.1007/s40273-018-0623-8 .
Download references
Dr. Cho is supported by NIH (1K23HL157610, 1R21NS135045). Dr. Brodie received research support from and consults for LivaNova.
Author information
Akram M. Zaaqoq and Roberto Lorusso have contributed equally as senior authors.
Authors and Affiliations
Divisions of Neuroscience Critical Care and Cardiac Surgery Departments of Neurology, Neurosurgery, and Anaesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Phipps 455, Baltimore, MD, 21287, USA
Sung-Min Cho, Jaeho Hwang & Pouya Tahsili-Fahadan
Division of Cardiac Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Sung-Min Cho & Glenn Whitman
Cardiothoracic Surgery Department, Heart and Vascular Centre, Maastricht University Medical Centre, Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands
Giovanni Chiarini & Roberto Lorusso
Division of Anaesthesiology, Intensive Care and Emergency Medicine, Spedali Civili University, Affiliated Hospital of Brescia, Brescia, Italy
Giovanni Chiarini
Medical/Critical Pharmacy Division, King Faisal Specialist Hospital and Research Center, 11564, Al Mathar Ash Shamali, Riyadh, Saudi Arabia
Marwa Amer & Alyaa Elhazmi
Alfaisal University College of Medicine, Riyadh, Saudi Arabia
Bufalini Hospital, AUSL della Romagna, Cesena, Italy
Marta V. Antonini
Department of Critical Care Medicine, Guy’s and St Thomas’ National Health Service Foundation Trust, London, UK
Nicholas Barrett
2nd Department of Medicine, Cardiology and Angiologiy, General University Hospital and 1st School of Medicine, Charles University, Prague, Czech Republic
Jan Belohlavek, Jan Pudil & Daniel Rob
Division of Pulmonary, and Critical Care Medicine, Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Daniel Brodie
Departments of Surgery and Pediatrics, Creighton University, Omaha, NE, USA
Heidi J. Dalton
Programa de Oxigenación Por Membrana Extracorpórea, Hospital San Juan de Dios Santiago, Santiago, Chile
Rodrigo Diaz
Medical Critical Care Service, Department of Medicine, Inova Fairfax Medical Campus, Falls Church, VA, USA
Pouya Tahsili-Fahadan
Critical Care Research Group, Adult Intensive Care Services, The Prince Charles Hospital and University of Queensland, Rode Rd, Chermside, QLD, 4032, Australia
Jonathon Fanning & John Fraser
Cardiorespiratory and Critical Care Division, Great Ormond Street Hospital for, Children National Health Service Foundation Trust, London, UK
Aparna Hoskote
Department of Thoracic and Cardiovascular Surgery, Korea University Medicine, Seoul, Republic of Korea
Jae-Seung Jung
Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Würzburg, Würzburg, Germany
Christopher Lotz
Cardiothoracic Intensive Care Unit, Department of Cardiac, Thoracic and Vascular Surgery, National University Health System, Singapore, Singapore
Graeme MacLaren & Kollengode Ramanathan
Congenital Heart Center, Departments of Surgery and Pediatrics, University of Florida, Gainesville, FL, USA
Pediatric Intensive Care Unit, Department of Woman, Child, and Adolescent Medicine, Geneva University Hospital, Geneva, Switzerland
Angelo Polito
Department of Pediatrics, Section Critical Care Medicine, Children’s Medical Center at Dallas, The University of Texas Southwestern Medical Center at Dallas, Dallas, TX, USA
Lakshmi Raman
Department of Intensive Care, Erasmus University Medical Center, Rotterdam, The Netherlands
Dinis Dos Reis Miranda
ECMO Department, Fundacion Cardiovascular de Colombia, Floridablanca, Santander, Colombia
Leonardo Salazar Rojas
Department of Intensive Care, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
Fabio Silvio Taccone
Department of Anesthesiology, Division of Critical Care, University of Virginia, Charlottesville, VA, USA
Akram M. Zaaqoq
You can also search for this author in PubMed Google Scholar
Contributions
S.-M.C. prepared the first draft, led the conceptualization and approach, and finalized the guidelines. A.M.Z. and R.L. provided critical revision and contributed in finalizing the guidelines as co-chairs. J.H. and G.C. provided tables and contributed to the first draft. M.A. created all figures and supplemental figures. M.A., N.B., J.B., D.B., H.J.D, R.D., A.E., P.T.F., H.F., J.F., A.H., J.-S.J., C.L., G.M., G.P., A.P., J.P., L.R., K.R., D.D., D.R., L.S., F.S.T., and G.W. were divided into 6 writing groups and prepared each section of the guidelines (6 sections).
Corresponding author
Correspondence to Sung-Min Cho .
Ethics declarations
Ethical approval and consent to participate.
Not applicable as this is a consensus guidelines article.
Competing interests
Dr. Cho is a consultant for Hyperfine, Inc. and supported by NIH (1K23HL157610 and 1R21NS135045). Dr. Brodie received research support from and consults for LivaNova. He has been on the medical advisory boards for Xenios, Medtronic, Inspira, and Cellenkos. He is the President-elect of the Extracorporeal Life Support Organization (ELSO) and the Chair of the Executive Committee of the International ECMO Network (ECMONet), and he writes for UpToDate. Dr. Daniel is supported by MH CZ (DRO-VFN64165) and receives consulting honoraria from Abiomed and Resuscitec. Dr. Lorusso received research support from Medtronic and LivaNova, is consultant for Medtronic and Livanova, Member of the Medical Advisory Board of Eurosets and Xenios, and receives speaker fee from Abiomed.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary Information
Additional file1, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cho, SM., Hwang, J., Chiarini, G. et al. Neurological monitoring and management for adult extracorporeal membrane oxygenation patients: Extracorporeal Life Support Organization consensus guidelines. Crit Care 28 , 296 (2024). https://doi.org/10.1186/s13054-024-05082-z
Download citation
Received : 17 July 2024
Accepted : 28 August 2024
Published : 06 September 2024
DOI : https://doi.org/10.1186/s13054-024-05082-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neuromonitoring
- Neurological care
- Neurological outcomes
Critical Care
ISSN: 1364-8535
- Submission enquiries: [email protected]
Periodic safety update reports (PSURs)
PSURs are pharmacovigilance documents intended to provide an evaluation of the risk-benefit balance of a medicinal product at defined time points after its authorisation.
The objective of the PSUR is to present a comprehensive and critical analysis of the risk-benefit balance of the product, taking into account new or emerging safety information in the context of cumulative information on risk and benefits
EMA and national competent authorities assess information in PSURs to determine if there are new risks identified for a medicine and/or if its risk-benefit balance has changed.
A PSUR assessment can determine if further investigations on a specific issue are needed, or if an action is necessary to protect public health (e.g. an update of the information provided to healthcare professionals and patients).
Marketing authorisation holders (MAHs) are legally required to submit PSURs, in line with current legislation.
Article 35 of the Commission Implementing Regulation describes the structure of PSURs. Guidance is available on the preparation, submission and assessment of PSURs. This format is a legal requirement for both nationally authorised products and centrally authorised products.
Guideline on good pharmacovigilance practices (GVP): Module VII – Periodic safety update report
English (EN) (1.45 MB - PDF)
For more information, see:
- Regulation (EU) No 1235/2010
- Directive 2010/84/EU
- Commission Implementing Regulation (EU) No 520/2012
Preparation of PSURs
MAHs should consult the following information when preparing a PSUR:
The Agency has also published an explanatory note to GVP module VII, which all MAHs should consult when preparing PSURs. It addresses specific challenges in the EU single assessment procedure for nationally authorised products, but the issues may also apply to centrally authorised products.
For more information on GVP modules, see Good pharmacovigilance practices .
- Guideline on good pharmacovigilance practices (GVP): Module VII – Periodic safety update report - Explanatory note
English (EN) (301.89 KB - PDF)
Specific guidance is available on preparing PSURs for COVID-19 vaccines :
- COVID-19 guidance: Periodic safety update reports for COVID-19 vaccines
Submission of PSURs
As of 13 June 2016, MAHs are required to submit all PSURs in the EU to the central PSUR repository using the eSubmission Gateway/ Web Client .
Use of the PSUR repository is mandatory for both centrally and nationally authorised medicines, whether they follow the EU single assessment or a purely national assessment procedure.
For more information on how to use the PSUR repository, see:
- Periodic Safety Update Report (PSUR) repository mandatory use: questions and answers
Further information on the PSUR repository, guidance on how to register and multimedia tutorials for MAHs on how to submit a PSUR in the correctly-structured electronic formats can be found on EMA's eSubmission website .
Submission requirements and EU reference dates: the EURD list
MAHs for active substances and combinations of active substances that are subject to assessment at EU level must submit the relevant PSURs according to the requirements set up in the list of EU reference dates (EURD) list:
List of European Union reference dates and frequency of submission of periodic safety update reports (PSURs)
English (EN) (926.84 KB - XLSX)
The list does not include substances assessed at national level , although their PSURs must nonetheless be submitted via the PSUR repository. For these active substances, the frequency of submission is established at national level. For more information, see:
- CMDh best practice guide
The EURD list provides the following information for each active substance/combination it contains:
- frequency of PSUR submission;
- data lock point;
- submission date;
- requirements for the submission of PSURs for generic, well-established use, homeopathic and traditional herbal products.
The EURD list is a legally binding document and MAHs are legally responsible for complying with its requirements. The list overrules the 'standard' PSUR submission cycle and any conditions related to the frequency of PSUR submission included in a marketing authorisation.
EMA updates the EURD list every month , following adoption by the Committee for Medicinal Products for Human Use (CHMP) and Coordination Group for Mutual Recognition and Decentralised Procedures - Human (CMDh), after consultation with the Pharmacovigilance Risk Assessment Committee (PRAC).
Any amendment to the EURD list becomes effective six months after its publication .
For more information on the EURD list and answers to frequently-asked-questions, see the following document:
Introductory cover note to the list of European Union reference dates and frequency of submission of periodic safety update reports
English (EN) (142.13 KB - PDF)
MAHs and other stakeholders can request amendments to the EURD list by emailing [email protected] with the following information:
Template for a request for amendments of or addition of active substances or combinations of active substances to the European Union reference-date list
English (EN) (103.03 KB - DOCX)
- extract of the relevant active substances/combinations from the Article 57 database for which the addition/amendment is requested.
Before submitting a request, EMA encourages stakeholders to carefully consult the introductory cover note. There may be some delay before EMA is able to respond due to the high volume of requests and necessary processing time.
Assessment of PSURs
PSURs submitted in accordance with the EURD list are subject to the EU PSUR single assessment (PSUSA) procedure.
EMA carries out PSUSA procedures to assess PSURs of medicines containing the same active substances or combinations, even if they are subject to different marketing authorisations and are authorised in different EU Member States. This aims to harmonise and strengthen benefit-risk review of medicines across the European Economic Area.
For more information, see the questions-and-answers below and Periodic safety update report single assessments .
Post-authorisation procedural advice: questions and answers
1. what is a periodic safety update report (psur).
Periodic safety update reports are pharmacovigilance documents intended to provide a safety update resulting in an evaluation of the impact of the reports on the risk-benefit balance of a medicinal product. They shall be submitted by marketing authorisation holders at defined time points during the post-authorisation phase.
The legal requirements for submission of PSURs are established in the Regulation (EC) No 726/2004 and the Directive 2001/83/EC.
The format of PSURs shall follow the structure described in the Commission implementing Regulation (EU) No 520/2012.
Further details and guidance for the submission of PSURs in the EU, including the list of Union references dates and frequency of submission are provided in Module VII “Periodic safety update report” of the guideline on good pharmacovigilance practices (GVP) and in the following questions and answers.
- Regulation (EC) 726/2004
- Directive 2001/83/EC
- Commission implementing Regulation (EU) No 520/2012 of 19 June 2012 on the performance of pharmacovigilance activities provided for in Regulation (EC) No 726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council
- European Commission questions and answers on transitional arrangements concerning the entering into force of the new pharmacovigilance rules provided by Directive 2010/84/EU amending Directive 2001/83/EC and Regulation (EU) No 1235/2010 amending Regulation (EC) No 726/2004 (SANCO/D5/FS/(2012)1014848)
- Questions and answers on practical transitional measures for the implementation of the pharmacovigilance legislation
- Guideline on good pharmacovigilance practices (GVP) – Module VII – PSURs
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guideline E2C (R2) on periodic benefit-risk evaluation report - Step 5
- Guideline on good pharmacovigilance practices: Annex I - Definitions (Rev. 4) (superseded)
2. What is the scope of PSUR assessment under the EU single assessment?
The Pharmacovigilance Risk Assessment Committee (PRAC) is in charge of issuing recommendation on the PSUR assessment for a single centrally authorised product and of the EU PSUR single assessment.
The EU PSUR single assessment, referred also as PSUSA, is the assessment of PSURs for medicinal products subject to different marketing authorisations containing the same active substance or the same combination of active substances and for which the frequency and dates of submission of PSURs have been harmonised in the list of EU reference dates (referred also as EURD list). These PSURs will be jointly assessed by the PRAC or a Member State appointed by the CMDh and result in one single assessment report, which will be shared amongst all the marketing authorisation holders (MAHs) whose medicinal product(s) are part of the PSUR single assessment procedure. It should be noted that the responsibility for the quality of the submitted documentation lies with the MAH(s) and is crucial to the overall assessment. The data presented in the submissions should be intended exclusively for the purposes of the concerned procedure. The information and data contained in the individual submissions will be assessed and reflected in the single assessment report. Such information and data will not be redacted from the single assessment report with respect to individual products prior to sharing them with all concerned MAHs. Indeed, PSUR related data presented in agreement with Guideline on good pharmacovigilance practices (GVP): Module VII – Periodic safety update report as such are not considered to be commercially confidential. Of note, MAHs cannot use the information and data contained in the submissions for any other purposes than those related to the concerned procedure.
Overall, the PRAC will issue a recommendation for the assessment of the following PSURs:
- PSURs of centrally authorised product(s);
- PSURs of any mix of centrally authorised products and nationally authorised products (including through the mutual recognition and decentralised procedures);
- PSURs of nationally authorised products.
Please note that, for nationally authorised medicinal products which are marketed in only one Member State and whose active substance or combination of active substances is included in the EURD list, the MAH should submit a PSUR as part of PSUSA procedure. Note that a PSUSA is foreseen for each active substance or combination of active substances registered in the EURD list.
For purely nationally authorised medicinal products, containing substances or combination of actives substances not included in the EURD list, for which no PSUSA procedure has been established, the assessment of the PSURs will remain at national level. For more information, see:
- CMDh best practic guide
Purely nationally authorised medicinal products are considered those which contain substances or a combination of actives substances which are only authorised in one Member State.
3. How shall I present my PSUR and in which format?
The format and content of the PSUR, is legally required according to Commission implementing Regulation (EU) No 520/2012 since January 2013 and is further described in the Guideline on good pharmacovigilance practices (GVP) Module VII – Periodic safety update report.
In addition, the required format and content of PSURs in the EU are based on those for the Periodic Benefit Risk Evaluation Report (PBRER) described in the ICH-E2C(R2) guideline (see Annex IV ICH-E2C(R2)). To keep the terminology consistent with the one used in the EU legislation, the new PBRER continues to be described as PSUR.
Unless otherwise requested by competent authorities, the marketing authorisation holder shall prepare a single PSUR for all its medicinal products containing the same active substance with information covering all the authorised indications, route of administration, dosage forms and dosing regiments, irrespective of whether authorised under different names and through separate procedures. Of note, the PSUR section “Worldwide marketing authorisation status" applies irrespectively to centrally authorised products and nationally authorised products. Regarding centrally authorised products, the marketing status should also be provided as a stand-alone report through the relevant mailbox and using the dedicated template as indicated in the EMA Post-authorisation Guidance on ‘marketing and cessation notification’ – What is the reporting format to the agency and to whom to report .
Even if a single PSUR is prepared for several products, please note that for medicinal products with documentation previously submitted in eCTD format, PSURs should be presented in a new eCTD sequence in the respective eCTD lifecycle of the concerned product. Where relevant, data relating to a particular indication, dosage form, and route of administration or dosing regimen, shall be presented in a separate section of the PSUR and any safety concerns shall be addressed accordingly.
Within the PSUR, the marketing authorisation holder is required to consider the impact of the data and evaluations presented within the report, on the marketing authorisation. Based on the evaluation of the cumulative safety data and the risk-benefit analysis, the marketing authorisation holder shall draw conclusions in the PSUR as to the need for changes to the product information of the products covered by the PSUR. For the purpose of analysing the impact of the PSUR data, the MAH can establish a so-called reference product information which should include “core safety” and “authorised indications” components, as explained in the GVP module VII on PSURs (section VII.B.4. ‘Reference information’). The changes proposed to the labelling can be based on the reference product information. However, as the reference product information might be different for the various EU product information, it is essential that the MAH considers the proposed changes for the reference product information in the context of the different EU product information for the products covered by the submitted PSUR. This should be clearly discussed in both the conclusions and actions section of the body of the PSUR as well as in the EU regional appendix.
In the EU regional appendix, sub-section “Proposed product information” of the PSUR, the marketing authorisation holder should provide their proposal for product information (SmPC and package leaflet) changes based on the above-mentioned evaluation. These should take into account all EU authorised indications for products containing that active substance or combination of active substances. For marketing authorisation holders of nationally authorised products with a large number of marketing authorisations with different product information, the Agency will also accept that the core message of the proposed changes to the product information be included in the EU regional appendix as described below (see question “ How can I propose changes to the Product Information within the PSUR for NAPs which are part of an EU single assessment? ”).
It is important that changes proposed to the product information which are based on the submitted PSUR data are not submitted in parallel via a separate variation procedure.
Additional clarification on the content of the PSUR can be found in the explanatory note to GVP Module VII and should be used by MAHs for the preparation of PSURs subject to single assessment. As it complements GVP Module VII, both documents should be consulted in conjunction. The explanatory note will form the basis of the upcoming revision of GPV VII; this update, once finalised, will therefore supersede this guidance document.
The explanatory note highlights and addresses challenges specific to the EU single assessment for nationally authorised products. Points highlighted in this document may nevertheless also apply to the assessment of centrally authorised products and hence it is recommended that all MAHs consult the document prior to finalisation of their PSUR.
In connection with the explanatory note to Guideline on good pharmacovigilance practices (GVP): Module VII – Periodic safety update report , an assessors question and answer guidance document has also been developed.
The submission should include a cover letter and in order to facilitate the registration of the submission, marketing authorisation holders are required to fill in all the submission attributes through the eSubmission delivery file UI . This delivery file should be completed in accordance with the published EURD list, where the procedure number is the combination of a unique ID and the applicable Data Lock Point (DLP) in YYYYMM format.
All the entries in the EURD list have been assigned a procedure number presented in the column “Procedure number of the PSUR single assessment”.
In order to facilitate the identification of procedures containing centrally and/or nationally authorised substances, the extra columns “Centrally Authorised Product (CAP) and “Nationally authorised product (NAP)” have been added in the EURD list”.
In line with article 57(2) of Regulation (EU) No 1235/2010 all holders of marketing authorisations for medicines in the European Union and the European Economic Area must submit information to the European Medicines Agency on authorised medicines and keep this information up to date. This is a legally binding requirement from the EU pharmaceutical legislation. The Agency uses this information to support the analysis of data, regulatory activities and communication. In relation to the submission of PSURs, this facilitates the processing of the submissions in the PSUR Repository.
Please see question “ To whom should I submit my PSUR?” for further details on submission requirements.
- Guideline on good pharmacovigilance practices (GVP) – Module VII –Periodic safety update report
- Guideline on good pharmacovigilance practices (GVP) – Module VII –Periodic safety update report - Explanatory note
- Commission implementing Regulation (EU) No 520/2012 on the performance of pharmacovigilance activities
- Harmonised Guidance for eCTD Submissions in the EU
4. What is the List of European Union reference dates (EURD list) and frequency of submission of PSURs?
The list of Union reference dates and frequency of submission of PSURs” (so-called the “EURD list”) consists of active substances and combinations of active substances, for which PSURs shall be submitted in accordance with the EU reference dates and frequencies determined by the Committee for Medicinal Products for Human Use (CHMP) and the Coordination Group for Mutual Recognition and Decentralised Procedures - Human (CMDh) following consultation with the Pharmacovigilance and Risk Assessment Committee (PRAC).
The European Union reference date (EURD) corresponds to the date of the first or the earliest known date of the marketing authorisation in the EU of a medicinal product containing the active substance or combination of active substances.
In addition to the EU reference dates and frequencies of PSURs, the EURD list also provides the Data Lock Point (DLP) of the next PSUR submissions.
The EURD list facilitates the harmonisation of DLPs and frequency of submission of PSURs for medicinal products containing the same active substance or the same combination of active substances subject to different marketing authorisations, authorised in more than one Member State. This will, where appropriate, allow one single assessment of PSURs for products containing the same active substance.
The PSUR frequency as published on the EURD list for a given active substance or combination of active substances overrules the standard submission cycle (i.e. 6-monthly, yearly and thereafter 3-yearly) set out in the legislation and any condition related to the frequency of submission of PSURs included in the Marketing Authorisation. However, national competent authorities (NCAs) may still request the submission of a PSUR at any given time.
The EURD list is a living document, meaning that it can be amended whenever considered necessary by the PRAC, CHMP or CMDh in response to the emergence of relevant new safety information, newly authorised substances or requests from the marketing authorisation holders.
Full information on the EURD list is included in the GVP Module VII – Periodic safety update report and the introductory cover note to the EURD list.
For guidance on submission of requests for amendment of the EURD list, please refer to the question “ How can I request to amend the list of EU reference dates ” .
- Guideline on good pharmacovigilance practices – Module VII –Periodic safety update report
5. When do changes to the EURD list become legally binding?
The EURD list is updated on a monthly basis and any changes in the EURD list, such as the PSUR submission frequencies, the dates of submission and the PSUR submission requirement for medicinal products referred to in Articles 10(1), 10a, 14 or 16a of Directive 2001/83/EC come into force 6 months after its publication. This publication occurs after adoption of the EURD list by the CHMP and CMDh following consultation of the PRAC.
Whilst changes become binding 6 months after publication, there might exceptionally be situations where PSUR submissions are necessary prior to the new frequency taking effect and this will be indicated in the EURD list as well.
It is the responsibility of the marketing authorisation holder to check regularly the list of EU reference dates and frequency of submission published in the European medicines web-portal to ensure compliance with the PSUR reporting requirements for their medicinal products.
6. How can I request to amend the list of EU reference dates? Rev. December 2019
Marketing authorisation holders can submit requests to the CHMP or the CMDh, as appropriate, to determine the Union reference dates or to change the frequency of submission of PSURs on one of the following grounds:
- for reasons relating to public health;
- in order to avoid a duplication of the assessment;
- in order to achieve international harmonisation.
The request and its grounds should be considered by the PRAC and the CHMP if it concerns at least one marketing authorisation granted in accordance with the centralised procedure or the CMDh otherwise, which will either approve or deny the request.
The list will then be amended accordingly when appropriate and published on the European medicines website.
For more details on how to submit amendments to the list, please refer to the Introductory cover note to the list of European Union reference dates and frequency of submission of periodic safety update reports .
- Guideline on good pharmacovigilance practices (GVP) Module VII – Periodic safety update report
7. Do I have to submit a PSUR if the active substance/combination of active substances of my medicinal product is not in the EURD list?
If the active substance contained in the medicinal product is not included in the EURD list, the MAH should submit the PSUR directly to the PSUR repository, using the non-EU single assessment functionality, via the eSubmission Gateway. The PSUR will then be considered delivered to the relevant national competent authority (NCA) where the product is authorized. The frequency of submission shall be in accordance with the conditions specified in the marketing authorisation (MA), or otherwise according to the standard submission schedule of PSURs (i.e. 6 month intervals, yearly and thereafter 3 yearly). Marketing authorisation holders for certain medicinal products containing active substances not in the EURD list, such as medicinal products authorised under Article 10(1) or 10a of Directive 2001/83/EC, a homeopathic simplified registration or a traditional-use registration are not required to submit PSURs, unless there are specific requirements in the MA for the product. PSURs shall also be submitted upon request of NCA.
For more details on PSUR submissions for generics, products containing well-established substances, homeopathic or herbal medicinal products, please refer to the question Do I have to submit a PSUR my medicinal product if it is a generic, a product containing a well-established substance, a homeopathic or herbal medicinal product?
8. Do I have to submit a PSUR if the combination of active substances of my product is not in the EURD list but one or more individual components are listed?
If the specific fixed dose combination is not listed in the EURD list, PSURs should not be submitted according to the EURD list entry of one or more individual components. However PSURs should be submitted as specified in the conditions of the marketing authorisation for the combination product (if any), or otherwise according to the standard submission cycle (i.e. 6-monthly, yearly and thereafter 3-yearly) unless the combination medicinal product falls within the categories of medicinal products exempted from the obligation to submit PSURs.
MAHs or national competent authorities can request the inclusion of the fixed combination in the EURD list for reasons related to public health, in order to avoid duplication of assessment or in order to achieve international harmonisation. Instructions on how to submit requests to amend the EURD list can be found on the EURD list webpage .
9. Do I have to submit a PSUR if my medicinal product is not marketed?
MAHs are required to submit PSURs once a medicinal product is authorised in the EU, regardless of its marketing status.
10. Do I have to submit a PSUR if the marketing authorisation for my product has been granted on or after the data lock point (DLP) in the EURD list?
The MAH is not obliged to submit a PSUR if the granting of the Marketing Authorisation (MA) was notified on or after the DLP. The first PSUR will either be due following the subsequent DLP in the EURD list or, depending on the newly approved MA, a first PSUR submission might be considered earlier than the next DLP. However, if the MA was granted before the DLP, the obligation to submit applies.
11. My company holds a Parallel Import Authorisation; do we have to submit PSUR for these product(s)? If a PSUR is submitted will it be assessed?
Only MAHs are required to submit PSURs as per the provisions of Article 107b of Directive 2001/83/EC and Article 28(2) of Regulation (EC) No 726/2004. Parallel importers do not qualify as MAHs, and therefore they are not subject to the obligation to submit PSURs.
If however a PSUR has been submitted by a company holding a parallel import authorisation, such PSUR might be taken into account by the Lead Member State/PRAC Rapporteur and assessed in terms of its impact on the risk-benefit balance of the medicinal product concerned. If the data contained in the PSUR contribute meaningfully to the scientific assessment, these data should be included in the scope of the PSUR procedure. However, the parallel importer will not become party to the PSUR procedure and will not receive a copy of the assessment report and outcome documentation as a MAH would.
If a parallel importer receives a notification of an adverse drug reaction from a patient, a doctor or any other source, the parallel importer should inform this person that the adverse drug reactions should be reported directly to the MAH of the medicinal product concerned. The parallel importer should also inform the MAH immediately.
12. Do I have to submit a PSUR for my medicinal product if it is a generic, a product containing a well-established substance, a homeopathic or herbal medicinal product?
Medicinal products authorised under Articles 10(1), 10a, 14 or 16a of Directive 2001/83/EC are exempted from routine submission of PSURs unless otherwise specified in the marketing authorisation or required through the EURD list (see dedicated column “ Are PSURs required for products referred to in Articles 10(1), 10a, 14, 16a of Directive 2001/83/EC as amended? Yes/No ”). National competent authorities can also request PSUR for generic medicinal products at any time on the grounds detailed in Article 107c (2) of the Directive.
The MAHs of such medicinal products should use alternative mechanisms such as signal management and emerging safety issues channels to communicate relevant new safety information to regulatory authorities(see GVP Module VI and Module IX).
Additionally,product information should be kept up-to-date by the MAH by submitting the appropriate variations taking account of the latest scientific knowledge or conclusions of assessments and recommendations made public by means of the EMA and national competent authority websites.
Medicinal products which have been authorised through the equivalent legal basis as the current Articles 10(1) and 10a legal basis before the re-codification of the Directive 2001/83/EC i.e. respectively Article 4.8 a(iii), first paragraph (essential similarity) of Directive 65/65/EEC / 10 a(iii), first paragraph of Directive 2001/83/EC and Art 4.8 a(ii) (well established use) of Directive 65/65/EEC / 10.1 a(ii) of Directive 2001/83/EC are, by analogy, not required to submit PSUR unless there is a specific condition in the authorisation or there is an indication in the EURD list that PSUR submission is required, or in response to a specific request.
13. Do I have to submit a PSUR for my hybrid medicinal product?
Medicinal products authorised under Article 10(3) of Directive 2001/83/EC (hybrid application) are not exempted from the obligation to submit PSURs.
14. Do I have to submit a PSUR if my medicinal product is authorised in accordance with Article 126(a) of Directive 2001/83/EC?
Only MAHs are required to submit PSURs as per the provisions of Article 107b of Directive 2001/83/EC and Article 28(2) of Regulation (EC) No 726/2004. Holders of authorisation under Art 126a of Directive 2001/83/EC are not subject to the obligation to submit PSURs with regards to such authorisation.
15. Will the withdrawal/non-renewal/revocation of the marketing authorisation of my product impact on the ongoing EU single PSUR assessment?
In case of withdrawal, non-renewal or revocation of a marketing authorisation (MA) while the EU single PSUR assessment (PSUSA) procedure is ongoing, the impact on the ongoing procedure can be either that:
- If the PSUSA procedure includes MAs remaining valid, or
- If there are other medicinal products which contain the same active substance or combination of active substances (e.g. generics) as the medicinal product covered by the withdrawn/non-renewed/revoked MA.
- the procedure will be stopped, if the withdrawn/non-renewed/revoked MA is the only MA covered by the ongoing PSUR assessment procedure, unless there are important safety concerns to consider the recall of any remaining medicinal products available on the market or the assessment could inform on public health concerns on long-term safety effects of the concerned product or evaluation of other medicinal products (e.g. same class of products) on the market regarding scientific and technical progress or future risk management or for other public health reasons.
For centrally authorised medicinal products, where the EU single PSUR AR will be completed, the information will be reflected in the EPAR of the concerned medicinal product.
16. Will I have to submit PSUR after withdrawal/non-renewal/revocation of the marketing authorisation of my product? Rev. Apr 2021
Where a marketing authorisation is withdrawn, revoked or not renewed, the former marketing authorisation holder is encouraged to continue to collect spontaneous reports of suspected adverse reactions occurring in the EU (see Draft guideline on good pharmacovigilance practices: Module VI - Management and reporting of adverse reactions to medicinal products ) to, for example, facilitate review of delayed onset adverse reactions or of retrospectively notified cases of adverse reactions.
Depending on the date of the EC decision on the revocation or withdrawal, or the date of expiry of the marketing authorization in case of non-renewal, marketing authorisation holders may still be required to submit a PSUR:
- If the date is after the submission deadline specified in the EURD list, submission is mandatory irrespective of whether the date is before or after the start date of the procedure.
- If the date is prior to the submission date specified in the EURD list, submission is no longer required except for exceptional cases for centrally authorised medicinal products, whereby the former marketing authorisation holder may be requested to submit a final / ad-hoc periodic safety update report (PSUR). An agreement on the procedural details of the PSUR submission should be reached between the marketing authorisation holder and the Agency. Since such PSUR will not be subject to a single PSUR assessment, it should not be submitted to the PSUR repository.
In the case when the marketing authorisation of a medicinal product is suspended, this would not affect the requirement to submit a PSUR, since this situation corresponds to a temporary marketing cessation which could be lifted. The usual requirements in terms of submission apply. If the product has not been marketed during the whole reporting interval this should be specified in the PSUR.
17. Do PSURs need to contain case narratives and line listings?
The PSUR should focus on summary information, scientific assessment and integrated benefit-risk evaluation.
Marketing authorisation holders are not required to systematically include listings of individual cases, including case narratives, in the PSUR. However, they shall provide case narratives in the relevant risk evaluation section of the PSUR where integral to the scientific analysis of a signal or safety concern in the relevant risk evaluation section.
In this context “case narrative” refers to clinical evaluations of individual cases rather than the CIOMS narratives included in the individual case safety report (ICSR).
During the assessment of the PSUR, line listings for adverse reactions of special interest may be requested by the PRAC.
18. How can I submit the proposed changes to the product information within the PSUR for NAPs which are part of an EU single assessment?
According to the guidance set out in the Guideline on good pharmacovigilance practices (GVP): Module VII – Periodic safety update report , proposed changes to the EU labels as a result of the PSUR data should be provided under Section VII.C.5.1. PSUR EU regional appendix, sub-section “Proposed product information” of the PSUR.
It should be presented as a tracked change version of each EU SmPCs and package leaflets of the products concerned and each product information should be translated into English language including the tracked changes proposed, in order to enable the EU single assessment.
This can result in having to submit a large number of sets of tracked change product information with the additional burden of providing translations. Hence MAHs can consider the option to focus on the proposed amendments to SmPC and package leaflet. In such case, only the amended parts of the SmPC and package leaflet should be provided in track changes and in English language under the EU regional appendix.
Where the proposed changes are not based on the data submitted within the PSUR, these will not be considered and a variation will have to be submitted as appropriate to the relevant national competent authority.
In case no changes to the product information are being proposed as part of the PSUR, the MAH should not include any product information within the EU regional appendix.
19. Can I submit a RMP update together with my PSUR?
A risk management plan (RMP) update can be submitted with a PSUR for single centrally authorised medicinal product (CAP) or a mixture of CAPs belonging to the same global marketing authorisation (GMA) when the changes to the RMP are a direct result of data presented in the PSUR. In this case no stand-alone RMP variation is necessary.
If the above does not apply, the updated RMP should be submitted as a stand-alone variation.
A stand-alone variation should also be submitted for transitions to RMP Template Rev. 2 when significant changes, not consequential to the PSUR data, are proposed in the RMP, i.e. safety specifications changes (reclassifications or removal of safety concerns) related to the implementation of GVP V “Risk Management Systems” Rev. 2 principles. For the cases where there are also changes to the RMP consequential to the PSUR data, the stand-alone variation should be submitted in parallel to the PSUR procedure, to facilitate the assessment of the RMP update including both the changes related to the PSUR data and the other significant changes in the RMP to the safety specifications.
As an interim measure, submission of RMP updates cannot be accepted with PSURs subject to a PSUSA of:
- a mixture of CAPs pertaining to different GMAs;
- a mixture of centrally and nationally authorised medicinal products;
- a mixture of NAPs.
In these cases, MAHs should submit the updated RMPs as part of another procedure affecting the RMP, if one such procedure is foreseen. Alternatively, MAHs should submit a separate variation to update their RMP.
The EMA will check at the beginning of the procedure whether the submission of an RMP has been correctly performed in accordance to the above criteria. In order to facilitate this check, the MAH should confirm in the cover letter that the RMP update is a direct result of data in the PSUR. To further facilitate the check and assessment, the MAH should present clean and tracked changes Word versions of the RMP in the submission.
If an RMP is incorrectly submitted with a PSUR, this will be identified at the start of the procedure and both the MAH and PRAC Rapporteur will be made aware that the RMP will not be assessed and should be submitted through another appropriate procedure. If the RMP was submitted as an eCTD the MAH will have to delete that version of the RMP in the next sequence to maintain the correct lifecycle of the product.
The assessment of a PSUR may result in a recommendation to update the content of the RMP through a subsequent variation.
For nationally authorised medicinal products (i.e. authorised through MRP, DCP or national procedures), any RMP update should be submitted via a variation procedure to the national competent authority for assessment, even if PSURs are part of a PSUSA.
20. Can I submit a clinical study report together with my PSUR? Rev. Aug. 2020
The PSUR should provide comprehensive information on the findings of all PASS, both interventional and non-interventional, in PSUR sections 7 and 8 respectively as an integrated summary.
Information regarding completed clinical trials provided in the PSUR section “Summaries of significant findings from clinical trials during the reporting interval” can be presented in the PSUR in either a narrative format or as a synopsis.
The PSUR is not the appropriate procedure for submitting final or interim study reports to the EU regulatory authorities. Final study reports should be submitted and assessed via the appropriate procedure in line with the guidelines on the details of the various categories of variations, on the operations of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008. For centrally authorised products interim study reports not impacting on the product information or on the condition as stated in the Annex II of the marketing authorisation can be submitted as a post-authorisation measure (PAM) as described in question How and to whom shall I submit my PAM data (see Post-authorisation measures: questions and answers)? . For nationally authorised products, interim study reports, if requested, need to be submitted to the relevant competent authority unless specified otherwise.
However, in case a study report is able to further support either the discussion by the MAH or the PRAC/LMS’ assessment of the PSUR sections dealing with data from clinical trials, findings from non-interventional studies, or other clinical trials and sources, the MAH may provide the study report (or relevant parts thereof) as an appendix to the PSUR. The inclusion as an appendix does not discharge the MAH from their obligation to submit procedure in line with the above-mentioned guidelines on the details of the various categories of variations.
Relevant interim and final study results should be included in the PSUR sections “Summaries of significant findings from clinical trials during the reporting interval” and “Findings from non-interventional studies”, as applicable, in line with GVP VII.
21. What are the timelines for the submission of PSURs?
Marketing authorisation holders should submit to the Agency PSURs as established in GVP Module VII as follows:
- within 70 calendar days of the data lock point (day 0) for PSURs covering intervals up to 12 months (including intervals of exactly 12 months); or
- within 90 calendar days of the data lock point (day 0) for PSURs covering intervals in excess of 12 months;
- the timeline for the submission of ad hoc PSURs requested by competent authorities will be normally specified in the request, otherwise the ad hoc PSURs should be submitted within 90 days of the data lock point.
The deadline for the submission of PSURs (Day 70 or Day 90 following the DLP) is published in the EURD list. This deadline is legally binding and must be adhered to. However, the PSUR repository allows for a submission window between the DLP and the submission deadline, there is therefore no technical restriction preventing MAHs to submit their PSUR in advance of the deadline.
The procedural start dates is published in the PSUR assessment timetable. It should be noted that the PSUSA cut-off date continues to apply to procedures containing nationally authorised medicinal products, and indicates the next upcoming start date for relevant submission deadlines in the EURD list.
The are published on the EMA website.
- Guideline on good pharmacovigilance practices (GVP) Module VII – Periodic safety update report, Rev 1 (EMA/816292/2011, 09 December 2013)
- Timetable: Periodic safety update report (PSUR) and PSUR single assessment (PSUSA)
22. What happens if I missed the submission deadline?
It is the responsibility of MAHs to ensure that they submit the necessary PSUR by the submission deadline as stated in the EURD list and that they are not in breach of their legal obligations with respect to the submission of PSURs.
If you have missed the submission deadline due to technical issues with the PSUR Repository, please contact EMA as soon as possible via the Service Desk portal in order to request a late submission ID. Please note that late submissions can no longer be accepted once the procedure has started.
- User Guidance for marketing authorisation holders (MAHs) for PSUR repository
23. To whom should I submit my PSUR?
The use of the PSUR Repository is mandatory for all PSUR submissions. MAHs are required to submit PSURs directly to the PSUR repository using the eSubmission Gateway; the submission of PSURs directly to national competent authorities is no longer accepted. This affects all PSURs irrespective whether they are for centrally or nationally authorised medicinal products and whether they follow the EU single assessment or purely national PSUR procedure.
The obligation to submit to the PSUR Repository does not apply to products that have been given a positive CHMP scientific opinion under Article 58 of Regulation (EC) No 726/2004. For further information on how to submit PSURs for Article 58 products please refer to the guidance on Dossier requirements for Centrally Authorised Products (CAPs).
The use of the xml delivery file for submissions to the PSUR Repository is mandatory for all PSURs and any related submissions via the eSubmission Gateway and/or the Web Client. PSURs and any related submissions using the existing file naming conventions are no longer possible. The mandatory use of the PSUR xml delivery file is introduced to harmonise the submission mechanism for all PSURs and it applies to all types of PSURs and any related submissions. For further instructions on creation of the xml delivery file, please refer to the MAH PSUR Repository User Guidance document.
- Dossier requirements for centrally authorised products
- CMDh PSUR submission guidance document
- PSUR repository MAH user guidance document
- eSubmission website
- eSubmission gateway / Web client website
- Common repository website
- PSUR repository website
- Harmonised guidance for eCTD submissions in the EU
24. How to identify the official contact person for the PSUR?
The official contact person for the PSUR procedure is the one provided in the xml delivery file. This person will be the sole recipient of any communication from EMA throughout this procedure, including the PRAC Recommendation, CHMP/CMDh output, and Commission Decision, as applicable. The contact information provided in the xml delivery file will always override any information provided in the cover letter.
25. What fee do I have to pay? Rev. Apr 2021
The EMA levies a fee for the assessment of PSUR(s) since 26 August 2014.
For the PSUR assessment involving only one marketing authorisation holder (MAH) the total amount of the fee will be levied on that MAH.
For the PSUR assessment under a PSUSA procedure involving more than one MAH, the total amount of the fee will be divided among all the MAHs concerned proportionately to the number of chargeable units.
The MAHs concerned will be established on the basis of the obligation to submit the PSUR(s) and not on the basis of the actual PSUR submission(s) received by the EMA.
The total of chargeable units in the procedure will be identified from the Art. 57 database. The share payable by each marketing authorisation will be calculated by the EMA. An advice note will be generated at the data lock point (DLP) date and sent accordingly to the relevant QPPVs in order to ensure the accurate identification of the chargeable units for the products involved in the procedure. At start of the procedure, the invoice will be sent to each MAH with the relevant chargeable units calculation. The fee will be due to the EMA within 30 calendar days from the date of the invoice.
Applicants requiring a purchase order number or similar references on their invoice are encouraged to issue a standing (blanket) purchase order covering pharmacovigilance fees levied by the Agency for a given period and to provide such reference to the Agency’s accounts receivable service at [email protected]. The Agency does not accept stand-alone notifications of purchase order numbers or purchase order numbers associated with a dossier for this type of procedure only.
For MAHs already qualified as an SME (i.e. micro-, small- or medium-sized enterprise) by the EMA or for those that will send a SME declaration in advance of the start date or by the latest after 30 days of the invoice date, the fee will be reduced (small- or medium-sized enterprise) or waived (micro-sized enterprise).
The EMA has published further guidance on how the fees are calculated and collected.
- SME declaration
- Data submission for authorised medicines
26. How will my PSUR submission be handled?
The PSUR assessment under a PSUSA procedure is as follows, regardless whether it refers to one or more centrally authorised medicinal products, a mix of centrally authorised medicinal products and nationally authorised products, or nationally authorised products only.
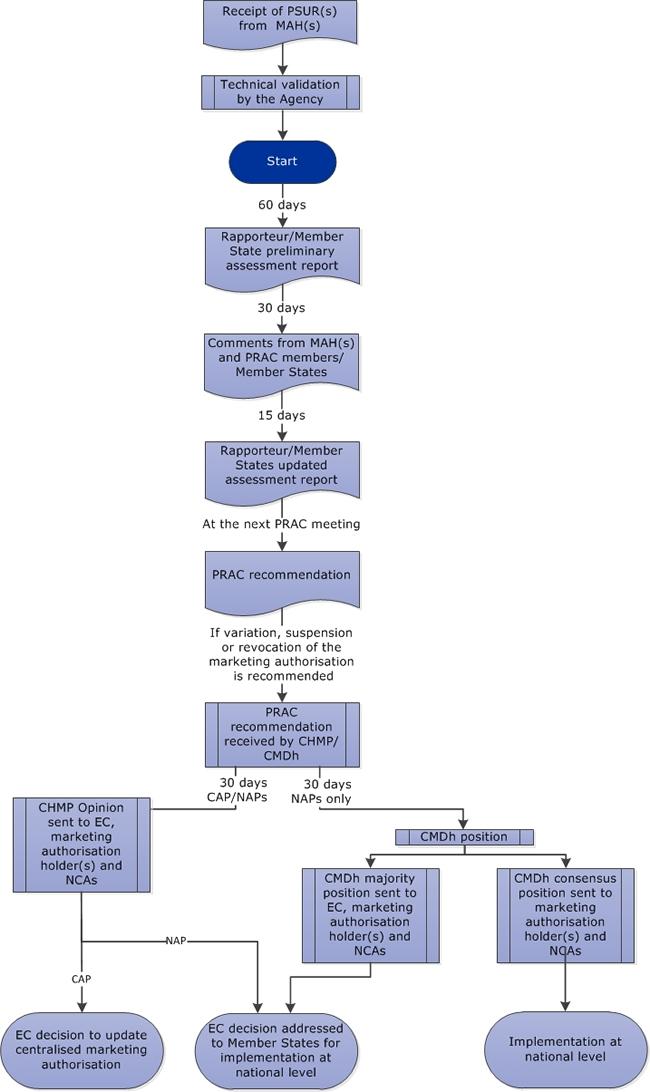
The assessment of a PSUR or several PSURs for the same active substance(s) is done by the PRAC or in case of nationally authorised products only by the appointed Lead Member State, respectively. The timelines for assessment are for up to 134 days followed by 67 days of Commission decision making process (if applicable). Upon technical* validation by the EMA of the submitted PSUR(s), the following timetable shall apply:
| Day 0 | Start of the procedure according to the |
| Day 60 | PRAC Rapporteur's / Lead Member State preliminary assessment report |
| Day 90 | MAH and PRAC members' / Lead Member States comments |
| Day 105 | PRAC Rapporteur's / Lead Member State updated assessment report (if necessary) |
| Day 120 | PRAC recommendation adoption with the PRAC assessment report |
| Day 134 | CHMP opinion / CMDh position (in case PRAC recommends a variation, suspension or revocation of the MA) |
*There is no validation of the content of the PSUR.
The MAH is expected to provide, as applicable, by Day 90:
- responses to the “request for supplementary information” as outlined in the relevant section of the PRAC Rapporteur / Lead Member State PSUR preliminary assessment report,
- comment on the proposed wording (in case the recommendation is a variation),
- propose a wording in case the recommendation is a variation but no exact wording is proposed by the PRAC Rapporteur / Lead Member State,
- provide a justification in case the MAH does not agree with the PRAC Rapporteur / Member State recommendation to vary, suspend or revoke the MA; and/or
- include additional comments or clarification deemed necessary by the MAH
The MAH's comments should be submitted as per the PSUR dossier submission requirements detailed in the question “ How shall I submit the response to a request for supplementary information during a PSUSA procedure?”.
In case of major disagreement with the PRAC Rapporteur’s/Lead Member State’s proposed Recommendation as stated in the updated assessment report, the MAH should contact the Product Lead (for CAPs) or Risk Management Specialist (for NAPs only) no later than two working days following receipt of the report. In this communication the MAH should indicate whether they would wish to make use of the opportunity of an oral explanation to defend their position before the PRAC. In the absence of a reply within two days, the EMA will assume that no oral explanation is requested
The MAH of centrally authorised medicinal products should submit a clean and a tracked version of the agreed amended product information prior to the adoption of the PRAC recommendation.
In case the PRAC adopts a recommendation on the maintenance of the marketing authorisation, such recommendation is not transmitted to the CHMP or CMDh and the procedure ends with the adoption of the PRAC recommendation.
In case the PRAC recommends any regulatory action i.e. variation, suspension or revocation of the marketing authorisation, the PRAC recommendation will be transmitted to the CHMP if it includes at least one CAP or to the CMDh if it includes only NAPs. At its next meeting following the PRAC recommendation, the CHMP or the CMDh, as applicable, will adopt an opinion or a position, respectively. Subsequently, where the procedure includes at least one CAP, the Commission will adopt a decision to the MAHs for the centrally authorised products and, as applicable, to the competent authorities of the Member States for nationally authorised products.
Where the procedure includes only NAPs, the procedure ends with the CMDh position in case of consensus and in case of a majority vote, the CMDh position will be followed by a Commission decision (CD) to the Member States, which respectively have to be implemented according to the timetable indicated in the CMDh position or within 30 days of the CD receipt by the Member States. For further details on the procedural aspects of the EU PSUSA for NAPs only, please refer to the relevant CMDh SOP .
The outcome of the PSUR assessment results in a legally binding decision or CMDh position and any action to vary, suspend or revoke the marketing authorisations must be implemented in a harmonised and timely manner for all products within the scope of the procedure across the EU.
There may be times when MAHs are requested to take action on the basis of aspects which was not necessarily part of the PSUSA procedural scope e.g. product information of some generics not in line with that of the originator, or products which have not implemented the outcome of a previous regulatory procedure. In such cases this information will be included in the “Other considerations” section of the PRAC assessment report. Any information included in this section, will be discussed by the CMDh (for nationally authorised products) with the aim of agreeing on any necessary action, which will then be transmitted to MAHs either in the CMDh minutes or as a press release (dependent on the issue). Guidance on the content of the “Other considerations” section may be found in the published PRAC PSUR assessment report templates ( Templates for assessors ).
For more information, see:
- CMDh Guidance on the Informal Work-Sharing procedure for follow-up for PSUSA for NAPs
Amendments to the SmPC, labelling and package leaflet as a result of the PSUR assessment are implemented without subsequent variation submission for centrally authorised products and through the appropriate variation at national level for nationally authorised products (including those authorised through the mutual recognition and decentralised procedures).
27. How shall I submit the response to a request for supplementary information during a PSUSA procedure?
No specific template needs to be followed for the responses to the request for supplementary information (RSI).
The submission requirements for responses to requests for supplementary information are the same as those for the submission of the PSURs. For the submission of responses to the PSUR Repository, the xml delivery file is filled in the same way as the original PSUR submission apart from the selection of 'response' as a regulatory activity (submission unit). This xml delivery file should be attached to the relevant eCTD/NeeS sequence submitted via the eSubmission Gateway/Web Client. The regulatory activity 'PSUR' can only be used for the 'initial' PSUR submission due to the built-in business rules linking to the submission deadline.
Please refer to the e-submission webpage and the PSUR Repository MAH user guide for more information on the creation of the delivery file.
28. How is the CHMP opinion/CMDh position structured and which annexes need to be translated? Re. Apr 2021
This section presents the translation process of procedures of the below Annexes to the CHMP opinion / CMDh position. The Annexes of both the CHMP opinion as well as the CMDh position will be translated into all EU languages following an agreed timetable.
In addition, a linguistic review by Member States of these Annexes in all EU languages is performed after adoption of the CHMP opinion and CMDh position.
Please be reminded that in accordance with Union data protection requirements, no personal data should be included in the annotated PIs. This applies to the English version submitted at the time of opinion, the draft translations submitted at D+5 and the final translations submitted at D+25. Please submit annotated PIs in an anonymised format (i.e. names of the reviewers removed from the track-changes). If you do not wish to do so, please ensure that the individuals whose data is included consented to its sharing with EMA and its further sharing by EMA with third parties such as other marketing authorisation applicants, marketing authorisation holders and National Competent Authorities, as relevant. EMA expressly disclaims any liability or accountability for the presence of unnecessary personal data in the annotated PI submitted by the marketing authorisation holder.
Procedures that contain centrally authorised products (CAP(s))
- Annex B: Annexes I, II, IIIA, IIIB, IV 1 (scientific conclusions and grounds for the variation of the marketing authorisation) and 127a (risk minimisation measures addressed to Member States)
Procedures that contain a mix of centrally authorised products (CAP(s)) and nationally authorised products (NAP(s))
For the CAP(s):
For the NAP(s):
- Annex I (scientific conclusions and grounds for variation to the terms of the marketing authorisations)
- Annex II (amendments to the product information of the nationally authorised medicinal products)
- Annex III (conditions to the marketing authorisations), as applicable
Procedures that contain nationally authorised products (NAP(s))
- Annex III or IV (timetable for implementation) 2 , as applicable
The preparation of the translation process
In view of the short timeframe for finalisation of the translations and in order to optimise the quality of the translations, the MAHs are strongly advised to prepare for the translation process well in advance in the pre-opinion / position stage, i.e. just following adoption of the PRAC recommendation for variation.
In case of a PSUSA procedure where several MAHs are involved, the EMA will coordinate the translation process by approaching the MAHs individually and provide the timelines accordingly. MAHs should translate all relevant Annexes for each procedure.
During the translation process
Depending on the type of outcome and whether a Commission Decision is required irrespective whether the procedure includes CAPs and/or NAPs, the timelines for the translation process vary depending on the need for a linguistic review as illustrated below:
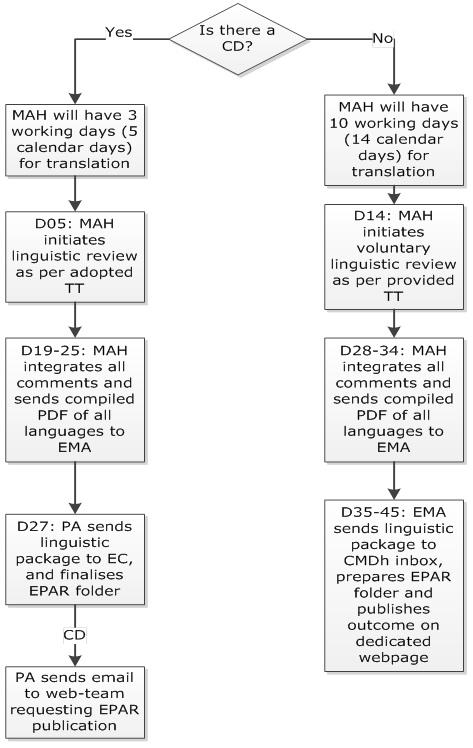
a. In case of CHMP opinion or CMDh position by majority i.e. followed a Commission Decision, the MAH has to provide the translations of the adopted Annexes in all EU languages (including Icelandic and Norwegian – if applicable as detailed below) according to the following timelines:
| Day 5 (5 days after opinion/ position) | Translations of the adopted Annexes in EN and in all other EU languages (Including Icelandic and Norwegian) are to be provided electronically (in one Eudralink package if applicable) to the Member states contact points for translations review and to the EMA's procedure assistant and the PSUSA Mailbox. |
| Day 19 (19 days after opinion/ position) | Member States will send linguistic comments on the Annexes to the MAH by e-mail with a copy to the PSUSA Mailbox. |
| Day 25 (25 days after opinion / position) | The MAH(s) will implement the required changes, compile the translations and send it back to the EMA. In case of disagreement between a Member State and the MAH, the EMA will not interfere in the translation process at this stage. Disagreements should be solved directly with the concerned MS. In order to facilitate and accelerate the check of the implementation of the' comments, the MAH should indicate in “ Submission of day +25 /235 final product information annexes (human and veterinary) - QRD Form 2 and checklist” for each language if all comments have been implemented or not. In the latter case, a justification should be provided for the appropriate language(s) stating why certain comments are not reflected in the final texts. |
b. In case of CMDh position by consensus, Member States may perform a voluntary linguistic review in the translation process, therefore the following timelines apply:
| Day 1 – 14 (1 to 14 days after position): | MAH translates the adopted Annexes in other EU languages based on the EN provided version. MAHs with marketing authorisations in Iceland and/or Norway will provide these languages as well. |
| Day 15 (15 days after the position): | Translations of the adopted Annexes in EN and all other EU languages (incl. Icelandic and Norwegian if applicable) are to be provided electronically (in one Eudralink package if applicable) to the Member States (MS) contact Points for Translations and to the EMA's procedure assistant and the PSUSA Mailbox for voluntary linguistic check. |
| Day 28-34 (28-34 days after position) | The MAH(s) will implement the required changes. Translation of the adopted Annexes in EN and in all other EU languages (Including Icelandic and Norwegian) are to be compiled and provided electronically (in one Eudralink package if applicable) to the EMA's procedure assistant and the PSUSA Mailbox. |
| Day 35-45 (35-45 days after position) | The EMA will send the package to the CMDh and prepare the translations for publication. |
After the translation process
Once the translations are received from the MAH, the Agency will check if all Member States' comments have been implemented.
- In case of a CHMP opinion or a CMDh majority position, the Agency will compile the Annexes in all languages and send the final copies to the Commission, members of the Standing Committee and the MAH(s) at Day 27 (27 days after opinion). Following receipt of the final compiled translations, the Commission will start the 22-day Standing Committee consultation, addressing only legal and public health matters (which means in principle no further linguistic review).
- In case of a CMDh position (by consensus), the Agency will compile the Annexes in all languages, send the final copies to the Member States and, where applicable, the full set of Annexes will be published on the EMA website.
Standards of translation of Annexes
- The structure of the English Annexes has to be strictly followed and should be exactly translated as per the adopted English version (i.e. full product information or only amendments to the relevant sections of the product information).
- For translations of Annexes QRD templates for each language should be used
- Make sure that the title pages are adjusted and all brackets (i.e. >) are taken out in the title.
- Do not leave sections out, do not update the Annex III, e.g. the sections [to be completed on a national level] simply to be translated as 'to be completed on a national level'.
- Good quality of the translations and compliance with the Member States' comments is required to facilitate the process.
If a translation is considered not to be of an acceptable quality, the Member State concerned will inform the MAH and the Agency within 3 days of receipt of the translation. The Agency will inform the MAH of the insufficient quality of the translations and the transmission to the Commission will be delayed until receipt of the amended translation (which would be expected within 1 week). A revised timetable will then be prepared.
The MAHs are also strongly advised to liaise directly with the Member States in case of disagreement with any of the comments made or in case further clarification on some comments is required, and to reflect the outcome in “ Submission of day +25 /235 final product information annexes (human and veterinary) - QRD Form 2 and checklist ”.
In addition, the MAHs are reminded that in case the complete product information is part of the Annex III, it should be presented in strict compliance with the Quality Review of Documents (QRD) convention to be followed for the European Medicines Agency QRD templates (e.g. format, layout and margins).
The Agency will monitor the quality of the translations, the review by the Member States and industry's compliance with the Member States' comments as part of the Performance Indicators.
- Quality Review of Documents (QRD) convention to be followed for the European Medicines Agency QRD templates
- Product-information templates
- Product information: Reference documents and guidelines
- The linguistic review process of product information in the centralised procedure - Human
- Member states contact points for translations review
- EC Guideline on the operation of the procedures laid down in Chapters II, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008
1 Annex IV are part of the next EPAR publication. However, they will not remain part of the EPAR and will become obsolete with the next following EPAR revision. They, however, remain part of the Commission Decision in the Union Registry on the Commission's webpage.
2 This time table is adopted in case a CMDh position reached by consensus and therefore not followed by a Commission Decision; in case of a majority position, the deadlines foreseen in the legislation for implementation after the Commission Decision apply.
29. To whom should I submit follow-up data requested after the conclusion of a PSUSA procedure?
The submission of post-authorisation measures (PAMs) for CAPs to address follow-up data request to a PSUSA must be done in eCTD format via the eSubmission Gateway/Web Client, and will be considered delivered to all national competent authorities’ representatives, alternates and scientific experts. PAMs must not be submitted to the PSUR Repository.
As a general principle no follow-up measures for NAPs should be submitted to the Agency outside a formal procedure as there is no regulatory/legal framework to conduct the assessment. Should there be exceptionally follow up data for NAPs to be submitted further to a PSUSA procedure, these must not be submitted to the PSUR Repository. Submission and assessment is expected to take place at national level and, as necessary, be coordinated across the Member States. See also Question ‘How will my PSUR be handled’ about section ‘Other considerations’ of the PSUSA assessment report. MAHs should contact the relevant Risk Management Specialist in case of such requests if there is a need for initial clarification on the process.
30. How can I know about the outcome of a PSUSA procedure?
Information on the outcome of centrally authorised medicinal products is made available in the European Public Assessment Report (EPAR) page of the relevant medicine.
Information regarding the variation of NAPs that are part of a CAP/NAP procedure is available in the Community Register for nationally authorised products.
Information on the outcome of the EU single assessment of PSURs involving nationally authorised medicinal products only is made available on the EMA website, on the ' Download medicine data ' page.
31. How shall I implement the outcome of a PSUSA procedure?
For PSUSA of CAPs the product information is varied as part of the Commission Decision issued to the MAHs, without the need for a variation. For CAPs outside the procedure (e.g. generics), the changes should be introduced through a variation IB C.I.3z.
For the NAPs included in the PSUSA procedure regardless whether NAP only, or mixed CAP and NAP products, the Commission decision is addressed to the Member States and therefore, it should be implemented by the NCAs within 30 days following its notification for all NAP products involved in the procedure (as listed in the Annex to the EC decision). By analogy to the implementation of referral procedures, the respective variations for the NAPs have to be submitted to the relevant NCA within 10 days after publication of the Commission Decision on the EC website.
For PSUSAs of NAPs, for which a CMDh position was adopted by consensus or majority (EC Commission Decision), a timetable for submission of the variations which is applicable for all affected products, including those that are not listed in the annex to the decision, is published on the EMA website. Changes to the product information after finalisation of a single PSUR assessment (with PRAC recommendation) may be implemented through the submission of a variation IA IN under category C.I.3.a if harmonised national translations are available and no further adaptation of the currently approved wording of the decision (EC Commission Decision or CMDh position) is necessary. In cases where the wording has to be adapted, a type IB under category C.I.3.z has to be submitted. In case the MAH wants to submit new data for assessment, a type II variation should be submitted.
For generic products or others not directly involved in the PSUSA procedure itself, the changes have to be submitted via a variation procedure according to the timelines indicated in the table below.
For NAPs, further guidance on implementing variation can be found on the CMDh website (Question Answers, Pharmacovigilance legislation) .
| CAP products | NAP products | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Not applicable;implemented through commission decision to MAH | Yes IB C.1.3.z | Yes - IA C.1.3.a (harmonised national translations available) - IB C.1.3.z (adaptation of wording needed) - II (new data submitted; classification dependent on proposed changes) | Yes - IA C.1.3.a (harmonised national translations available) - IB C.1.3.z (adaptation of wording needed) - II (new data submitted; classification dependent on proposed changes) | |
| Not applicable | MAHs to submit variations within two months after receipt of the EMA communication encompassing the safety updates referred to in the relevant PSUSA procedure | For CMDh position as per the dateindicated in the translation timetable i.e. 105 calendar days after adoption of the CMDh position. For CMDh position 10 days after publication of CD on EC website. For CHMP Opinion: 10 days after publication of CD on EC website. | For CMDh position as per the date indicated in the translation timetable i.e. 105 calendar days after adoption of the CMDh position. For CMDh position 60 days after publication of CD on EC website. For CHMP Opinion: 60 days after publication of CD on EC website. |
3 45 calendar days for translation publication + 60 calendar days from publication of translations
4 See also Q 3.3 of the QA list for the submission of variations according to Commission Regulation (EC) 1234/2008
32. Can PSURs still be submitted with renewal application?
PSURs, PSUR addendums, summary bridging reports and line listings should no longer be submitted as part of a renewal application. The clinical overview submitted in the renewal application should include relevant information to support the benefit-risk re-evaluation of the medicinal product. Please refer to the Guideline on the processing of renewals in the centralised procedure .
- Guideline on the processing of renewals in the centralised procedure
33. Who should I contact if I have a question regarding the preparation of a PSUR submission and during the procedure?
For centrally authorised products (CAPs), if you cannot find the answer to your question in the Q&A when preparing your application or during the procedure, please contact the Product Lead responsible for your product.
For nationally authorised products (NAPs), please submit your query using the Ask EMA feature.
In the web form you will be asked to provide your name, the name of your employer or organisation, contact details and the subject of your enquiry. You should type the full details of your query in the appropriate space. The use of key word 'PSUR' as a minimum in the subject line will help the Agency allocate your query to the correct person.
Please give as much detail as possible when completing your request (the procedure number of the PSUR single assessment as per the EURD list, the name of the product and the name of the active substance/combination of active substances) and be sure to include your correct and complete contact details. If the contact details you provide are incomplete or inaccurate this may prevent the Agency from communicating with you. In case of incomplete or incorrect data in the web form, the request may not be processed.
PSURs for NAPs will be handled by the Risk Management Specialist . You will be able to contact the RMS throughout the procedure.
34. Who should I contact if I have a technical issue with the submission of the PSUR?
For PSUR repository interface, eSubmission Gateway and/or the Web Client questions, issues, change requests and requests for services, please contact us through the Service Desk portal . This portal improves the efficiency of the technical support by allowing users to report issues, track progress of their queries and obtain answers to frequently asked questions. This portal replaces the following mailboxes ([email protected] [email protected], and [email protected]) which are to be used for all technical queries concerning these IT systems, such as e.g. Web Client/Gateway set-up, registration details or the transmission failures of files in the production or test environment.
The Agency aims to respond to your query within 5 working days. To help us deal with your enquiry, please provide as much information as possible including whether your query refers to a NAP or a CAP, the procedure number of the PSUR single assessment as per the EURD list, the name of the product and the name of the active substance/combination of active substances in your correspondence.
Please refer to the e submission webpage and the PSUR Repository MAH user guide for additional information.
35. Who should I contact if I have an issue related to the EURD list?
For information on how to submit requests for amendments of the EURD list or any other questions related to the EURD list, please refer to the EURD list submission requirements .
36. Who should I contact if I have an issue related to the payment of fee and QPPV advice notes? Rev. Apr 2021
For queries on fees and QPPV advice notes (please refer to question “ What fee do I have to pay? ”), you can contact us using the designated EMA fees query form available on the Pharmacovigilance fees: questions and answers webpage .
For queries on invoices already issued, please go to the Agency's How to pay page.
- Regulatory and procedural guidance
- Pharmacovigilance
Share this page
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Dietary and lifestyle management of functional hypothalamic amenorrhea: a comprehensive review.

Graphical Abstract
1. Introduction
2. methods and aim of work, 3.1. the impact of energy availability on the menstrual cycle, 3.2. dietary intervention, 3.3. the role of micronutrients in the management of fha, 3.4. psychological interventions in fha, 3.5. other nutritional and lifestyle interventions, 4. discussion, 5. conclusions, author contributions, data availability statement, conflicts of interest.
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003 , 88 , 297–311. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lieberman, J.L.; DE Souza, M.J.; Wagstaff, D.A.; Williams, N.I. Menstrual Disruption with Exercise Is Not Linked to an Energy Availability Threshold. Med. Sci. Sports Exerc. 2018 , 50 , 551–561. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Considine, R.V. Female Reproductive System. In Medical Physiology: Principles for Clinical Medicine , 4th ed.; Rhoades, R., Bell, D.R., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 693–709. [ Google Scholar ]
- Miller, P.B. Infrequent Menstrual Bleeding and Amenorrhea during the Reproductive Years. In Disorders of Menstruation ; Marshburn, P.B., Hurst, B.S., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2011; p. 140. [ Google Scholar ] [ CrossRef ]
- Gibson, M.E.S.; Fleming, N.; Zuijdwijk, C.; Dumont, T. Where Have the Periods Gone? The Evaluation and Management of Functional Hypothalamic Amenorrhea. J. Clin. Res. Pediatr. Endocrinol. 2020 , 12 , 18–27. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017 , 102 , 1413–1439. [ Google Scholar ] [ CrossRef ]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A.; Maciejewska-Jeske, M. Functional hypothalamic amenorrhea and its influence on women’s health. J. Endocrinol. Investig. 2014 , 37 , 1049–1056. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Podfigurna, A.; Meczekalski, B. Functional Hypothalamic Amenorrhea: A Stress-Based Disease. Endocrines 2021 , 2 , 203–211. [ Google Scholar ] [ CrossRef ]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014 , 1842 , 414–423. [ Google Scholar ] [ CrossRef ]
- Reed, J.L.; De Souza, M.J.; Mallinson, R.J.; Scheid, J.L.; I Williams, N. Energy availability discriminates clinical menstrual status in exercising women. J. Int. Soc. Sports Nutr. 2015 , 12 , 11. [ Google Scholar ] [ CrossRef ]
- Ortiga-Carvalho, T.M.; Oliveira, K.J.; Soares, B.A.; Pazos-Moura, C.C. The role of leptin in the regulation of TSH secretion in the fed state: In vivo and in vitro studies. J. Endocrinol. 2002 , 174 , 121–125. [ Google Scholar ] [ CrossRef ]
- Warren, M.P.; Sundgot-Borgen, J.; Fried, J.L. Amenorrhea, Osteoporosis, and Eating Disorders in Athletes. In Textbook of Sports Medicine: Basic Science and Clinical Aspects of Sports Injury and Physical Activity ; Kjaer, M., Krogsgaard, M., Magnusson, P., Engebretsen, L., Roos, H., Takala, T., Woo, S.L., Eds.; Blackwell Science: Oxford, UK, 2003; pp. 462–480. [ Google Scholar ]
- Bortoletto, P.; Reichman, D. Secondary Amenorrhea. In Reproductive Endocrinology and Infertility: Integrating Modern Clinical and Laboratory Practice ; Carrell, D.T., Peterson, C.M., Eds.; Springer: New York, NY, USA, 2010; pp. 33–37. [ Google Scholar ]
- Koltun, K.J.; De Souza, M.J.; Scheid, J.L.; Williams, N.I. Energy availability is associated with luteinizing hormone pulse frequency and induction of luteal phase defects. J. Clin. Endocrinol. Metab. 2020 , 105 , 185–193. [ Google Scholar ] [ CrossRef ]
- Practice Committee of the American Society for Reproductive Medicine. Current clinical irrelevance of luteal phase deficiency: A committee opinion. Fertil. Steril. 2015 , 103 , e27–e32. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Williams, N.I.; Leidy, H.J.; Hill, B.R.; Lieberman, J.L.; Legro, R.S.; De Souza, M.J. Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am. J. Physiol. Endocrinol. Metab. 2015 , 308 , E29–E39. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, D.; Hitchcock, C.L.; Barr, S.I.; Yu, T.; Prior, J.C. Negative Spinal Bone Mineral Density Changes and Subclinical Ovulatory Disturbances—Prospective Data in Healthy Premenopausal Women With Regular Menstrual Cycles. Epidemiologic Rev. 2013 , 36 , 137–147. [ Google Scholar ] [ CrossRef ]
- Fahrenholtz, I.L.; Sjödin, A.; Benardot, D.; Tornberg, Å.B.; Skouby, S.; Faber, J.; Sundgot-Borgen, J.K.; Melin, A.K. Within-day energy deficiency and reproductive function in female endurance athletes. Scand. J. Med. Sci. Sports 2018 , 28 , 1139–1146. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ryterska, K.; Kordek, A.; Załęska, P. Has Menstruation Disappeared? Functional Hypothalamic Amenorrhea—What Is This Story about? Nutrients 2021 , 13 , 2827. [ Google Scholar ] [ CrossRef ]
- Coelho, A.R.; Cardoso, G.; Brito, M.E.; Gomes, I.N.; Cascais, M.J. The female athlete Triad/Relative energy deficiency in sports (RED-S). Rev. Bras. Ginecol. Obstet. 2021 , 43 , 395–402. [ Google Scholar ] [ CrossRef ]
- De Souza, M.J.; Mallinson, R.J.; A Strock, N.C.; Koltun, K.J.; Olmsted, M.P.; A Ricker, E.; Scheid, J.L.; Allaway, H.C.; Mallinson, D.J.; Don, P.K.; et al. Randomised controlled trial of the effects of increased energy intake on menstrual recovery in exercising women with menstrual disturbances: The ‘REFUEL’ study. Hum. Reprod. 2021 , 36 , 2285–2297. [ Google Scholar ] [ CrossRef ]
- Łagowska, K.; Kapczuk, K.; Jeszka, J. Nine–month nutritional intervention improves restoration of menses in young female athletes and ballet dancers. J. Int. Soc. Sports Nutr. 2014 , 11 , 52. [ Google Scholar ] [ CrossRef ]
- De Souza, M.J.; Ricker, E.A.; Mallinson, R.J.; Allaway, H.C.M.; Koltun, K.J.; Strock, N.C.; Gibbs, J.C.; Don, P.K.; Williams, N.I. Bone mineral density in response to increased energy intake in exercising women with oligomenorrhea/amenorrhea: The REFUEL randomized controlled trial. Am. J. Clin. Nutr. 2022 , 115 , 1457–1472. [ Google Scholar ] [ CrossRef ]
- Mallinson, R.J.; Williams, N.I.; Olmsted, M.P.; Scheid, J.L.; Riddle, E.S.; De Souza, M.J. A case report of recovery of menstrual function following a nutritional intervention in two exercising women with amenorrhea of varying duration. J. Int. Soc. Sports Nutr. 2013 , 10 , 34. [ Google Scholar ] [ CrossRef ]
- Cominato, L.; da Silva, M.M.X.; Steinmetz, L.; Pinzon, V.; Fleitlich-Bilyk, B.; Damiani, D. Menstrual cycle recovery in patients with anorexia nervosa: The importance of insulin-like growth factor 1. Horm. Res. Paediatr. 2014 , 82 , 319–323. [ Google Scholar ] [ CrossRef ]
- Dempfle, A.; Herpertz-Dahlmann, B.; Timmesfeld, N.; Schwarte, R.; Egberts, K.M.; Pfeiffer, E.; Fleischhaker, C.; Wewetzer, C.; Bühren, K. Predictors of the resumption of menses in adolescent anorexia nervosa. BMC Psychiatry 2013 , 13 , 308. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jukic, A.M.Z.; Wilcox, A.J.; McConnaughey, D.R.; Weinberg, C.R.; Steiner, A.Z. 25-Hydroxyvitamin D and Long Menstrual Cycles in a Prospective Cohort Study. Epidemiology 2018 , 29 , 388–396. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, V.; Tamar, N.; Lone, Z.; Das, E.; Sahu, R.; Majumdar, S. Association between serum 25-hydroxy vitamin D level and menstrual cycle length and regularity: A cross-sectional observational study. Int. J. Reprod. Biomed. 2021 , 19 , 979–986. [ Google Scholar ] [ CrossRef ]
- Misra, M.; Klibanski, A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014 , 2 , 581–592. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium status and stress: The vicious circle concept revisited. Nutrients 2020 , 12 , 3672. [ Google Scholar ] [ CrossRef ]
- Parazzini, F.; Di Martino, M.; Pellegrino, P. Magnesium in the gynecological practice: A literature review. Magnes. Res. 2017 , 30 , 1–7. [ Google Scholar ] [ CrossRef ]
- Boyle, N.B.; Lawton, C.; Dye, L. The effects of magnesium supplementation on subjective anxiety and stress—A systematic review. Nutrients 2017 , 9 , 429. [ Google Scholar ] [ CrossRef ]
- Ji, X.; Grandner, M.A.; Liu, J. The relationship between micronutrient status and sleep patterns: A systematic review. Public Health Nutr. 2017 , 20 , 687–701. [ Google Scholar ] [ CrossRef ]
- Reid, B.M.; Georgieff, M.K. The Interaction between Psychological Stress and Iron Status on Early-Life Neurodevelopmental Outcomes. Nutrients 2023 , 15 , 3798. [ Google Scholar ] [ CrossRef ]
- Tian, X.; Diaz, F.J. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 2012 , 153 , 873–886. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lopresti, A.L. The effects of psychological and environmental stress on micronutrient concentrations in the body: A review of the evidence. Adv. Nutr. 2020 , 11 , 103–112. [ Google Scholar ] [ CrossRef ]
- Tranoulis, A.; Soldatou, A.; Georgiou, D.; Mavrogianni, D.; Loutradis, D.; Michala, L. Adolescents and young women with functional hypothalamic amenorrhoea: Is it time to move beyond the hormonal profile? Arch. Gynecol. Obstet. 2020 , 301 , 1095–1101. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Berga, S.L.; Marcus, M.D.; Loucks, T.L.; Hlastala, S.; Ringham, R.; A Krohn, M. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil. Steril. 2003 , 80 , 976–981. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hakimi, O.; Cameron, L.-C. Effect of exercise on ovulation: A systematic review. Sports Med. 2017 , 47 , 1555–1567. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Sports Endocrinol. 2016 , 47 , 12–26. [ Google Scholar ] [ CrossRef ]
- Hackney, A.C. Exercise as a stressor to the human neuroendocrine system. Medicina 2006 , 1 , 783–792. [ Google Scholar ] [ CrossRef ]
- Sanders, K.M.; Kawwass, J.F.; Loucks, T.; Berga, S.L. Heightened cortisol response to exercise challenge in women with functional hypothalamic amenorrhea. Am. J. Obstet. Gynecol. 2018 , 218 , 230.e1–230.e6. [ Google Scholar ] [ CrossRef ]
- Huhmann, K. Menses Requires energy: A review of how disordered eating, excessive exercise, and high stress lead to menstrual irregularities. Clin. Ther. 2020 , 42 , 401–407. [ Google Scholar ] [ CrossRef ]
- Kyriakidis, M.; Caetano, L.; Anastasiadou, N.; Karasu, T.; Lashen, H. Functional hypothalamic amenorrhoea: Leptin treatment, dietary intervention and counselling as alternatives to traditional practice—Systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016 , 198 , 131–137. [ Google Scholar ] [ CrossRef ]
- Roberts, R.E.; Farahani, L.; Webber, L.; Jayasena, C. Current understanding of hypothalamic amenorrhoea. Ther. Adv. Endocrinol. Metab. 2020 , 11 , 2042018820945854. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Syed-Abdul, M.M.; Soni, D.S.; Wagganer, J.D. Impact of a Professional Nutrition Program on a Female Cross Country Collegiate Athlete: A Case Report. Sports 2018 , 6 , 82. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011 , 29 (Suppl. S1), S7–S15. [ Google Scholar ] [ CrossRef ]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014 , 48 , 491–497. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Loucks, A.B.; Verdun, M.; Heath, E.M. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J. Appl. Physiol. 1998 , 84 , 37–46. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Joy, E.; De Souza, M.J.; Nattiv, A.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 Female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Optom. Vis. Sci. 2014 , 13 , 219–232. [ Google Scholar ] [ CrossRef ]
- Silva, M.R.; Paiva, T. Low energy availability and low body fat of female gymnasts before an international competition. Eur. J. Sport Sci. 2015 , 15 , 591–599. [ Google Scholar ] [ CrossRef ]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Faber, J.; Ritz, C.; Sjödin, A.; Sundgot-Borgen, J. The LEAF questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 2014 , 48 , 540–545. [ Google Scholar ] [ CrossRef ]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018 , 76 , 243–259. [ Google Scholar ] [ CrossRef ]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011 , 29 (Suppl. S1), S17–S27. [ Google Scholar ] [ CrossRef ]
- Mumford, S.L.; Chavarro, J.E.; Zhang, C.; Perkins, N.J.; Sjaarda, L.A.; Pollack, A.Z.; Schliep, K.C.; A Michels, K.; Zarek, S.M.; Plowden, T.C.; et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am. J. Clin. Nutr. 2016 , 103 , 868–877. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie (eng. Nutrition Standards for the Polish Population and Their Application) , 1st ed.; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny: Warsaw, Poland, 2020; pp. 68–437. [ Google Scholar ]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019 , 30 , 67–77.e3. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Study | Energy Availability (EA) Levels | Findings on LH Pulses | Findings on Menstrual Disorders | Notes |
|---|---|---|---|---|
| Loucks et al. [ ] | 30 kcal/kg FFM/day 20 kcal/kg FFM/day 10 kcal/kg FFM/day | −20 kcal/kg FFM/day, 16% decrease in LH pulse frequency, a 21% increase in amplitude, −10 kcal/kg FFM/day, 39% decrease in LH pulse frequency, 109% increase in amplitude | EA below 30 kcal/kg FFM/day is linked to a higher likelihood of menstrual disorders, such as oligo/amenorrhea | Establishes a threshold for EA below which LH pulsatility and menstrual function are impaired |
| Koltun et al. [ ] | No specific threshold identified. Reduction from 38 to 28 kcal/kg FFM/day | Decrease in LH pulse frequency by 0.017 pulses/hour for each unit decrease in EA. Lower EA also significantly reduces LH secretion frequency | Increased risk of luteal phase defects with lower EA. Significant EA reductions heighten the likelihood of menstrual disorders | No clear threshold for EA, but findings suggest more severe impacts with greater EA reduction |
| Liberman et al. [ ] | EA < 30 kcal/kg FFM/day | LH pulse frequency decreases and amplitude increases with reduced EA | Menstrual disorders (luteal phase defects, anovulation, oligomenorrhea) become more likely as EA decreases but can occur even above 30 kcal/kg FFM/day | Highlights that menstrual disorders can occur even above 30 kcal/kg FFM/day, challenging the strict threshold concept |
| Reed et al. [ ] | FHA group: 30.9 ± 2.4 kcal/kg FFM/day vs. 36.9 ± 1.7 kcal/kg FFM/day in control | No specific findings on LH pulses were provided | Women with functional hypothalamic amenorrhea (FHA)had significantly lower EA compared to regularly menstruating women | EA of 30 kcal/kg FFM/day does not clearly differentiate between regular menstruation and menstrual disorders |
| Study | Population | Intervention | Results | Conclusion |
|---|---|---|---|---|
| De Souza et al. [ ] | Thirty-three women (age 18–35) with secondary amenorrhea or oligomenorrhea, BMI 16–25 kg/m , exercising >2 h/week | Increased caloric intake by 330 ± 65 kcal/day (20–40%) over 12 months | Weight gain: 2.6 ± 0.4 kg, Fat mass gain: 2.0 ± 0.3 kg, Increase in T3 concentration by 9 ± 4 ng/dL | A modest caloric surplus (~300–350 kcal/day) is sufficient for restoring menstrual cycles. Improved energy balance leads to menstrual recovery |
| Łagowska et al. [ ] | Fifty-two athletes and ballet dancers with menstrual disorders, training >4 times/week | Increased caloric intake by 20–30%, energy availability increased by >30 kcal/kg FFM/day over 9 months | Weight gain: 1.3 kg (ballet dancers), no significant weight changes (athletes), Increased LH and LH/FSH ratio, Menstrual recovery in 3 dancers and 7 athletes | Increased caloric intake is critical for hormonal improvement and menstrual recovery. Menstrual function can be restored when body fat mass reaches 22% |
| Mallinson et al. [ ] | Two women with FHA of different durations | A 12-month nutritional intervention with individualized caloric increases | Weight gain: 4.3 kg (long-term FHA) and 2.8 kg (short-term FHA), Improvements in leptin and T3 concentrations | Weight gain and improved hormone levels are crucial for menstrual recovery, with individual variations of response |
| Cominato et al. [ ] | Adolescents with eating disorders | A 20-week nutritional intervention | Recovery of menstrual function linked to increases in BMI, LH, IGF-1, and estradiol | IGF-1 may serve as a potential marker for menstrual recovery. Nutritional rehabilitation is a key to restoring menstrual function |
| Deampfle et al. [ ] | One hundred and fifty-two girls (age 11–18) with eating disorders and underweight | Observational study followed participants over 12 months | Forty-seven percent regained menstrual function, Strong correlation between %EBW and resumption of menstruation | Achieving expected body weight is strongly associated with menstrual recovery. BMI is not a reliable predictor of menstrual function |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Dobranowska, K.; Plińska, S.; Dobosz, A. Dietary and Lifestyle Management of Functional Hypothalamic Amenorrhea: A Comprehensive Review. Nutrients 2024 , 16 , 2967. https://doi.org/10.3390/nu16172967
Dobranowska K, Plińska S, Dobosz A. Dietary and Lifestyle Management of Functional Hypothalamic Amenorrhea: A Comprehensive Review. Nutrients . 2024; 16(17):2967. https://doi.org/10.3390/nu16172967
Dobranowska, Katarzyna, Stanisława Plińska, and Agnieszka Dobosz. 2024. "Dietary and Lifestyle Management of Functional Hypothalamic Amenorrhea: A Comprehensive Review" Nutrients 16, no. 17: 2967. https://doi.org/10.3390/nu16172967
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

IMAGES
VIDEO
COMMENTS
The guideline is intended to assist sponsors in the development of a report that is complete, free from ambiguity, well organized and easy to review. ICH E3 Questions and Answers (PDF - 141KB)
Efficacy Guidelines
Literature review Revise guideline to clarify how this emerging area can contribute to ... ICH Efficacy Related Guidelines: M12, Drug Interaction Studies Keywords: FDA, CDER, SBIA, ICH, Health ...
This ICH GCP Guidance Integrated Addendum provides a unified standard for the European Union, Japan, the United States, Canada, and Switzerland to facilitate the mutual acceptance of data from ...
The revised version of the ICH M4E(R1) Guideline on Enhancing the Format and Structure of Benefit-Risk Information in ICH, was approved by the Assembly under Step 4 of the ICH Process at the Lisbon meeting in June 2016. ... The document proposes a review and revision in some parts of the Section 2.5 Clinical Overview of the Module 2 of the ...
GENERAL CONSIDERATIONS FOR CLINICAL STUDIES ...
Electronic Standards (ESTRI) Reflection Papers & Discussion Groups. Consideration Documents. CIOMS Glossary of ICH Terms & Definitions. ICH PQKM Task Force. Meetings. ICH Calendar. Assembly. Management Committee.
The ICH guideline 'General considerations for clinical studies' is intended to describe internationally accepted principles and practices in the design and conduct of clinical studies that will facilitate acceptance of data and results by regulatory authorities, provide guidance on the consideration of quality in the design and conduct of clinical studies across the product lifecycle ...
ICH E6(R3) Guideline. 1 1 I. INTRODUCTION. 2 Good Clinical Practice (GCP) is an international, ethical, scientific and quality standard for the 3 conduct of trials that involve human participants. Clinical trials conducted in accordance with 4 this standard will help to assure that the rights, safety and well-being of trial participants are 5 ...
The steps critical to planning a clinical trial include the idea, review of the available literature, identifying a problem, formulating the hypothesis, writing a synopsis, identifying the investigators, ... Quality assurance, according to the ICH and GCP guidelines, is necessary to be implemented during clinical research to generate quality ...
Published studies: drug Z is a nitric oxide (NO)-dependent vasodilator in canine models. 21 reports of syncope in drug Z-treated subjects to date in the development program (21/632, 3.3%), versus 3 (1.4%) in placebo. Most cases orthostatic, and/or associated with coadministration of nitrates or vasodilators.
The Technical Specification that are acceptable to all regulatory authorities of the ICH regions presents the conformance, cardinality, and other technical attributes that enable the interoperable electronic exchange of protocol content with a view to develop an open, non-proprietary standard to enable electronic exchange of clinical protocol ...
When searching the literature for pertinent papers and reviews, the usual rules apply: be thorough, use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and. look at who has cited past relevant papers and book chapters.
15 The Management Committee provides: Recommendations on the selection of new topics for harmonisation as well as on the adoption, withdrawal or amendments of ICH Guidelines The Assembly takes decisions: By consensus In the absence of consensus: vote in accordance with the Articles of Association, where only regulatory members have
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
ICH guidelines -"Q" series (quality guidelines) - A review. Khagga Bhavyasri 1, *, Kaitha Manisha Vishnumurthy 1, Dammu Rambabu 2 and Mogili Su makanth 1. 1 RBVRR Women's College of Pharmacy ...
Safety Guidelines. ICH has produced a comprehensive set of safety Guidelines to uncover potential risks like carcinogenicity, genotoxicity and reprotoxicity. A recent breakthrough has been a non-clinical testing strategy for assessing the QT interval prolongation liability: the single most important cause of drug withdrawals in recent years.
Another area where this ICH guideline interfaces with prior ischemic stroke guidelines is the challenging area of antithrombotic agent use in patients after ICH who are at risk for both recurrent ICH and ischemic stroke (Section 9.1.3, Management of Antithrombotic Agents). ... In a systematic review of the literature, including 11 patients with ...
for approving and authorising new. medicinal products [1]. The birth of ICH took p lace at a meeting in. April 1990, hosted by EFPIA in Brussels. Representatives of the regulatory agencies. and ...
Q8 (R2) Pharmaceutical development - Scientific. guideline. This document describes the suggested contents for the 3.2.P.2 (Pharmaceutical Development) section of a regulatory submission in the ICH M4 Common Technical Document (CTD) format. The guideline also indicates areas where the demonstration of greater understanding of pharmaceutical and ...
We highlight the ICH guidelines and CPMP points to consider on missing data. Specifically, we outline how we should consider missing data issues when designing, planning and conducting studies to minimize missing data impact. ... Review of guidelines and literature for handling missing data in longitudinal clinical trials with a case study ...
1. Introduction. The Development Safety Update Report (DSUR) proposed in this guideline is intended to be a common standard for periodic reporting on drugs under development (including marketed drugs that are under further study) among the ICH regions.
In the post-pandemic context, there has been an increasing demand for technology-based interventions in education and healthcare systems, such as augmented and mixed reality technologies. Despite the promising outcomes of applying mixed reality (MR), there is limited aggregated evidence focusing on child-patient interventions in hospital-based or clinical settings. This literature review ...
Critical care of patients on extracorporeal membrane oxygenation (ECMO) with acute brain injury (ABI) is notable for a lack of high-quality clinical evidence. Here, we offer guidelines for neurological care (neurological monitoring and management) of adults during and after ECMO support. These guidelines are based on clinical practice consensus recommendations and scientific statements.
This literature review, in light of the presented case report, explores the complex interplay between gabapentin (GBP), a gamma-aminobutyric acid (GABA) analog, and the hypothalamic-pituitary-adrenal (HPA) axis in patients undergoing major surgical procedures. It specifically investigates the potential impact of GBP on cortisol levels, stress responses, and infection risk, illustrated by a ...
This format is a legal requirement for both nationally authorised products and centrally authorised products. Guideline on good pharmacovigilance practices (GVP): Module VII - Periodic safety update report. Consultation dates: 21/02/2012 to 18/04/2012 Adopted Legal effective date: 13/12/2013 Reference Number: EMA/816292/2011 Rev.1*.
Functional Hypothalamic Amenorrhea (FHA) is a condition characterized by the absence of menstruation, which is increasingly affecting young women. However, specific recommendations for treating and preventing this condition are lacking. Based on a review of the available literature, this article provides practical and feasible dietary management recommendations for healthcare professionals and ...